Application of the In Vitro HoxB8 Model System to Characterize the Contributions of Neutrophil–LPS Interaction to Periodontal Disease
Abstract
1. Introduction
2. Results
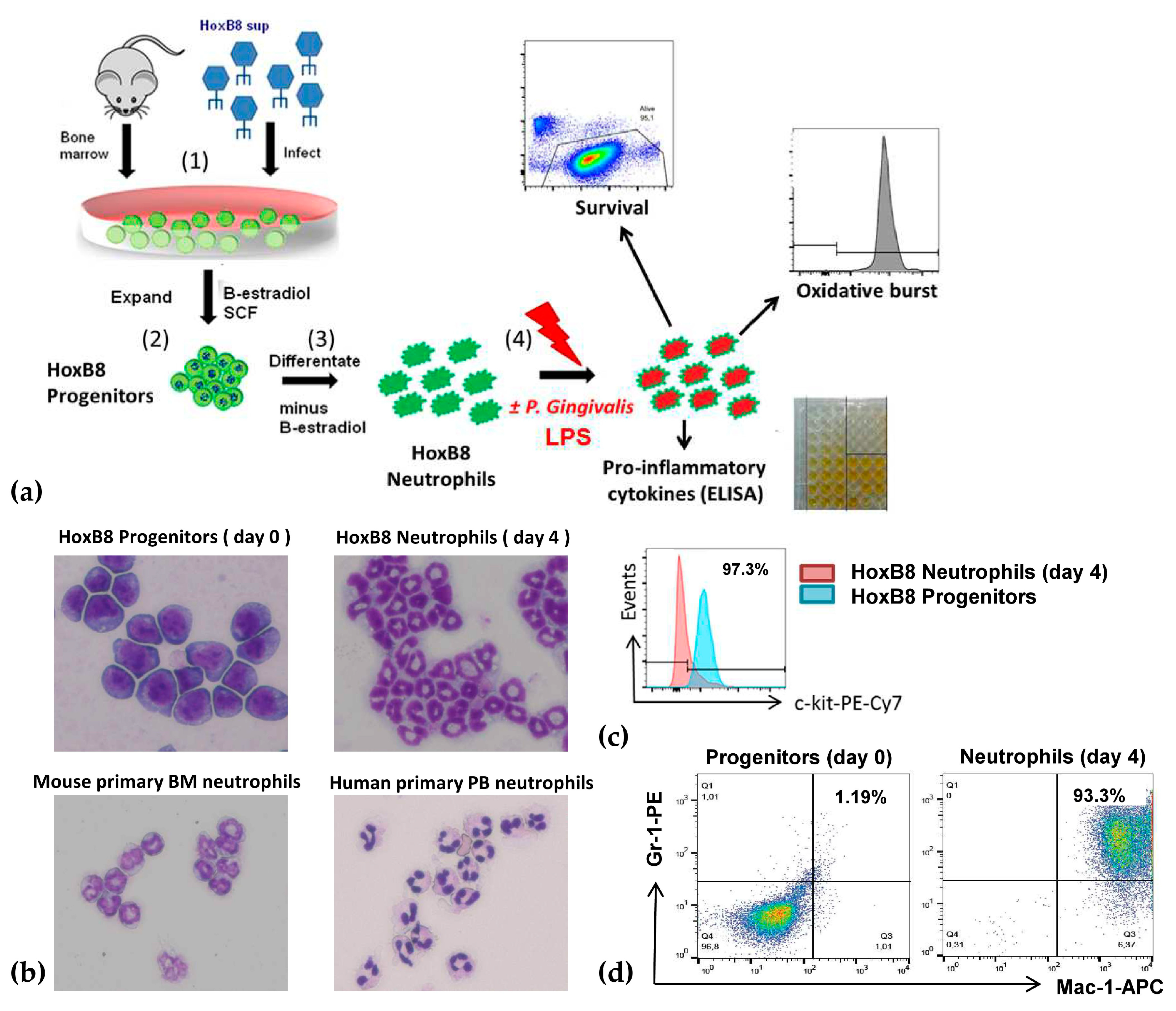
2.1. A New Perspective in Periodontitis Research: The HoxB8 System
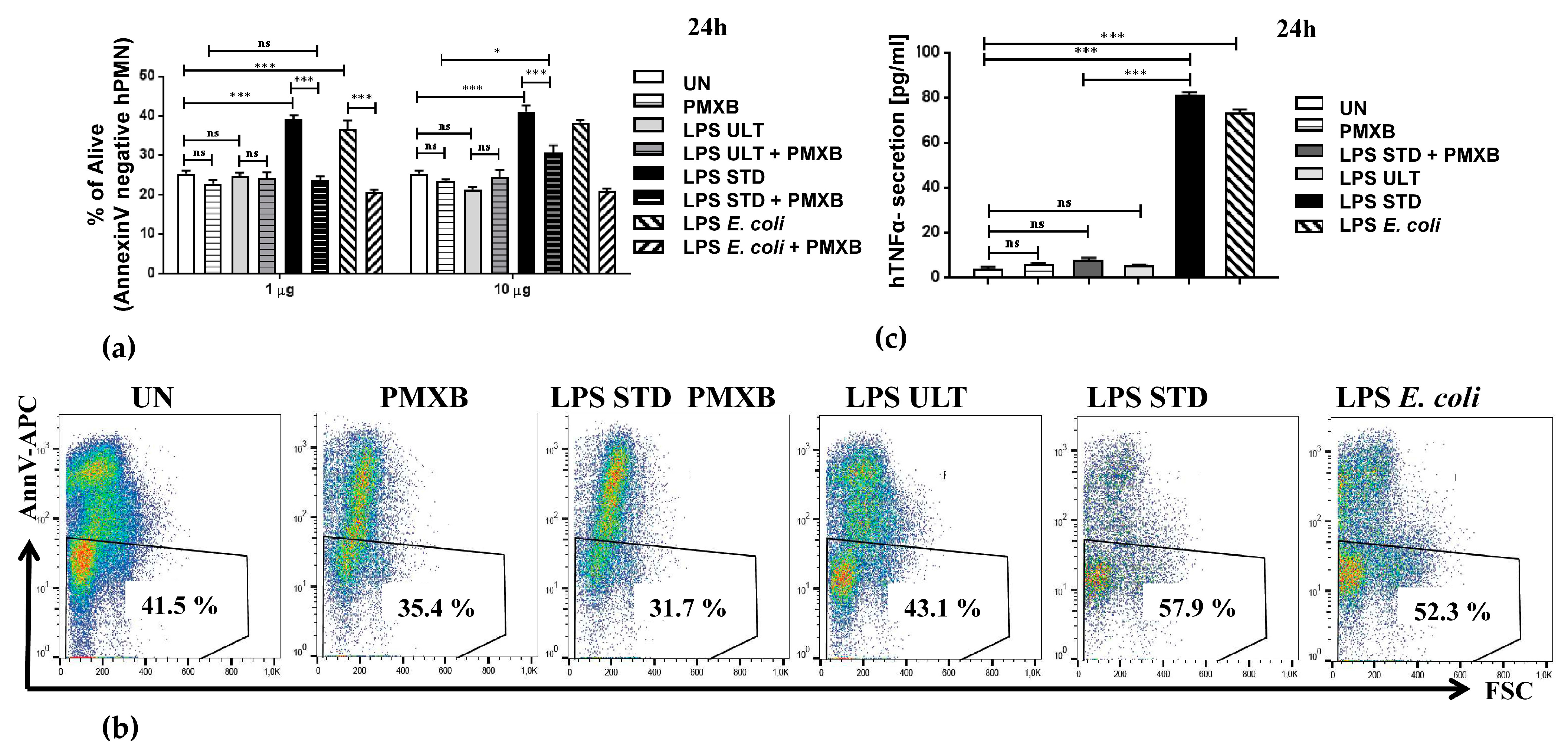
2.2. Two Variants of LPSs Isolated from P. gingivalis Differentially Impact Neutrophil Survival
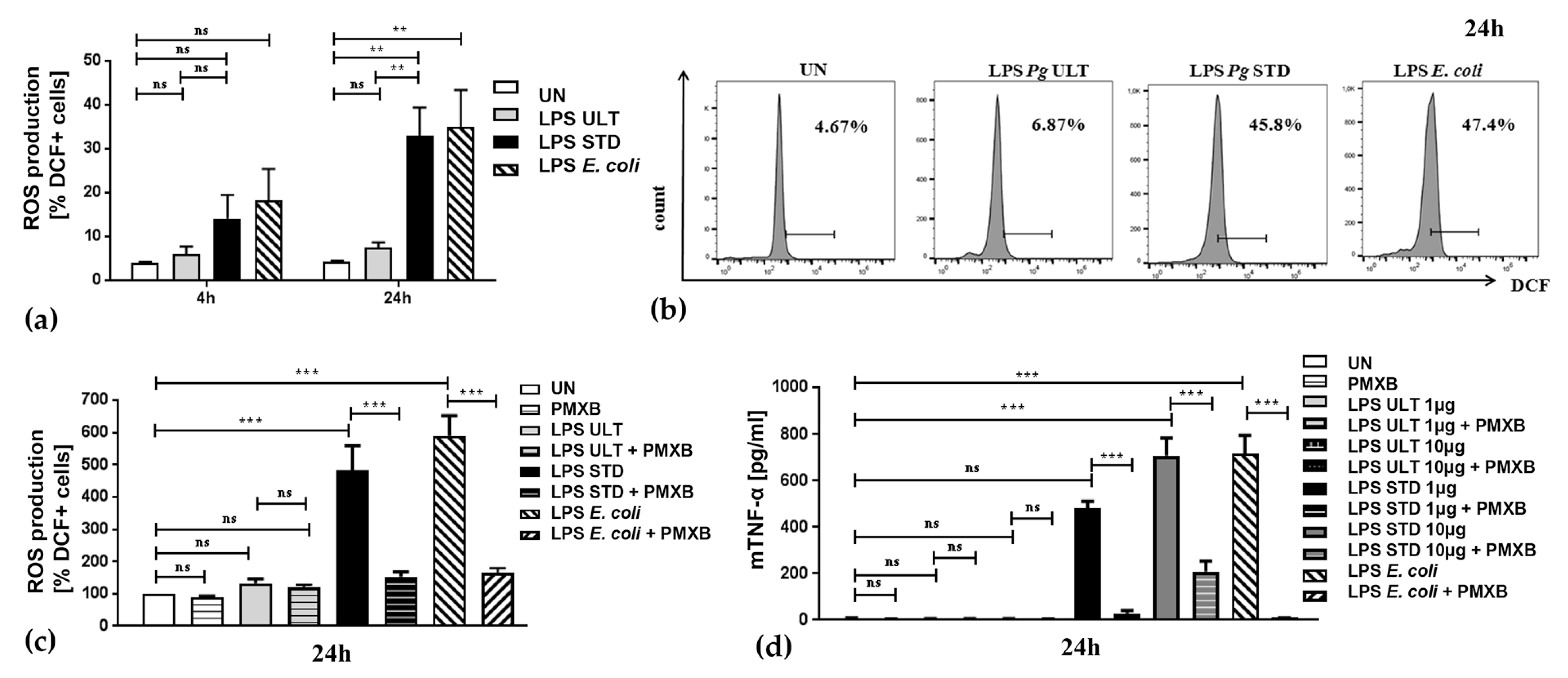
2.3. Neutrophil Inflammatory Functions are Differentially Affected by Two Purification Variants of P. gingivalis Endotoxin
3. Discussion
4. Materials and Methods
4.1. HoxB8 Cell Lines and Cell Cultures
4.2. Murine and Human Neutrophil Isolations
4.3. Immunofluorescence and Microscopic Analysis
4.4. Neutrophil Stimulations
4.5. Functional Analysis
4.6. Ethics Statement
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gallagher, R.; Collins, S.; Trujillo, J.; McCredie, K.; Ahearn, M.; Tsai, S.; Metzgar, R.; Aulakh, G.; Ting, R.; Ruscetti, F.; et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 1979, 54, 713–733. [Google Scholar] [CrossRef]
- Jensen, H.A.; Bunaciu, R.; Ibabao, C.N.; Myers, R.; Varner, J.D.; Yen, A. Retinoic acid therapy resistance progresses from unilineage to bilineage in HL-60 leukemic blasts. PLoS ONE 2014, 9, e98929. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Krause, D.S.; Berliner, N. Normal neutrophil differentiation and secondary granule gene expression in the EML and MPRO cell lines. Exp. Hematol. 1998, 26, 1178–1185. [Google Scholar] [PubMed]
- Guchhait, P.; Tosi, M.F.; Smith, C.W.; Chakaraborty, A. The murine myeloid cell line 32Dcl3 as a model system for studying neutrophil functions. J. Immunol. Methods 2003, 283, 195–204. [Google Scholar] [CrossRef]
