Entomological Assessment of Onchocerca Species Transmission by Black Flies in Selected Communities in the West Region of Cameroon
Abstract
1. Introduction
2. Results
2.1. Percentage of Infective and Infected Pools of Black Flies in Surveyed Communities
2.2. Frequency of Infective and Infected Black Flies
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethical Considerations and Participant Enlistment
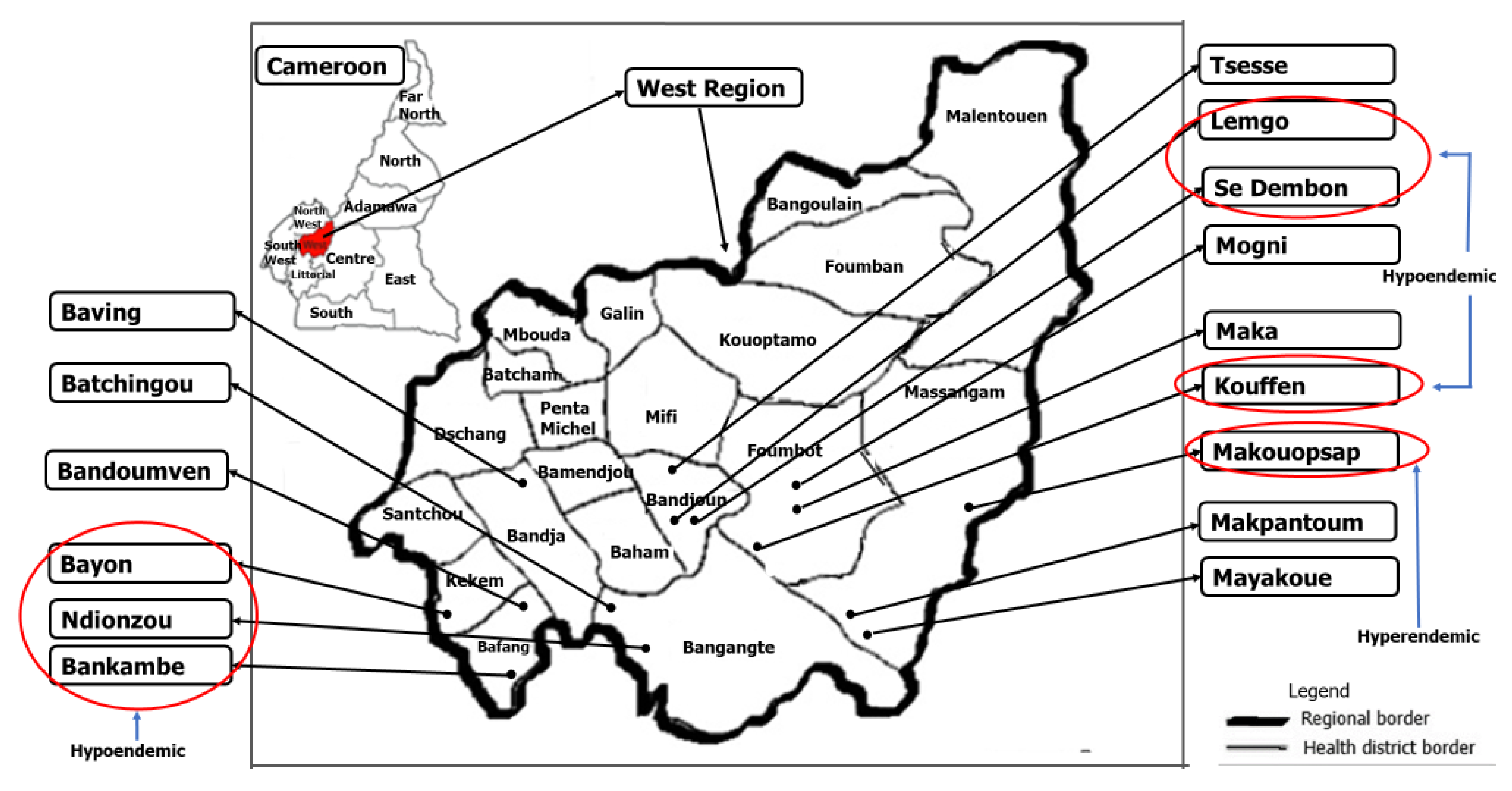
5.2. Selection of Study Sites and Black Fly Collection
5.3. DNA Extraction
5.4. Amplification by PCR
5.5. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shintouo, C.M.; Shey, R.A.; Nebangwa, D.N.; Esoh, K.K.; Nongley, N.F.; Nguve, J.E.; Giron, P.; Mutesa, L.; Vanhamme, L.; Souopgui, J.; et al. In Silico Design and Validation of OvMANE1, a Chimeric Antigen for Human Onchocerciasis Diagnosis. Pathogens 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Njume, F.N.; Ghogomu, S.M.; Shey, R.A.; Gainkam, L.O.T.; Poelvoorde, P.; Humblet, P.; Kamgno, J.; Robert, A.; Mutesa, L.; Lelubre, C.; et al. Identification and characterization of the Onchocerca volvulus Excretory Secretory Product Ov28CRP, a putative GM2 activator protein. PLoS Negl. Trop. Dis. 2019, 13, e0007591. [Google Scholar] [CrossRef] [PubMed]
- Lustigman, S.; Makepeace, B.L.; Klei, T.R.; Babayan, S.A.; Hotez, P.; Abraham, D.; Bottazzi, M.E. Onchocerca volvulus: The Road from Basic Biology to a Vaccine. Trends Parasitol. 2018, 34, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Boursou, D.; Ndjonka, D.; Eisenbarth, A.; Manchang, K.; Paguem, A.; Ngwasiri, N.N.; Vildina, J.D.; Abanda, B.; Krumkamp, R.; van Hoorn, S.; et al. Onchocerca-infected cattle produce strong antibody responses to excretory-secretory proteins released from adult male Onchocerca ochengi worms. BMC Infect. Dis. 2018, 18, 200. [Google Scholar] [CrossRef]
- Wahl, G.; Ekale, D.; Schmitz, A. Onchocerca ochengi: Assessment of the Simulium vectors in north Cameroon. Parasitology 1998, 116 Pt 4, 327–336. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Ssonko, V.N.; Oyet, W.; Lakwo, T.; Idro, R. Neurological manifestations in Onchocerca volvulus infection: A review. Brain Res. Bull. 2019, 145, 39–44. [Google Scholar] [CrossRef]
- World Health Organisation. Onchocerciasis Key Facts. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/onchocerciasis (accessed on 28 April 2020).
