Effects of Oral Glutamine Supplementation, Birthweight and Age on Colonic Morphology and Microbiome Development in Male Suckling Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design and Sample Collection
2.2. Colonic Morphometry, Histochemistry and Immunohistochemistry
2.3. Chemical Analyses
2.4. DNA Extraction and 16S rDNA Sequencing
2.5. Data Evaluation and Statistical Analysis
3. Results
3.1. Morphology of the Colon and Frequency of Goblet Cells, Intraepithelial Lymphocytes and IgA-Positive Cells
3.2. Bacterial Metabolites in the Colon Digesta
3.3. Impact of AA Supplementation on the Colonic Microbiota
3.4. Impact of Birthweight on the Colon Microbiota
3.5. Impact of Age on the Colon Microbiota
3.6. Interaction of Supplementation, Birthweight and Age Effects on Bacterial Phyla, Order and Genera
3.7. Quantitative Analysis, Ecological Indices and Principal Component Analysis of the Colonic Microbiota
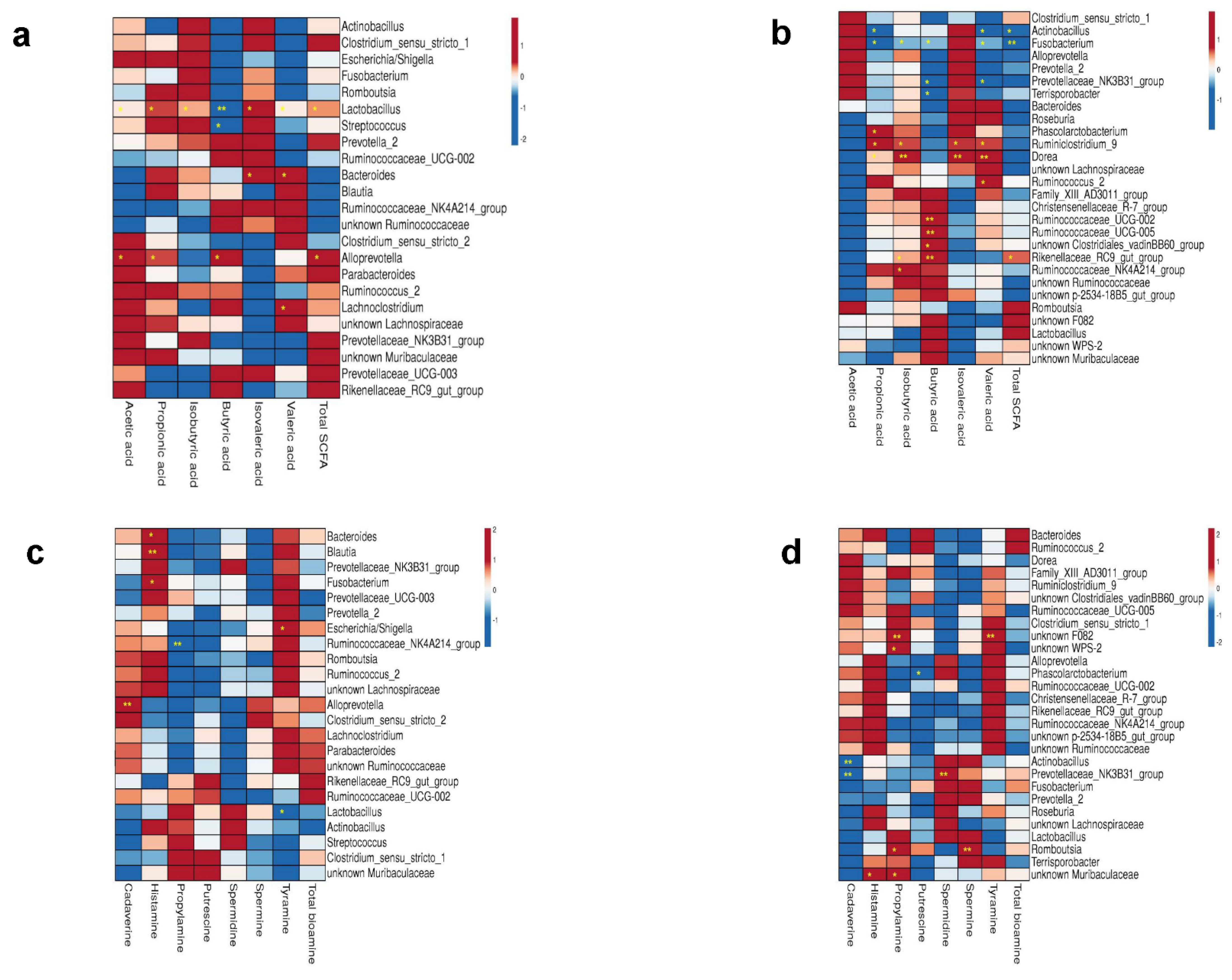
3.8. Correlations between Microbiota and Bacterial Metabolites in Colon Digesta
4. Discussion
4.1. Effects of Gln Supplementation
4.2. Effects of Birthweight
4.3. Comparison of Age Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.W. Milk and Dairy Products in Human Nutrition Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; Wiley: New York, NY, USA, 2013; Chapter 28; pp. 614–626. [Google Scholar]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, D.; Yin, Y.; Wang, X.; Li, P.; Dangott, L.J.; Hu, W.; Wu, G. Intrauterine Growth Restriction Affects the Proteomes of the Small Intestine, Liver, and Skeletal Muscle in Newborn Pigs. J. Nutr. 2008, 138, 60–66. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The Maturing Development of Gut Microbiota in Commercial Piglets during the Weaning Transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Ma, S.; Zhu, Z.; Su, Y.; Zoetendal, E.G.; Mackie, R.; Liu, J.; Mu, C.; Huang, R.; Smidt, H.; et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ. Microbiol. 2016, 18, 1566–1577. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Zhang, L.-L.; Vastenhouw, S.A.; Heilig, H.G.H.J.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Early-Life Environmental Variation Affects Intestinal Microbiota and Immune Development in New-Born Piglets. PLoS ONE 2014, 9, e100040. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Dattilo, A.M. Early Development of Intestinal Microbiota: Implications for Future Health. Gastroenterol. Clin. North Am. 2012, 41, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, A.P.; Rome, V.; Richard, M.; Formal, M.; Gall, S.D.; Boudry, G. Post-natal co-development of the microbiota and gut barrier function follows different paths in the small and large intestine in piglets. FASEB J. 2019, 34, 1430–1446. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Qiu, X.; Du, L.; Wang, J.; Wang, Q.; Huang, J.; Liu, Z. Changes of Gut Microbiota and Its Correlation With Short Chain Fatty Acids and Bioamine in Piglets at the Early Growth Stage. Front. Veter- Sci. 2021, 7, 617259. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Jiang, L.; Dai, Z.; Li, T.; Han, D.; Wang, J. Characterization of the Early Life Microbiota Development and Predominant Lactobacillus Species at Distinct Gut Segments of Low- and Normal-Birth-Weight Piglets. Front. Microbiol. 2019, 10, 797. [Google Scholar] [CrossRef] [Green Version]
