Adaptive Immunity to Dengue Virus: Slippery Slope or Solid Ground for Rational Vaccine Design?
Abstract
1. Background
1.1. Dengue Epidemiology, Clinical Disease and Immunopathogenesis
1.2. Biology of DENV
1.3. Dengue Vaccines
1.4. Scope of This Review
2. Neutralising Antibodies against DENV
2.1. The Neutralising Antibody Response to DENV Infection
2.2. Impact of the Structural Heterogeneity and Dynamics of DENV on Antibody-Mediated Neutralisation
2.3. Antibodies That Target the Fusion Loop Epitope in the E Protein
2.4. PrM Protein–Specific Antibodies
2.5. Antibodies that Bind E Protein Domain III
2.5.1. Insights from EDIII-Specific Mouse MAbs
2.5.2. Human Antibodies to EDIII
2.5.3. EDIII as a Vaccine Candidate
2.6. Antibodies that Target Quaternary Epitopes on the Virion
2.6.1. Human MAbs to Serotype-Specific Quaternary Epitopes
2.6.2. Antibody Responses to Serotype-Specific Quaternary Epitopes in Human Polyclonal Sera
2.6.3. Antibodies that Bind the E Protein Dimer Epitope
2.6.4. E Protein Dimer–Based Vaccine Candidates
3. Antibodies to NS1
3.1. Structure and Pathogenic Roles of NS1
3.2. NS1-Specific Antibodies and Their Protective Effects
3.3. Vaccine Candidates Based on Full-Length NS1
3.4. Molecular Mimicry between NS1 and Host Proteins
3.5. Modified NS1-Based Vaccines
4. T-Cell Responses to DENV Infection and Vaccination
4.1. DENV Proteins Recognised by T Cells
4.2. Involvement of T Cells in Dengue Pathogenesis
4.3. Protective Role of T Cells During DENV Infection
4.3.1. Evidence from Murine Studies
4.3.2. Evidence from Human Studies
4.4. Importance of DENV-Specific T Cells in Vaccine-Mediated Protection
4.4.1. T-Cell Responses to Live-Attenuated Dengue Vaccines
4.4.2. T-Cell Responses to Experimental Dengue Vaccines
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Vasilakis, N. Dengue–Quo tu et quo vadis? Viruses 2011, 3, 1562–1608. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors. Aedes Aegypti Ae. Albopictus. Elife 2015, 4, e08347. [Google Scholar] [PubMed]
- Simmons, C.P.; Farrar, J.J.; van Vinh Chau, N.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef]
- Gossner, C.M.; Ducheyne, E.; Schaffner, F. Increased risk for autochthonous vector-borne infections transmitted by Aedes albopictus in continental Europe. Eurosurveillance 2018, 23, 1800268. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Prim. 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef]
- Sabin, A.B. Research on dengue during World War II. Am. J. Trop. Med. Hyg. 1952, 1, 30–50. [Google Scholar] [CrossRef]
- Imrie, A.; Meeks, J.; Gurary, A.; Sukhbaatar, M.; Truong, T.T.; Cropp, C.B.; Effler, P. Antibody to dengue 1 detected more than 60 years after infection. Viral Immunol. 2007, 20, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, J.J.; Balmaseda, A.; Gresh, L.; Sahoo, M.K.; Montoya, M.; Wang, C.; Abeynayake, J.; Kuan, G.; Pinsky, B.A.; Harris, E. Homotypic dengue virus reinfections in Nicaraguan children. J. Infect. Dis. 2016, 214, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Forshey, B.M.; Reiner, R.C.; Olkowski, S.; Morrison, A.C.; Espinoza, A.; Long, K.C.; Vilcarromero, S.; Casanova, W.; Wearing, H.J.; Halsey, E.S.; et al. Incomplete protection against dengue virus type 2 re-infection in Peru. PLoS Negl. Trop. Dis. 2016, 10, e0004398. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.; Gresh, L.; Mercado, J.C.; Williams, K.L.; Vargas, M.J. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl. Trop. Dis. 2013, 7, 2357. [Google Scholar] [CrossRef]
- Anderson, K.B.; Gibbons, R.V.; Cummings, D.A.T.; Nisalak, A.; Green, S.; Libraty, D.H.; Jarman, R.G.; Srikiatkhachorn, A.; Mammen, M.P.; Darunee, B.; et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J. Infect. Dis. 2014, 209, 360–368. [Google Scholar] [CrossRef]
- Sangkawibha, N.; Rojanasuphot, S.; Ahandrik, S.; Viriyapongse, S.; Salttul, V.; Phanthumachinda, B.; Halstead, S.B.; Ro, S.; Ahandrlk, S.; Vlrlyapongso, S.; et al. Risk factors in dengue shock syndrome: A prospective epldemlologlc study in Rayong, Thailand. I. The 1980 outbreak. Am. J. Epidemiol. 1984, 120, 653–669. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Rothman, A.L.; Ennis, F.A.; Nisalak, A. Dengue in the early febrile phase: Viremia and antibody responses. J. Infect. Dis. 1997, 176, 322–330. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