- Mounchili, S.C.; Ghogomu, M.A.; Ghogomu, S.M.; Njume, F.N.; Shey, R.A.; Souopgui, J. Analysis of Onchocerca volvulus β-tubulin gene polymorphism in the Mbonge sub-division of Cameroon: Evidence of gene selection by ivermectin. J. Genet. Mol. Biol. 2018, 2, 1–6. [Google Scholar]
- Fobi, G.; Yameogo, L.; Noma, M.; Aholou, Y.; Koroma, J.B.; Zouré, H.M.; Ukety, T.; Lusamba-Dikassa, P.-S.; Mwikisa, C.; Boakye, D.A.; et al. Managing the Fight against Onchocerciasis in Africa: APOC Experience. PLoS Negl. Trop. Dis. 2015, 9, e0003542. [Google Scholar] [CrossRef]
- Katabarwa, M.N.; Eyamba, A.; Nwane, P.; Enyong, P.; Kamgno, J.; Kuété, T.; Yaya, S.; Aboutou, R.; Mukenge, L.; Kafando, C.; et al. Fifteen years of annual mass treatment of onchocerciasis with ivermectin have not interrupted transmission in the west region of cameroon. J. Parasitol. Res. 2013, 2013, 420928. [Google Scholar] [CrossRef]
- Tekle, A.H.; Zoure, H.G.M.; Mounkaila, N.; Boussinesq, M.; Coffeng, L.E.; Stolk, W.; Remme, J.H. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: Epidemiological evaluation results. Infect. Dis. Poverty 2016, 5, 66. [Google Scholar] [CrossRef]
- Kamga, G.-R.; Dissak-Delon, F.N.; Djeunga, H.N.; Biholong, B.D.; Ghogomu, S.M.; Souopgui, J.; Kamgno, J.; Robert, A. Important progress towards elimination of onchocerciasis in the West Region of Cameroon. Parasites Vectors 2017, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Guidelines for Stopping Mass Drug Administration and Verifying Elimination of Human Onchocerciasis; World Health Organisation: Geneva, Switzerland, 2016. [Google Scholar]
- Oskam, L.; Schoone, G.J.; Kroon, C.C.M.; Luján, R.; Davies, J.B. Polymerase chain reaction for detecting Onchocerca volvulus in pools of blackflies. Trop. Med. Int. Health 1996, 1, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Koala, L.; Nikièma, A.S.; Paré, A.B.; Drabo, F.; Toé, L.D.; Belem, A.M.G.; Boakye, D.A.; Traoré, S.; Dabire, R.K. Entomological assessment of the transmission following recrudescence of onchocerciasis in the Comoe Valley, Burkina Faso. Parasit Vectors 2019, 12, 34. [Google Scholar] [CrossRef]
- Bennuru, S.; Oduro-Boateng, G.; Osigwe, C.; Del Valle, P.; Golden, A.; Ogawa, G.M.; Cama, V.; Lustigman, S.; Nutman, T.B. Integrating multiple biomarkers to increase sensitivity for the detection of Onchocerca volvulus infection. J. Infect. Dis. 2019, 221, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Bakajika, D.; Senyonjo, L.; Enyong, P.; Oye, J.; Biholong, B.; Elhassan, E.; Boakye, D.; Dixon, R.; Schmidt, E. On-going transmission of human onchocerciasis in the Massangam health district in the West Region of Cameroon: Better understanding transmission dynamics to inform changes in programmatic interventions. PLoS Negl. Trop. Dis. 2018, 12, e0006904. [Google Scholar] [CrossRef] [PubMed]