- D’Inca, R.; Guen, C.G.-L.; Che, L.; Sangild, P.T.; Huërou-Luron, I. Intrauterine Growth Restriction Delays Feeding-Induced Gut Adaptation in Term Newborn Pigs. Neonatology 2011, 99, 208–216. [Google Scholar] [CrossRef] [PubMed]
- D’Inca, R.; Kloareg, M.; Guen, C.G.; le Huërou-Luron, I. Intrauterine Growth Restriction Modifies the Developmental Pattern of Intestinal Structure, Transcriptomic Profile, and Bacterial Colonization in Neonatal Pigs. J. Nutr. 2010, 140, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, S.; Jiang, L.; Wang, W.; Li, T.; Zuo, B.; Li, Z.; Wang, J. Differences in the Gut Microbiota Establishment and Metabolome Characteristics Between Low- and Normal-Birth-Weight Piglets During Early-Life. Front. Microbiol. 2018, 9, 1798. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. (Lausanne) 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N. Biogenic Amines: Signals Between Commensal Microbiota and Gut Physiology. Front. Endocrinol. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sciascia, Q.L.; Görs, S.; Nguyen, N.; Baghal, F.R.; Schregel, J.; Tuchscherer, A.; Zentek, J.; Metges, C.C. Glutamine supplementation moderately affects growth, plasma metabolite and free amino acid patterns in neonatal low birth weight piglets. Br. J. Nutr. 2022, 1–11. [Google Scholar] [CrossRef]
- Wu, G.; Knabe, D.A.; Yan, W.; Flynn, N.E. Glutamine and glucose metabolism in enterocytes of the neonatal pig. Am. J. Physiol. Integr. Comp. Physiol. 1995, 268, R334–R342. [Google Scholar] [CrossRef]
- Wu, G.; Meier, S.A.; Knabe, D.A. Dietary Glutamine Supplementation Prevents Jejunal Atrophy in Weaned Pigs. J. Nutr. 1996, 126, 2578–2584. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Li, X.-L.; Xi, P.-B.; Zhang, J.; Wu, G.; Zhu, W.-Y. l-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 2013, 45, 501–512. [Google Scholar] [CrossRef] [PubMed]
- A Cabrera, R.; Usry, J.L.; Arrellano, C.; Nogueira, E.T.; Kutschenko, M.; Moeser, A.J.; Odle, J. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J. Anim. Sci. Biotechnol. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Ji, F.; Wang, L.; Yang, H.; Hu, A.; Yin, Y. Review: The roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal 2019, 13, 2727–2735. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, P.; Li, X.; Zhou, H.; Wang, F.; Li, D.; Yin, Y.; Wu, G. Gene Expression Is Altered in Piglet Small Intestine by Weaning and Dietary Glutamine Supplementation. J. Nutr. 2008, 138, 1025–1032. [Google Scholar] [CrossRef]
- Aescht, E.; Büchl-Zimmermann, S.; Burmester, A.; Dänhardt-Pfeiffer, S.; Desel, C.; Hamers, C.; Jach, G.; Kässens, M.; Makovitzky, J.; Mulisch, M.; et al. Romeis Mikroskopische Technik; Mulisch, M., Welsch, U., Eds.; Spektrum Akademischer Verlag Heidelberg: Heidelberg, Germany, 2010; Volume 18, Chapter 2; pp. 39–179. [Google Scholar]
- Mowry, R.W. Special Value of Methods That Color Both Acidic and Vicinal Hydroxl Groups in Histochemical Study of Mucins—With Revised Directions for Colloidal Iron Stain, Use of Alcian Blue G8x and Their Combinations with Periodic Acid-Schiff Reaction. Ann. N. Y. Acad. Sci. 1963, 106, 402. [Google Scholar] [CrossRef]