- Wang, G.G.; Calvo, K.R.; Pasillas, M.P.; Sykes, D.B.; Häcker, H.; Kamps, M.P. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 2006, 3, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Redecke, V.; Wu, R.; Zhou, J.; Finkelstein, D.; Chaturvedi, V.; High, A.A.; Häcker, H. Hematopoietic progenitor cell lines with myeloid and lymphoid potential. Nat. Methods 2013, 10, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Darveau, R.P.; Pham, T.-T.T.; Lemley, K.; Reife, R.A.; Bainbridge, B.W.; Coats, S.R.; Howald, W.N.; Way, S.S.; Hajjar, A.M. Porphyromonas gingivalis Lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect. Immun. 2004, 72, 5041–5051. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2013, 35, 3–11. [Google Scholar] [CrossRef]
- Nativel, B.; Couret, D.; Giraud, P.; Meilhac, O.; D’Hellencourt, C.L.; Viranaïcken, W.; Da Silva, C.R. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci. Rep. 2017, 7, 15789. [Google Scholar] [CrossRef]
- Castillo, L.A.; Birnberg-Weiss, F.; Rodriguez-Rodrigues, N.; Martire-Greco, D.; Bigi, F.; Landoni, V.I.; Gomez, S.A.; Fernandez, G.C. Klebsiella pneumoniae ST258 Negatively Regulates the Oxidative Burst in Human Neutrophils. Front. Immunol. 2019, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Frankenberg, T.; Kirschnek, S.; Obermaier, B.; Häcker, H.; Paul, R.; Häcker, G. Apoptosis Is Essential for Neutrophil Functional Shutdown and Determines Tissue Damage in Experimental Pneumococcal Meningitis. PLoS Pathog. 2009, 5, e1000461. [Google Scholar] [CrossRef] [PubMed]
- Vier, J.; Groth, M.; Sochalska, M.; Kirschnek, S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell Death Dis. 2016, 7, e2103. [Google Scholar] [CrossRef] [PubMed]
- Kirschnek, S.; Vier, J.; Gautam, S.; Frankenberg, T.; Rangelova, S.; Eitz-Ferrer, P.; Grespi, F.; Ottina, E.; Villunger, A.; Hacker, H.; et al. Molecular analysis of neutrophil spontaneous apoptosis reveals a strong role for the pro-apoptotic BH3-only protein Noxa. Cell Death Differ. 2011, 18, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Nazir, M. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Van Dyke, T.E.; Sheilesh, D.; Dave, S. Risk factors for periodontitis. J. Int. Acad. Periodontol. 2005, 7, 3–7. [Google Scholar]