- Osei-Atweneboana, M.Y.; Boakye, D.A.; Awadzi, K.; Gyapong, J.; Prichard, R.K. Genotypic analysis of β-tubulin in Onchocerca volvulus from communities and individuals showing poor parasitological response to ivermectin treatment. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 20–28. [Google Scholar] [CrossRef]
- Kamga, G.-R.; Dissak-Delon, F.N.; Djeunga, H.N.; Biholong, B.D.; Ghogomu, S.M.; Souopgui, J.; Zoure, H.G.M.; Boussinesq, M.; Kamgno, J.; Robert, A. Still mesoendemic onchocerciasis in two Cameroonian community-directed treatment with ivermectin projects despite more than 15 years of mass treatment. Parasit Vectors 2016, 9, 581. [Google Scholar] [CrossRef]
- Barbazan, P.; Escaffre, H.; Mbentengam, R.; Boussinesq, M. Entomologic study on the transmission of onchocerciasis in a forest-savanna transition area of Cameroon. Bull. Soc. Pathol. Exot. 1998, 91, 178–182. [Google Scholar]
- Cupp, E.W.; Sauerbrey, M.; Cama, V.A.; Eberhard, M.; Lammie, P.J.; Unnasch, T.R. Elimination of onchocerciasis in Africa by 2025: The need for a broad perspective. Infect. Dis. Poverty 2019, 8, 50. [Google Scholar] [CrossRef]
- Higazi, T.B.; Zarroug, I.M.A.; Mohamed, H.A.; ElMubark, W.A.; DeRan, T.C.M.; Aziz, N.; Katabarwa, M.; Hassan, H.K.; Unnasch, T.R.; MacKenzie, C.D.; et al. Interruption of Onchocerca volvulus Transmission in the Abu Hamed Focus, Sudan. Am. J. Trop. Med. Hyg. 2013, 89, 51–57. [Google Scholar] [CrossRef]
- Kim, Y.E.; Remme, J.H.; Steinmann, P.; Stolk, W.A.; Roungou, J.B.; Tediosi, F. Control, elimination, and eradication of river blindness: Scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Negl. Trop. Dis. 2015, 9, e0003664. [Google Scholar]
- Rebollo, M.P.; Zouré, H.; Ogoussan, K.; Sodahlon, Y.; A Ottesen, E.; Cantey, P.T. Onchocerciasis: Shifting the target from control to elimination requires a new first-step-elimination mapping. Int. Health 2018, 10 (Suppl. 1), i14–i19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Guidelines for Stopping Mass Drug Administration and Verifying Elimination of Human Onchocerciasis, Criteria and Procedures Annexes. 2016. Available online: https://www.who.int/onchocerciasis/resources/ISBN9789241510011_Annexes.pdf (accessed on 30 May 2020).