- Liu, P.; Pieper, R.; Rieger, J.; Vahjen, W.; Davin, R.; Plendl, J.; Meyer, W.; Zentek, J.; Liu, P. Effect of Dietary Zinc Oxide on Morphological Characteristics, Mucin Composition and Gene Expression in the Colon of Weaned Piglets. PLoS ONE 2014, 9, e91091. [Google Scholar] [CrossRef]
- Luise, D.; Bertocchi, M.; Motta, V.; Salvarani, C.; Bosi, P.; Luppi, A.; Fanelli, F.; Mazzoni, M.; Archetti, I.; Maiorano, G.; et al. Bacillus sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J. Anim. Sci. Biotechnol. 2019, 10, 74. [Google Scholar] [CrossRef]
- Waly, N.; Gruffydd-Jones, T.; Stokes, C.; Day, M. The Distribution of Leucocyte Subsets in the Small Intestine of Healthy Cats. J. Comp. Pathol. 2001, 124, 172–182. [Google Scholar] [CrossRef]
- German, A.; Hall, E.; Day, M. Analysis of Leucocyte Subsets in the Canine Intestine. J. Comp. Pathol. 1999, 120, 129–145. [Google Scholar] [CrossRef]
- Ferrara, F.; Tedin, L.; Pieper, R.; Meyer, W.; Zentek, J. Influence of medium-chain fatty acids and short-chain organic acids on jejunal morphology and intra-epithelial immune cells in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2017, 101, 531–540. [Google Scholar] [CrossRef]
- Hornickel, I.N.; Kacza, J.; Schnapper, A.; Beyerbach, M.; Schoennagel, B.; Seeger, J.; Meyer, W. Demonstration of Substances of Innate Immunity in the Esophageal Epithelium of Domesticated Mammals. Part I—Methods and Evaluation of Comparative Fixation. Acta Histochem. 2011, 113, 163–174. [Google Scholar] [CrossRef]
- Kröger, S.; Vahjen, W.; Zentek, J. Influence of Lignocellulose and Low or High Levels of Sugar Beet Pulp on Nutrient Digestibility and the Fecal Microbiota in Dogs. J. Anim. Sci. 2017, 95, 1598–1605. [Google Scholar] [CrossRef]
- Pieper, R.; Kröger, S.; Richter, J.F.; Wang, J.; Martin, L.; Bindelle, J.; Htoo, J.K.; von Smolinski, D.; Vahjen, W.; Zentek, J.; et al. Fermentable Fiber Ameliorates Fermentable Protein-Induced Changes in Microbial Ecology, but Not the Mucosal Response, in the Colon of Piglets. J. Nutr. 2012, 142, 661–667. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using Qiime 2 (Vol 37, Pg 852, 2019). Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glockner, F.O. The Silva and “All-Species Living Tree Project (Ltp)” Taxonomic Frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Vahjen, W.; Dadi, T.; Saliu, E.-M.; Boroojeni, F.G.; Zentek, J. Synergistic Effects of Probiotics and Phytobiotics on the Intestinal Microbiota in Young Broiler Chicken. Microorganisms 2019, 7, 684. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Haynes, T.E.; Li, P.; Li, X.; Shimotori, K.; Sato, H.; Flynn, N.E.; Wang, J.; Knabe, D.A.; Wu, G. l-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 2009, 37, 131–142. [Google Scholar] [CrossRef]
- Wiarda, J.E.; Trachsel, J.M.; Bond, Z.F.; Byrne, K.A.; Gabler, N.K.; Loving, C.L. Intraepithelial T Cells Diverge by Intestinal Location as Pigs Age. Front. Immunol. 2020, 11, 1139. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, H.; Liu, G.; Chen, S.; Tan, B.; Ren, W.; Bazer, F.W.; Wu, G.; Yin, Y. Glutamine Promotes Intestinal Siga Secretion through Intestinal Microbiota and Il-13. Mol. Nutr. Food Res. 2016, 60, 1637–1648. [Google Scholar] [CrossRef]