- Makkawi, H.; Hoch, S.; Burns, E.; Hosur, K.; Hajishengallis, G.; Kirschning, C.J.; Nussbaum, G. Porphyromonas gingivalis Stimulates TLR2-PI3K Signaling to Escape Immune Clearance and Induce Bone Resorption Independently of MyD88. Front. Microbiol. 2017, 7, 359. [Google Scholar] [CrossRef]
- Rossi, A.G.; Sawatzky, D.A.; Walker, A.; Ward, C.; Sheldrake, T.A.; Riley, N.A.; Caldicott, A.; Martinez-Losa, M.; Walker, T.R.; Duffin, R.; et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 2006, 12, 1056–1064. [Google Scholar] [CrossRef]
- Villunger, A.; O’Reilly, L.A.; Holler, N.; Adams, J.; Strasser, A. Fas ligand, Bcl-2, granulocyte colony-stimulating factor, and p38 mitogen-activated protein kinase: Regulators of distinct cell death and survival pathways in granulocytes. J. Exp. Med. 2000, 192, 647–658. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Velsko, I.M.; Rivera-Kweh, M.F.; Larjava, H.; Lucas, A.; Kesavalu, L. Global TLR2 and 4 deficiency in mice impacts bone resorption, inflammatory markers and atherosclerosis to polymicrobial infection. Mol. Oral Microbiol. 2016, 32, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, M.; Weis, J.J.; Toshchakov, V.Y.; Salkowski, C.A.; Cody, M.J.; Ward, D.C.; Qureshi, N.; Michalek, S.M.; Vogel, S.N. Signaling by Toll-Like Receptor 2 and 4 Agonists Results in Differential Gene Expression in Murine Macrophages. Infect. Immun. 2001, 69, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.E.M.; Jabr, C.L.; Dejani, N.N.; Saraiva, A.C.; De Aquino, S.G.; Medeiros, A.I.; Rossa, C., Jr. Inhibition of 5-Lipoxygenase (5-Lo) Attenuates Inflammation and Bone Resorption in Lipopolysaccharide (Lps)-Induced Periodontal Disease. J. Periodontol. 2017, 1–18. [Google Scholar] [CrossRef]