- OhAinle, M.; Balmaseda, A.; Macalalad, A.R.; Tellez, Y.; Zody, M.C.; Saborío, S.; Nuñez, A.; Lennon, N.J.; Birren, B.W.; Gordon, A.; et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci. Transl. Med. 2011, 3, 114ra128. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 1977, 265, 739–741. [Google Scholar] [CrossRef]
- Halstead, S.B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J. Infect. Dis. 1979, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Venkateshan, C.N.; Halstead, S.B. Dengue 4 virus monoclonal antibodies identify epitopes that mediate immune infection enhancement of dengue 2 viruses. J. Gen. Virol. 1987, 68, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Littaua, R.; Kurane, I.; Ennis, F.A. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 1990, 144, 3183–3186. [Google Scholar]
- Goncalvez, A.P.; Engle, R.E.; Claire, M.S.; Purcell, R.H.; Lai, C.-J. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 2007, 104, 9422–9427. [Google Scholar] [CrossRef] [PubMed]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010, 6, 1000790. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 2010, 7, 128–139. [Google Scholar] [CrossRef]
- Williams, K.L.; Sukupolvi-Petty, S.; Beltramello, M.; Johnson, S.; Sallusto, F.; Lanzavecchia, A.; Diamond, M.S.; Harris, E. Therapeutic efficacy of antibodies lacking FcγR against lethal dengue virus infection is due to neutralizing potency and blocking of enhancing antibodies. PLoS Pathog. 2013, 9, e1003157. [Google Scholar] [CrossRef]
- Nimmanitya, S.; Kliks, S.C.; Burke, D.S.; Nisalak, A. Evidence That maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 1988, 38, 411–419. [Google Scholar]
- Chau, T.N.B.; Hieu, N.T.; Anders, K.L.; Wolbers, M.; Lien, L.B.; Lu, H.; Minh, T.; Hien, T.T.; Hung, N.T.; Farrar, J.; et al. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese Infants Europe PMC Funders Group. J. Infect. Dis. 2009, 200, 1893–1900. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Katzelnick, L.C.; Burger-Calderon, R.; Gallini, J.; Moore, R.H.; Kuan, G.; Balmaseda, A.; Pinsky, B.A.; Harris, E. Antibody-dependent enhancement of severe disease is mediated by serum viral load in pediatric dengue virus infections. J. Infect. Dis. 2020, 221, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Francis, T.J. On the doctrine of original antigenic sin. Proc. Am. Philos. Soc. 1960, 104, 572–578. [Google Scholar]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S.; et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003, 9, 921–927. [Google Scholar] [CrossRef]
- Duangchinda, T.; Dejnirattisai, W.; Vasanawathana, S.; Limpitikul, W.; Tangthawornchaikul, N.; Malasit, P.; Mongkolsapaya, J.; Screaton, G. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. USA 2010, 107, 16922–16927. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; De Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; De Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, P.; Yu, X.; Brannan, J.M.; Bi, G.; Zhang, Q.; Schein, S.; Zhou, Z.H. Cryo-EM structure of the mature dengue virus at 3.5-Å resolution. Nat. Struct. Mol. Biol. 2013, 20, 105–110. [Google Scholar] [CrossRef]
- Kostyuchenko, V.A.; Zhang, Q.; Tan, J.L.; Ng, T.-S.; Lok, S.-M. Immature and mature dengue serotype 1 virus structures provide insight into the maturation process. J. Virol. 2013, 87, 7700–7707. [Google Scholar] [CrossRef]
- Kostyuchenko, V.A.; Chew, P.L.; Ng, T.-S.; Lok, S.-M. Near-atomic resolution cryo-electron microscopic structure of dengue serotype 4 virus. J. Virol. 2014, 88, 477–482. [Google Scholar] [CrossRef]