- Schregel, J.; Holthausen, J.S.; Sciascia, Q.L.; Li, Z.; Görs, S.; Eggert, A.; Tuchscherer, A.; Zentek, J.; Metges, C.C. Effects of Oral Glutamine Supplementation on Jejunal Morphology, Development, and Amino Acid Profiles in Male Low Birth Weight Suckling Piglets. PLoS ONE 2022, 17, e0267357. [Google Scholar] [CrossRef]
- Ren, W.; Duan, J.; Yin, J.; Liu, G.; Cao, Z.; Xiong, X.; Chen, S.; Li, T.; Yin, Y.; Hou, Y.; et al. Dietary l-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids 2014, 46, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Portune, K.J.; Beaumont, M.; Davila, A.-M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.J.; de Boer, V.C.J. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients 2020, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Han, X.; Fang, J.; Jiang, H. Role of dietary amino acids and microbial metabolites in the regulation of pig intestinal health. Anim. Nutr. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Yi, D.; Li, B.; Hou, Y.; Wang, L.; Zhao, D.; Chen, H.; Wu, T.; Zhou, Y.; Ding, B.; Wu, G. Dietary supplementation with an amino acid blend enhances intestinal function in piglets. Amino Acids 2018, 50, 1089–1100. [Google Scholar] [CrossRef]
- Chamorro, S.; de Blas, C.; Grant, G.; Badiola, I.; Menoyo, D.; Carabaño, R. Effect of dietary supplementation with glutamine and a combination of glutamine-arginine on intestinal health in twenty-five-day-old weaned rabbits1. J. Anim. Sci. 2009, 88, 170–180. [Google Scholar] [CrossRef]
- Van den Berg, A.; Fetter, W.P.; Westerbeek, E.A.; van der Vegt, I.M.; van der Molen, H.R.; van Elburg, R.M. The Effect of Glutamine-Enriched Enteral Nutrition on Intestinal Permeability in Very-Low-Birth-Weight Infants: A Randomized Controlled Trial. JPEN J. Parenter Enteral Nutr. 2006, 30, 408–414. [Google Scholar] [CrossRef]
- Loh, G.; Eberhard, M.; Brunner, R.M.; Hennig, U.; Kuhla, S.; Kleessen, B.; Metges, C.C. Inulin Alters the Intestinal Microbiota and Short-Chain Fatty Acid Concentrations in Growing Pigs Regardless of Their Basal Diet. J. Nutr. 2006, 136, 1198–1202. [Google Scholar] [CrossRef]
- He, S.; Kahles, F.; Rattik, S.; Nairz, M.; McAlpine, C.S.; Anzai, A.; Selgrade, D.; Fenn, A.M.; Chan, C.T.; Mindur, J.E.; et al. Gut Intraepithelial T Cells Calibrate Metabolism and Accelerate Cardiovascular Disease. Nature 2019, 566, 115–119. [Google Scholar] [CrossRef]
- Blachier, F.; Andriamihaja, M.; Kong, X. Fate of Undigested Proteins in the Pig Large Intestine: What Impact on the Large Intestine Epithelium? Anim. Nutr. 2021, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Bardocz, S. The role of dietary polyamines. Eur. J. Clin. Nutr. 1993, 47, 683–690. [Google Scholar] [PubMed]
- Gaukroger, C.H.; Stewart, C.J.; Edwards, S.A.; Walshaw, J.; Adams, I.P.; Kyriazakis, I. Changes in Faecal Microbiota Profiles Associated With Performance and Birthweight of Piglets. Front. Microbiol. 2020, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.; Ingerslev, H.-C.; Sturek, M.; Alloosh, M.; Cirera, S.; Christoffersen, B.; Moesgaard, S.G.; Larsen, N.; Boye, M. Characterisation of Gut Microbiota in Ossabaw and Göttingen Minipigs as Models of Obesity and Metabolic Syndrome. PLoS ONE 2013, 8, e56612. [Google Scholar] [CrossRef] [PubMed]