- Eisenbarth, A.; Achukwi, M.D.; Renz, A. Ongoing Transmission of Onchocerca volvulus after 25 Years of Annual Ivermectin Mass Treatments in the Vina du Nord River Valley, in North Cameroon. PLoS Negl. Trop. Dis. 2016, 10, e0004392. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Post, R.J.; Gomulski, L.M. Multivariate morphotaxonomy in the identification of adult females of the Simulium damnosum Theobald complex (Diptera: Simuliidae) in the Onchocerciasis Control Programme area of West Africa. Ann. Trop. Med. Parasitol. 1993, 87, 65–82. [Google Scholar] [CrossRef]
- Dissak-Delon, F.N.; Kamga, G.-R.; Humblet, P.C.; Robert, A.; Souopgui, J.; Kamgno, J.; Ghogomu, S.M.; Godin, I. Barriers to the National Onchocerciasis Control Programme at operational level in Cameroon: A qualitative assessment of stakeholders’ views. Parasites Vectors 2019, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, M.A.; Danis-Lozano, R.; Rodriguez, M.H.; Unnasch, T.R.; Bradley, J. Detection of Onchocerca volvulus infection in Simulium ochraceum sensu lato: Comparison of a PCR assay and fly dissection in a Mexican hypoendemic community. Parasitology 1999, 119 Pt 6, 613–619. [Google Scholar] [CrossRef]
- Rodriguez-Perez, M.A.; Unnasch, T.R.; Lizarazo-Ortega, C.; Domínguez-Vázquez, A.; Mendoza-Herrera, A.; Lilley, B.G.; Segura-Arenas, R.; Reyes-Villanueva, F. Polymerase chain reaction monitoring of transmission of Onchocerca volvulus in two endemic states in Mexico. Am. J. Trop. Med. Hyg. 2004, 70, 38–45. [Google Scholar] [CrossRef]
- Rodriguez-Perez, M.A.; Gopal, H.; Adeleke, M.A.; De Luna-Santillana, E.J.; Gurrola, J.N.; Guo, X. Detection of Onchocerca volvulus in Latin American black flies for pool screening PCR using high-throughput automated DNA isolation for transmission surveillance. Parasitol. Res. 2013, 112, 3925–3931. [Google Scholar] [CrossRef]
- Guevara, A.G.; Lilley, B.G.; Rumbea, J.; Unnasch, T.R.; López, A.; Collins, R.; Vieira, J.C.; Katholi, C.R.; Vieira, N. Entomological evaluation by pool screen polymerase chain reaction of Onchocerca volvulus transmission in Ecuador following mass Mectizan distribution. Am. J. Trop. Med. Hyg. 2003, 68, 222–227. [Google Scholar] [CrossRef][Green Version]
- Karija Vlahović, M.; Kubat, M. DNA extraction method from bones using Maxwell® 16. Leg. Med. 2012, 14, 272–275. [Google Scholar] [CrossRef]
- Gopal, H.; Rodríguez-Pérez, M.A. Purification of O. Volvulus gDNA from S. Ochraceum s.l. Black Fly Head or Body Pools Using the Maxwell® 16 Instrument. 2010. Available online: https://worldwide.promega.com/resources/pubhub/purification-of-o-volvulus-gdna-from-s-ochraceum-sl-black-fly-using-the-maxwell-16-instrument/#ArticleBody-e114f3eb-e9ed-4cb7-bc15–1ccd1036850a (accessed on 29 May 2020).