- Zhang, W.; Chipman, P.R.; Corver, J.; Johnson, P.R.; Zhang, Y.; Mukhopadhyay, S.; Baker, T.S.; Strauss, J.H.; Rossmann, M.G.; Kuhn, R.J.; et al. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat. Struct. Biol. 2003, 10, 907–912. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA. 2003, 100, 6986–6991. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J. Virol. 2005, 79, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Ogata, S.; Clements, D.; Strauss, J.H.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Conformational changes of the flavivirus E glycoprotein. Structure 2004, 12, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Maguire, T.; Hileman, R.E.; Fromm, J.R.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef]
- Crill, W.D.; Roehrig, J.T. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 2001, 75, 7769–7773. [Google Scholar] [CrossRef]
- Hung, J.-J.; Hsieh, M.-T.; Young, M.-J.; Kao, C.-L.; King, C.-C.; Chang, W. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J. Virol. 2004, 78, 378–388. [Google Scholar] [CrossRef]
- Chin, J.F.L.; Chu, J.J.H.; Ng, M.L. The envelope glycoprotein domain III of dengue virus serotypes 1 and 2 inhibit virus entry. Microbes Infect. 2007, 9, 1–6. [Google Scholar] [CrossRef]
- Watterson, D.; Kobe, B.; Young, P.R. Residues in domain III of the dengue virus envelope glycoprotein involved in cell-surface glycosaminoglycan binding. J. Gen. Virol. 2012, 93, 72–82. [Google Scholar] [CrossRef]
- Pokidysheva, E.; Zhang, Y.; Battisti, A.J.; Bator-Kelly, C.M.; Chipman, P.R.; Xiao, C.; Gregorio, G.G.; Hendrickson, W.A.; Kuhn, R.J.; Rossmann, M.G. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 2006, 124, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; de Wet, B.J.M.; deWet, B.J.M.; Martinez-Pomares, L.; Radcliffe, C.M.; Dwek, R.A.; Rudd, P.M.; Gordon, S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008, 4, e17. [Google Scholar] [CrossRef]
- Navarro-Sanchez, E.; Altmeyer, R.; Amara, A.; Schwartz, O.; Fieschi, F.; Virelizier, J.-L.; Arenzana-Seisdedos, F.; Desprès, P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003, 4, 723–728. [Google Scholar] [CrossRef]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L.; et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef] [PubMed]
- van der Schaar, H.M.; Rust, M.J.; Chen, C.; van der Ende-Metselaar, H.; Wilschut, J.; Zhuang, X.; Smit, J.M. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008, 4, e1000244. [Google Scholar] [CrossRef] [PubMed]
- Bressanelli, S.; Stiasny, K.; Allison, S.L.; Stura, E.A.; Duquerroy, S.; Lescar, J.; Heinz, F.X.; Rey, F.A. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004, 23, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Nour, A.M.; Li, Y.; Wolenski, J.; Modis, Y. Viral membrane fusion and nucleocapsid delivery into the cytoplasm are distinct events in some flaviviruses. PLoS Pathog. 2013, 9, e1003585. [Google Scholar] [CrossRef]
- Mackenzie, J.M.; Westaway, E.G.; Sakzewski, A. Assembly and maturation of the flavivirus kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J. Virol. 2001, 75, 10787–10799. [Google Scholar] [CrossRef]
- Welsch, S.; Miller, S.; Romero-Brey, I.; Merz, A.; Bleck, C.K.E.; Walther, P.; Fuller, S.D.; Antony, C.; Krijnse-Locker, J.; Bartenschlager, R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 2009, 5, 365–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Corver, J.; Chipman, P.R.; Zhang, W.; Pletnev, S.V.; Sedlak, D.; Baker, T.S.; Strauss, J.H.; Kuhn, R.J.; Rossmann, M.G. Structures of immature flavivirus particles. EMBO J. 2003, 22, 2604–2613. [Google Scholar] [CrossRef]
- Li, L.; Lok, S.-M.; Yu, I.-M.; Zhang, Y.; Kuhn, R.J.; Chen, J.; Rossmann, M.G. The flavivirus precursor membrane-envelope protein complex: Structure and maturation. Science 2008, 319, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Allison, S.L.; Schalich, J.; Heinz, F.X. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 1997, 71, 8475–8481. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.-M.; Zhang, W.; Holdaway, H.A.; Li, L.; Kostyuchenko, V.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 2008, 319, 1834–1837. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Heinz, F.X.; Mandl, C.W.; Holzmann, H.; Kunz, C. Fusion activity of flaviviruses: Comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J. Gen. Virol. 1991, 72, 1323–1329. [Google Scholar] [CrossRef]