- Karasova, D.; Crhanova, M.; Babak, V.; Jerabek, M.; Brzobohaty, L.; Matesova, Z.; Rychlik, I. Development of piglet gut microbiota at the time of weaning influences development of postweaning diarrhea—A field study. Res. Veter- Sci. 2020, 135, 59–65. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Morise, A.; Louveau, I.; Le Huërou-Luron, I. Growth and development of adipose tissue and gut and related endocrine status during early growth in the pig: Impact of low birth weight. Animal 2008, 2, 73–83. [Google Scholar] [CrossRef]
- Nebendahl, C.; Krüger, R.; Görs, S.; Albrecht, E.; Martens, K.; Hennig, S.; Storm, N.; Höppner, W.; Pfuhl, R.; Metzler-Zebeli, B.U.; et al. Effects on Transcriptional Regulation and Lipid Droplet Characteristics in the Liver of Female Juvenile Pigs after Early Postnatal Feed Restriction and Refeeding Are Dependent on Birth Weight. PLoS ONE 2013, 8, e76705. [Google Scholar] [CrossRef]
- Zhao, Y.; Albrecht, E.; Sciascia, Q.; Li, Z.; Görs, S.; Schregel, J.; Metges, C.; Maak, S. Effects of Oral Glutamine Supplementation on Early Postnatal Muscle Morphology in Low and Normal Birth Weight Piglets. Animals 2020, 10, 1976. [Google Scholar] [CrossRef]
- Radka, C.D.; Frank, M.W.; Rock, C.O.; Yao, J. Fatty acid activation and utilization by Alistipes finegoldii, a representative Bacteroidetes resident of the human gut microbiome. Mol. Microbiol. 2020, 113, 807–825. [Google Scholar] [CrossRef]
- Tang, S.; Xin, Y.; Ma, Y.; Xu, X.; Zhao, S.; Cao, J. Screening of Microbes Associated With Swine Growth and Fat Deposition Traits Across the Intestinal Tract. Front. Microbiol. 2020, 11, 586776. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Y.; Fu, H.; Xiong, X.; Fang, S.; Jiang, H.; Wu, J.; Yang, H.; Gao, J.; Huang, L. Expanded catalog of microbial genes and metagenome-assembled genomes from the pig gut microbiome. Nat. Commun. 2021, 12, 1106. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Everaert, N.; Van Cruchten, S.; Weström, B.; Bailey, M.; Van Ginneken, C.; Thymann, T.; Pieper, R. A review on early gut maturation and colonization in pigs, including biological and dietary factors affecting gut homeostasis. Anim. Feed Sci. Technol. 2017, 233, 89–103. [Google Scholar] [CrossRef]
- Jang, K.B.; Kim, S.W. Role of milk carbohydrates in intestinal health of nursery pigs: A review. J. Anim. Sci. Biotechnol. 2022, 13, 6. [Google Scholar] [CrossRef]
- Verdile, N.; Mirmahmoudi, R.; Brevini, T.; Gandolfi, F. Evolution of pig intestinal stem cells from birth to weaning. Animal 2019, 13, 2830–2839. [Google Scholar] [CrossRef]
- Alam, M.; Midtvedt, T.; Uribe, A. Differential cell kinetics in the ileum and colon of germfree rats. Scand. J. Gastroenterol. 1994, 29, 445–451. [Google Scholar] [CrossRef]