- Gutiérrez-López, R.; La Puente, J.M.-D.; Gangoso, L.; Soriguer, R.C.; Figuerola, J. Comparison of manual and semi-automatic DNA extraction protocols for the barcoding characterization of hematophagous louse flies (Diptera: Hippoboscidae). J. Vector Ecol. 2015, 40, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Richards, F., Jr.; Klein, R.; Rios, D.; Valdez, C.G.C.; Cupp, E.; Dominguez, A.; Hassan, H.K.; De Leon, O.; Espinoza, C.E.D.; Monroy, Z.M.; et al. One Hundred Years After Its Discovery in Guatemala by Rodolfo Robles, Onchocerca volvulus Transmission Has Been Eliminated from the Central Endemic Zone. Am. J. Trop. Med. Hyg. 2015, 93, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Higazi, T.B.; Zarroug, I.M.A.; Mohamed, H.A.; Mohamed, W.A.; DeRan, T.C.M.; Aziz, N.; Katabarwa, M.; Hassan, H.K.; Unnasch, T.R.; MacKenzie, C.D.; et al. Polymerase chain reaction pool screening used to compare prevalence of infective black flies in two onchocerciasis foci in northern Sudan. Am. J. Trop. Med. Hyg. 2011, 84, 753–756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marchon-Silva, V.; Caër, J.C.; Post, R.J.; Maia-Herzog, M.; Fernandes, O. Detection of Onchocerca volvulus (Nematoda: Onchocercidae) infection in vectors from Amazonian Brazil following mass Mectizan distribution. Mem. Inst. Oswaldo Cruz 2007, 102, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Katholi, C.R.; Toé, L.; Merriweather, A.; Unnasch, T.R. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J. Infect. Dis. 1995, 172, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, M.A.; Katholi, C.R.; Hassan, H.K.; Unnasch, T.R. Large-scale entomologic assessment of Onchocerca volvulus transmission by poolscreen PCR in Mexico. Am. J. Trop. Med. Hyg. 2006, 74, 1026–1033. [Google Scholar] [CrossRef][Green Version]

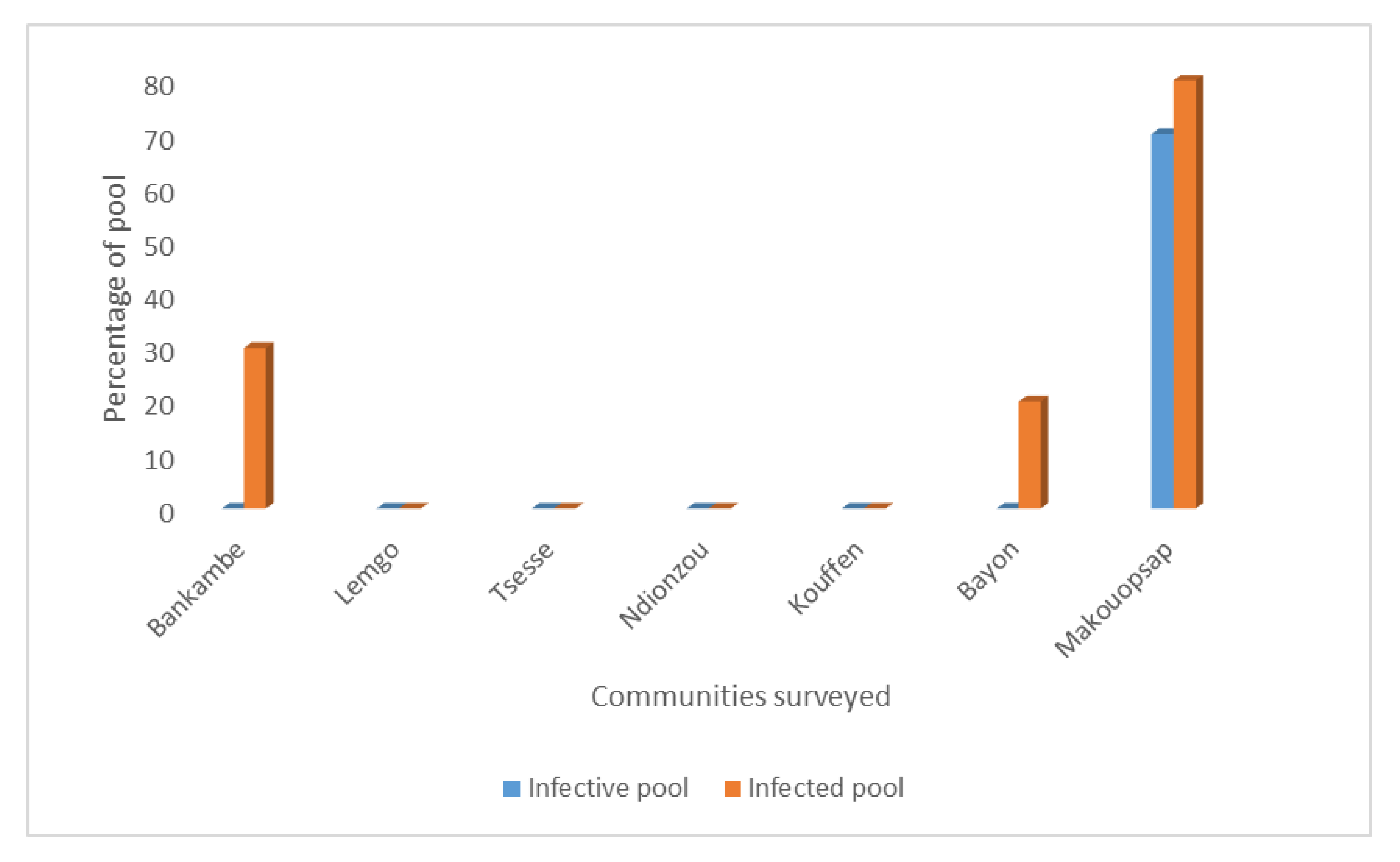
| Community | Infective Pool | Infected Pool | ||
|---|---|---|---|---|
| Frequency | 95% Confidence Interval a | Frequency | 95% Confidence Interval a | |