- Nogueira-Filho, G.; Rosa, B.T.; Santos, P.F.; Tunes, U.R.; Freire, S.M.; Meyer, R.; Darveau, R.P. Whole-Blood Cultures From Patients With Chronic Periodontitis Respond Differently to Porphyromonas gingivalis but not Escherichia coli Lipopolysaccharide. J. Periodontol. 2014, 85, e18–e23. [Google Scholar] [CrossRef]
- Al-Qutub, M.N.; Braham, P.H.; Karimi-Naser, L.M.; Liu, X.; Genco, C.A.; Darveau, R.P. Hemin-Dependent Modulation of the Lipid A Structure of Porphyromonas gingivalis Lipopolysaccharide. Infect. Immun. 2006, 74, 4474–4485. [Google Scholar] [CrossRef]
- Olsen, I.; Hajishengallis, G. Major neutrophil functions subverted by Porphyromonas gingivalis. J. Oral Microbiol. 2016, 8, 30936. [Google Scholar] [CrossRef]
- Popadiak, K.; Potempa, J.; Riesbeck, K.; Blom, A.M. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J. Immunol. 2007, 178, 7242–7250. [Google Scholar] [CrossRef]
- Lehr, S.; Vier, J.; Häcker, G.; Kirschnek, S. Activation of neutrophils by Chlamydia trachomatis-infected epithelial cells is modulated by the chlamydial plasmid. Microbes Infect. 2018, 20, 284–292. [Google Scholar] [CrossRef]
- Bryzek, D.; Ciaston, I.; Dobosz, E.; Gasiorek, A.; Makarska, A.; Sarna, M.; Eick, S.; Puklo, M.; Lech, M.; Potempa, B.; et al. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. 2019, 15, e1007773. [Google Scholar] [CrossRef]
- Golda, A.; Kosikowska-Adamus, P.; Babyak, O.; Lech, M.; Wysocka, M.; Lesner, A.; Potempa, J.; Koziel, J. Conjugate of Enkephalin and Temporin Peptides as a Novel Therapeutic Agent for Sepsis. Bioconjugate Chem. 2018, 29, 4127–4139. [Google Scholar] [CrossRef] [PubMed]
- Haschka, M.D.; Soratroi, C.; Kirschnek, S.; Häcker, G.; Hilbe, R.; Geley, S.; Villunger, A.; Fava, L. The NOXA–MCL1–BIM axis defines lifespan on extended mitotic arrest. Nat. Commun. 2015, 6, 6891. [Google Scholar] [CrossRef] [PubMed]