- Umesaki, Y.; Okada, Y.; Matsumoto, S.; Imaoka, A.; Setoyama, H. Segmented Filamentous Bacteria Are Indigenous Intestinal Bacteria That Activate Intraepithelial Lymphocytes and Induce MHC Class II Molecules and Fucosyl Asialo GM1 Glycolipids on the Small Intestinal Epithelial Cells in the Ex-Germ-Free Mouse. Microbiol. Immunol. 1995, 39, 555–562. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides Thetaiotaomicron and Faecalibacterium Prausnitzii Influence the Production of Mucus Glycans and the Development of Goblet Cells in the Colonic Epithelium of a Gnotobiotic Model Rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Van Kaer, L.; Olivares-Villagómez, D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J. Immunol. 2018, 200, 2235–2244. [Google Scholar] [CrossRef]
- Burkey, T.E.; Skjolaas, K.A.; Minton, J.E. BOARD-INVITED REVIEW: Porcine mucosal immunity of the gastrointestinal tract1. J. Anim. Sci. 2009, 87, 1493–1501. [Google Scholar] [CrossRef]
- Rothkotter, H.J.; Ulbrich, H.; Pabst, R. The Postnatal Development of Gut Lamina Propria Lymphocytes: Number, Proliferation, and T and B Cell Subsets in Conventional and Germ-Free Pigs. Pediatr. Res. 1991, 29, 237–242. [Google Scholar] [CrossRef]
- Collinder, E.; Cardona, M.E.; Kozakova, H.; Norin, E.; Stern, S.; Midtvedt, T. Biochemical Intestinal Parameters in Pigs Reared Outdoors and Indoors, and in Germ-Free Pigs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002, 49, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Hedemann, M.S.; Eskildsen, M.; Lærke, H.N.; Pedersen, C.; Lindberg, J.E.; Laurinen, P.; Knudsen, K.E.B. Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting fiber concentrations and fiber properties1. J. Anim. Sci. 2006, 84, 1375–1386. [Google Scholar] [CrossRef]
- Hurley, W.L. The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, NL, USA, 2015; Volume 1, Chapter 9; pp. 193–230. [Google Scholar]
- Beaumont, M.; Cauquil, L.; Bertide, A.; Ahn, I.; Barilly, C.; Gil, L.; Canlet, C.; Zemb, O.; Pascal, G.; Samson, A.; et al. Gut Microbiota-Derived Metabolite Signature in Suckling and Weaned Piglets. J. Proteome Res. 2021, 20, 982–994. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and Cultivation Study of Muribaculaceae Reveals Novel Species, Host Preference, and Functional Potential of This yet Undescribed Family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Lührmann, A.; Ovadenko, K.; Hellmich, J.; Sudendey, C.; Belik, V.; Zentek, J.; Vahjen, W. Characterization of the fecal microbiota of sows and their offspring from German commercial pig farms. PLoS ONE 2021, 16, e0256112. [Google Scholar] [CrossRef] [PubMed]


| Supp | BiW | Age | p Values 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Gln | Ala | LBW | NBW | 5 d | 12 d | SEM | Supp | BiW | Age | Supp × BiW | Supp × Age | BiW × Age | Supp × BiW × Age |
| Morphometry | ||||||||||||||
| CD, μm | 237 | 236 | 234 | 238 | 210 | 263 | 2.13 | 0.730 | 0.197 | <0.001 | 0.490 | 0.001 | <0.001 | 0.026 |
| CA, μm2 | 10,950 | 10,993 | 10,964 | 10,979 | 9389 | 12,554 | 144 | 0.842 | 0.947 | <0.001 | 0.259 | 0.010 | 0.002 | 0.008 |
| AB—PAS staining of Goblet cells 2 | ||||||||||||||
| Acid | 21.9 | 24.7 | 22.7 | 23.8 | 21.7 | 24.8 | 1.07 | 0.196 | 0.609 | 0.145 | 0.234 | 0.684 | 0.244 | 0.681 |
| Neutral | 67.1 | 69.2 | 70.9 | 65.4 | 75.4 | 60.9 | 2.64 | 0.686 | 0.280 | 0.006 | 0.296 | 0.915 | 0.527 | 0.128 |
| Mixed | 84.1 | 87.1 | 89.2 | 82.0 | 96.4 | 74.8 | 2.60 | 0.513 | 0.130 | <0.001 | 0.330 | 0.759 | 0.303 | 0.080 |