| Bankambe | 0.0 | 0–0.00096 | 0.2 | 0.00034–0.00521 |
| Lemgo | 0.0 | 0–0.00096 | 0.0 | 0–0.00096 |
| Tsesse | 0.0 | 0–0.00096 | 0.0 | 0–0.00096 |
| Ndionzou | 0.0 | 0–0.00096 | 0.0 | 0–0.00096 |
| Kouffen | 0.0 | 0–0.00096 | 0.0 | 0–0.00096 |
| Bayon | 0.0 | 0–0.00096 | 0.1 | 0.00131–0.00393 |
| Makouopsap | 0.6 | 0.00216–0.01347 | 0.8 | 0.00303–0.1809 |
| Health District | Community | n | Mf + (n) | Microfilaria Prevalence (%) |
|---|---|---|---|---|
| Bafang | Bankambe | 203 | 9 | 2.6 |
| Bandjoun | Lemgo | 75 | 0 | 0.0 |
| Tsesse | 123 | 0 | 0.0 | |
| Bangangté | Ndionzou | 141 | 0 | 0.0 |
| Foumbot | Kouffen | 107 | 0 | 0.0 |
| Kékem | Bayon | 231 | 6 | 2.3 |
| Massangam | Makouopsap | 123 | 53 | 41.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shintouo, C.M.; Nguve, J.E.; Asa, F.B.; Shey, R.A.; Kamga, J.; Souopgui, J.; Ghogomu, S.M.; Njemini, R. Entomological Assessment of Onchocerca Species Transmission by Black Flies in Selected Communities in the West Region of Cameroon. Pathogens 2020, 9, 722. https://doi.org/10.3390/pathogens9090722
Shintouo CM, Nguve JE, Asa FB, Shey RA, Kamga J, Souopgui J, Ghogomu SM, Njemini R. Entomological Assessment of Onchocerca Species Transmission by Black Flies in Selected Communities in the West Region of Cameroon. Pathogens. 2020; 9(9):722. https://doi.org/10.3390/pathogens9090722
Chicago/Turabian StyleShintouo, Cabirou Mounchili, Joel Ebai Nguve, Fru Bertha Asa, Robert Adamu Shey, Joseph Kamga, Jacob Souopgui, Stephen Mbigha Ghogomu, and Rose Njemini. 2020. "Entomological Assessment of Onchocerca Species Transmission by Black Flies in Selected Communities in the West Region of Cameroon" Pathogens 9, no. 9: 722. https://doi.org/10.3390/pathogens9090722
APA StyleShintouo, C. M., Nguve, J. E., Asa, F. B., Shey, R. A., Kamga, J., Souopgui, J., Ghogomu, S. M., & Njemini, R. (2020). Entomological Assessment of Onchocerca Species Transmission by Black Flies in Selected Communities in the West Region of Cameroon. Pathogens, 9(9), 722. https://doi.org/10.3390/pathogens9090722