- Yu, I.-M.; Holdaway, H.A.; Chipman, P.R.; Kuhn, R.J.; Rossmann, M.G.; Chen, J. Association of the pr peptides with dengue virus at acidic pH blocks membrane fusion. J. Virol. 2009, 83, 12101–12107. [Google Scholar] [CrossRef] [PubMed]
- Junjhon, J.; Lausumpao, M.; Supasa, S.; Noisakran, S.; Songjaeng, A.; Saraithong, P.; Chaichoun, K.; Utaipat, U.; Keelapang, P.; Kanjanahaluethai, A.; et al. Differential modulation of prM cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-M junction. J. Virol. 2008, 82, 10776–10791. [Google Scholar] [CrossRef]
- Zybert, I.A.; van der Ende-Metselaar, H.; Wilschut, J.; Smit, J.M. Functional importance of dengue virus maturation: Infectious properties of immature virions. J. Gen. Virol. 2008, 89, 3047–3051. [Google Scholar] [CrossRef]
- Cherrier, M.V.; Kaufmann, B.; Nybakken, G.E.; Lok, S.-M.; Warren, J.T.; Chen, B.R.; Nelson, C.A.; Kostyuchenko, V.A.; Holdaway, H.A.; Chipman, P.R.; et al. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J. 2009, 28, 3269–3276. [Google Scholar] [CrossRef]
- Junjhon, J.; Edwards, T.J.; Utaipat, U.; Bowman, V.D.; Holdaway, H.A.; Zhang, W.; Keelapang, P.; Puttikhunt, C.; Perera, R.; Chipman, P.R.; et al. Influence of pr-M Cleavage on the Heterogeneity of Extracellular Dengue Virus Particles. J. Virol. 2010, 84, 8353–8358. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Randolph, V.B.; Winkler, G.; Stollar, V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 1990, 174, 450–458. [Google Scholar] [CrossRef]
- Plevka, P.; Battisti, A.J.; Junjhon, J.; Winkler, D.C.; Holdaway, H.A.; Keelapang, P.; Sittisombut, N.; Kuhn, R.J.; Steven, A.C.; Rossmann, M.G. Maturation of flaviviruses starts from one or more icosahedrally independent nucleation centres. EMBO Rep. 2011, 12, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Armenia, I.; Molla, G. The Protein Imager: A full-featured online molecular viewer interface with server-side HQ-rendering capabilities. Bioinformatics 2020, 36, 2909–2911. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Arroyo, J.; Pugachev, K.V.; Miller, C.; Zhang, Z.X.; Weltzin, R.; Georgakopoulos, K.; Catalan, J.; Ocran, S.; Soike, K.; et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J. Virol. 2001, 75, 7290–7304. [Google Scholar] [CrossRef]
- Morrison, D.; Legg, T.J.; Billings, C.W.; Forrat, R.; Yoksan, S.; Lang, J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus -naive adults. J. Infect. Dis. 2010, 201, 370–377. [Google Scholar] [CrossRef]
- Capeding, R.Z.; Luna, I.A.; Bomasang, E.; Lupisan, S.; Lang, J.; Forrat, R.; Wartel, A.; Crevat, D. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: Randomized controlled phase I trial in the Philippines. Vaccine 2011, 29, 3863–3872. [Google Scholar] [CrossRef]
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; Jiwariyavej, V.; Dulyachai, W.; Pengsaa, K.; Wartel, T.A.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 2012, 380, 1559–1567. [Google Scholar] [CrossRef]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.S.; Ismail, H.I.H.M.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Ismail, H.I.H.M.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016, 14, 45–54. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Flasche, S.; Smith, P.G. Vaccine-attributable severe dengue in the Philippines. Lancet 2019, 394, 2151–2152. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Hombach, J.; Ferguson, N.; Selgelid, M.; Brien, K.O.; Vannice, K.; Barrett, A.; Ferdinand, E.; Flasche, S.; Guzman, M.; et al. Deliberations of the strategic advisory group of experts on immunization on the use of CYD-TDV dengue vaccine. Lancet Infect. Dis. 2019, 19, e31–e38. [Google Scholar] [CrossRef]
- Butrapet, S.; Huang, C.Y.; Pierro, D.J.; Bhamarapravati, N.; Gubler, D.J.; Kinney, R.M. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5′ noncoding region and nonstructural proteins 1 and 3. J. Virol. 2000, 74, 3011–3019. [Google Scholar] [CrossRef]