| Total | 173 | 181 | 183 | 171 | 194 | 161 | 5.25 | 0.430 | 0.248 | 0.001 | 0.213 | 0.911 | 0.578 | 0.130 |
| CD3+ lymphocytes 3 | ||||||||||||||
| CD3+ IEL | 1.97 | 1.76 | 1.77 | 1.96 | 1.15 | 2.60 | 0.07 | 0.028 | 0.047 | <0.001 | 0.255 | 0.107 | 0.165 | 0.043 |
| CD3+ lamina propria | 8.70 | 8.28 | 8.83 | 8.65 | 5.90 | 11.2 | 0.18 | 0.054 | 0.109 | <0.001 | 0.911 | 0.495 | 0.514 | 0.603 |
| IgA-positive cells in lamina propria 4 | ||||||||||||||
| IgA-positive cells | 8.17 | 8.71 | 8.04 | 8.83 | n.d | 8.44 | 0.54 | 0.627 | 0.479 | n.a | 0.911 | n.a | n.a | n.a |
| Supp | BiW | Age | p Values 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item, µmol/g Wet Weight | Gln | Ala | LBW | NBW | 5 d | 12 d | SEM | Supp | BiW | Age | Supp × BiW | Supp × Age | BiW × Age | Supp × BiW × Age |
| Spermine | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.00 | 0.275 | 0.534 | 0.764 | 0.351 | 0.840 | 0.744 | 0.076 |
| Cadaverine | 0.05 | 0.26 | 0.04 | 0.27 | 0.04 | 0.27 | 0.07 | 0.036 | 0.026 | 0.253 | 0.023 | 0.163 | 0.189 | 0.258 |
| Tyramine | 0.06 | 0.17 | 0.05 | 0.17 | 0.05 | 0.17 | 0.05 | 0.087 | 0.057 | 0.019 | 0.058 | 0.152 | 0.086 | 0.049 |
| Propylamine | 0.06 | 0.03 | 0.04 | 0.05 | 0.04 | 0.05 | 0.01 | 0.111 | 0.676 | 0.198 | 0.931 | 0.067 | 0.672 | 0.712 |
| Histamine | 0.07 | 0.09 | 0.07 | 0.09 | 0.07 | 0.09 | 0.02 | 0.698 | 0.611 | 0.546 | 0.192 | 0.725 | 0.901 | 0.319 |
| Spermidine | 0.29 | 0.39 | 0.36 | 0.33 | 0.36 | 0.33 | 0.03 | 0.020 | 0.205 | <0.001 | 0.099 | 0.485 | 0.034 | 0.427 |
| Putrescine | 0.58 | 0.46 | 0.46 | 0.58 | 0.46 | 0.58 | 0.07 | 0.152 | 0.208 | 0.018 | 0.470 | 0.074 | 0.189 | 0.819 |
| Total biogenic amines | 1.14 | 1.43 | 1.05 | 1.52 | 1.05 | 1.52 | 0.15 | 0.130 | 0.011 | <0.001 | 0.061 | 0.816 | 0.098 | 0.188 |
| Supp | BiW | Age | p Values 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item, mmol/L | Gln | Ala | LBW | NBW | 5 d | 12 d | SEM | Supp | BiW | Age | Supp × BiW | Supp × Age | BiW × Age | Supp × BiW × Age |
| Acetic Acid | 26.2 | 27.2 | 26.8 | 26.6 | 27.1 | 26.2 | 1.39 | 0.726 | 0.948 | 0.753 | 0.173 | 0.804 | 0.531 | 0.621 |
| Propionic acid | 7.94 | 9.49 | 8.19 | 9.24 | 8.07 | 9.36 | 0.83 | 0.359 | 0.532 | 0.445 | 0.071 | 0.737 | 0.893 | 0.222 |
| Isobutyric acid | 1.39 | 1.49 | 1.38 | 1.49 | 1.39 | 1.48 | 0.10 | 0.645 | 0.594 | 0.671 | 0.252 | 0.627 | 0.894 | 0.597 |
| Butyric acid | 3.00 | 3.60 | 3.03 | 3.57 | 2.86 | 3.73 | 0.39 | 0.436 | 0.487 | 0.263 | 0.101 | 0.520 | 0.870 | 0.090 |
| Isovaleric acid | 1.25 | 1.39 | 1.23 | 1.41 | 1.23 | 1.40 | 0.09 | 0.451 | 0.328 | 0.372 | 0.363 | 0.417 | 0.616 | 0.641 |
| Valeric acid | 1.13 | 1.19 | 1.13 | 1.18 | 1.07 | 1.24 | 0.09 | 0.718 | 0.767 | 0.325 | 0.174 | 0.203 | 0.865 | 0.132 |
| Total SCFA | 40.85 | 44.34 | 41.73 | 43.46 | 41.77 | 43.41 | 2.43 | 0.486 | 0.728 | 0.742 | 0.074 | 0.649 | 0.738 | 0.289 |
| Supp | BiW | Age | p Values 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item, % | Gln | Ala | LBW | NBW | 5 d | 12 d | SEM | Supp | BiW | Age | Supp × BiW | Suppl × Age | BiW × Age | Suppl × BiW × Age |
| Richness 3 | 179 | 167 | 172 | 173 | 164 | 181 | 5.56 | 0.391 | 0.974 | 0.114 | 0.794 | 0.343 | 0.204 | 0.550 |
| Shannon.Index 3 | 3.73 | 3.72 | 3.72 | 3.72 | 3.67 | 3.77 | 0.05 | 0.792 | 0.956 | 0.389 | 0.694 | 0.763 | 0.657 | 0.633 |