- Doeing, D.C.; Borowicz, J.L.; Crockett, E.T. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin. Pathol. 2003, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Krauss, J.L. Neutrophils in periodontal inflammation. Front. Oral Biol. 2011, 15, 56–83. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Shaw, A.; Antonelli, M.; Foster, D.; Klein, D.J.; Marshall, J.C.; Palevsky, P.M.; Weisberg, L.S.; Schorr, C.; Trzeciak, S.; et al. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients With Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA 2018, 320, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lee, W.; Kwa, A.L. Polymyxin B versus colistin: An update. Expert Rev. Anti-Infect. Ther. 2015, 13, 1481–1497. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sochalska, M.; Stańczyk, M.B.; Użarowska, M.; Zubrzycka, N.; Kirschnek, S.; Grabiec, A.M.; Kantyka, T.; Potempa, J. Application of the In Vitro HoxB8 Model System to Characterize the Contributions of Neutrophil–LPS Interaction to Periodontal Disease. Pathogens 2020, 9, 530. https://doi.org/10.3390/pathogens9070530
Sochalska M, Stańczyk MB, Użarowska M, Zubrzycka N, Kirschnek S, Grabiec AM, Kantyka T, Potempa J. Application of the In Vitro HoxB8 Model System to Characterize the Contributions of Neutrophil–LPS Interaction to Periodontal Disease. Pathogens. 2020; 9(7):530. https://doi.org/10.3390/pathogens9070530
Chicago/Turabian StyleSochalska, Maja, Magdalena B. Stańczyk, Maria Użarowska, Natalia Zubrzycka, Susanne Kirschnek, Aleksander M. Grabiec, Tomasz Kantyka, and Jan Potempa. 2020. "Application of the In Vitro HoxB8 Model System to Characterize the Contributions of Neutrophil–LPS Interaction to Periodontal Disease" Pathogens 9, no. 7: 530. https://doi.org/10.3390/pathogens9070530
APA StyleSochalska, M., Stańczyk, M. B., Użarowska, M., Zubrzycka, N., Kirschnek, S., Grabiec, A. M., Kantyka, T., & Potempa, J. (2020). Application of the In Vitro HoxB8 Model System to Characterize the Contributions of Neutrophil–LPS Interaction to Periodontal Disease. Pathogens, 9(7), 530. https://doi.org/10.3390/pathogens9070530