| Evenness 3 | 0.72 | 0.73 | 0.72 | 0.73 | 0.72 | 0.73 | 0.01 | 0.613 | 0.801 | 0.621 | 0.867 | 0.775 | 0.959 | 0.695 |
| Firmicutes | 68.8 | 66.6 | 64.4 | 71.1 | 65.1 | 70.4 | 1.82 | 0.429 | 0.049 | 0.156 | 0.009 | 0.429 | 0.105 | 0.032 |
| Bacteroidetes | 22.1 | 21.9 | 23.6 | 20.4 | 23.8 | 20.2 | 1.40 | 0.301 | 0.423 | 0.052 | 0.412 | 0.175 | 0.180 | 0.420 |
| Fusobacteria | 3.75 | 6.42 | 5.71 | 4.35 | 6.89 | 3.17 | 1.17 | 0.538 | 0.374 | 0.362 | 0.371 | 0.670 | 0.625 | 0.635 |
| Proteobacteria | 3.07 | 3.77 | 4.01 | 2.80 | 3.65 | 3.16 | 0.57 | 0.322 | 0.258 | 0.307 | 0.504 | 0.510 | 0.486 | 0.804 |
| Verrucomicrobia | 0.78 | 0.00 | 0.81 | 0.00 | 0.00 | 0.81 | 0.40 | 0.973 | 0.360 | 0.002 | 0.741 | 0.025 | 0.009 | 0.098 |
| WPS-2 | 0.42 | 0.10 | 0.06 | 0.47 | 0.00 | 0.53 | 0.17 | 0.179 | 0.138 | 0.173 | 0.233 | 0.281 | 0.256 | 0.473 |
| Actinobacteria | 0.33 | 0.24 | 0.32 | 0.26 | 0.36 | 0.21 | 0.06 | 0.524 | 0.904 | 0.869 | 0.779 | 0.796 | 0.717 | 0.836 |
| Spirochaetes | 0.29 | 0.32 | 0.43 | 0.17 | 0.05 | 0.55 | 0.11 | 0.380 | 0.917 | 0.003 | 0.784 | 0.024 | 0.019 | 0.121 |
| Planctomycetes | 0.27 | 0.17 | 0.26 | 0.18 | 0.07 | 0.38 | 0.06 | 0.054 | 0.248 | 0.113 | 0.166 | 0.074 | 0.293 | 0.309 |
| Tenericutes | 0.12 | 0.04 | 0.10 | 0.06 | 0.00 | 0.16 | 0.05 | 0.590 | 0.582 | 0.002 | 0.897 | 0.022 | 0.023 | 0.198 |
| Epsilonbacteraeota | 0.03 | 0.23 | 0.19 | 0.06 | 0.03 | 0.23 | 0.08 | 0.465 | 0.819 | <0.001 | 0.896 | 0.004 | 0.006 | 0.054 |
| Kiritimatiellaeota | 0.02 | 0.11 | 0.06 | 0.07 | 0.00 | 0.12 | 0.03 | 0.396 | 0.973 | 0.021 | 0.757 | 0.037 | 0.127 | 0.242 |
| Lentisphaerae | 0.01 | 0.04 | 0.02 | 0.03 | 0.05 | 0.01 | 0.01 | 0.937 | 0.080 | 0.230 | 0.145 | 0.499 | 0.083 | 0.191 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze Holthausen, J.; Schregel, J.; Sciascia, Q.L.; Li, Z.; Tuchscherer, A.; Vahjen, W.; Metges, C.C.; Zentek, J. Effects of Oral Glutamine Supplementation, Birthweight and Age on Colonic Morphology and Microbiome Development in Male Suckling Piglets. Microorganisms 2022, 10, 1899. https://doi.org/10.3390/microorganisms10101899
Schulze Holthausen J, Schregel J, Sciascia QL, Li Z, Tuchscherer A, Vahjen W, Metges CC, Zentek J. Effects of Oral Glutamine Supplementation, Birthweight and Age on Colonic Morphology and Microbiome Development in Male Suckling Piglets. Microorganisms. 2022; 10(10):1899. https://doi.org/10.3390/microorganisms10101899
Chicago/Turabian StyleSchulze Holthausen, Johannes, Johannes Schregel, Quentin L. Sciascia, Zeyang Li, Armin Tuchscherer, Wilfried Vahjen, Cornelia C. Metges, and Jürgen Zentek. 2022. "Effects of Oral Glutamine Supplementation, Birthweight and Age on Colonic Morphology and Microbiome Development in Male Suckling Piglets" Microorganisms 10, no. 10: 1899. https://doi.org/10.3390/microorganisms10101899
APA StyleSchulze Holthausen, J., Schregel, J., Sciascia, Q. L., Li, Z., Tuchscherer, A., Vahjen, W., Metges, C. C., & Zentek, J. (2022). Effects of Oral Glutamine Supplementation, Birthweight and Age on Colonic Morphology and Microbiome Development in Male Suckling Piglets. Microorganisms, 10(10), 1899. https://doi.org/10.3390/microorganisms10101899