- Huang, C.Y.-H.; Butrapet, S.; Tsuchiya, K.R.; Bhamarapravati, N.; Gubler, D.J.; Kinney, R.M. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J. Virol. 2003, 77, 11436–11447. [Google Scholar] [CrossRef]
- Osorio, J.E.; Velez, I.D.; Thomson, C.; Lopez, L.; Jimenez, A.; Haller, A.A.; Silengo, S.; Scott, J.; Boroughs, K.L.; Stovall, J.L.; et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: A randomised, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2014, 14, 830–838. [Google Scholar] [CrossRef]
- Sirivichayakul, C.; Barranco-Santana, E.A.; Esquilin-Rivera, I.; Oh, H.M.L.; Raanan, M.; Sariol, C.A.; Shek, L.P.; Simasathien, S.; Smith, M.K.; Velez, I.D.; et al. Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: A randomized, placebo-controlled phase 2 study. J. Infect. Dis. 2016, 213, 1562–1572. [Google Scholar] [CrossRef]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; Sirivichayakul, C.; Watanaveeradej, V.; Rivera, L.; Espinoza, F.; et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N. Engl. J. Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Borja-Tabora, C.; Vargas, L.M.; Velásquez, H.; Alera, M.T.; Sierra, V.; Rodriguez-Arenales, E.J.; Yu, D.; Wickramasinghe, V.P.; Moreira, E.D.; et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: A randomised, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1423–1433. [Google Scholar] [CrossRef]
- Blaney, J.E.; Durbin, A.P.; Murphy, B.R.; Whitehead, S.S. Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol. 2006, 19, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P.; Mcarthur, J.H.; Marron, J.A.; Blaney, J.E.; Thumar, B.; Wanionek, K.; Murphy, B.R.; Whitehead, S.S. Chimeric dengue serotype 2 vaccine, is safe and highly immunogenic in healthy dengue-naïve adults. Hum. Vaccin. 2006, 2, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P.; Kirkpatrick, B.D.; Pierce, K.K.; Elwood, D.; Larsson, C.J.; Lindow, J.C.; Tibery, C.; Sabundayo, B.P.; Shaffer, D.; Talaat, K.R.; et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: A randomized, double-blind clinical trial. J. Infect. Dis. 2013, 207, 957–965. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Durbin, A.P.; Pierce, K.K.; Carmolli, M.P.; Tibery, C.M.; Grier, P.L.; Hynes, N.; Diehl, S.A.; Elwood, D.; Jarvis, A.P.; et al. Robust and balanced immune responses to all 4 dengue virus serotypes following administration of a single dose of a live attenuated tetravalent dengue vaccine to healthy, flavivirus-naive adults. J. Infect. Dis. 2015, 212, 702–710. [Google Scholar] [CrossRef]
- Whitehead, S.S.; Durbin, A.P.; Pierce, K.K.; Elwood, D.; McElvany, B.D.; Fraser, E.A.; Carmolli, M.P.; Tibery, C.M.; Hynes, N.A.; Jo, M.; et al. In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl. Trop. Dis. 2017, 11, e0005584. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Whitehead, S.S.; Pierce, K.K.; Tibery, C.M.; Grier, P.L.; Hynes, N.A.; Larsson, C.J.; Sabundayo, B.P.; Talaat, K.R.; Janiak, A.; et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 2016, 8, 330ra36. [Google Scholar] [CrossRef]
- Halstead, S.B.; Russell, P.K. Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine 2016, 34, 1643–1647. [Google Scholar] [CrossRef]
- Henein, S.; Swanstrom, J.; Byers, A.M.; Moser, J.M.; Shaik, S.F.; Bonaparte, M.; Jackson, N.; Guy, B.; Baric, R.; de Silva, A.M. Dissecting antibodies induced by a chimeric yellow fever-dengue, live-attenuated, tetravalent dengue vaccine (CYD-TDV) in naïve and dengue exposed individuals. J. Infect. Dis. 2016, 215, 351–358. [Google Scholar] [CrossRef]
- Schlesinger, J.J.; Brandriss, M.W.; Walsh, E.E. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J. Gen. Virol. 1987, 68, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Zellweger, R.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A protective role for dengue virus-specific CD8 + T Cells. J. Immunol. 2009, 182, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Tsai, W.-Y.; Lin, S.-R.; Kao, C.-L.; Hu, H.-P.; King, C.-C.; Wu, H.-C.; Chang, G.-J.; Wang, W.-K. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J. Virol. 2008, 82, 6631–6643. [Google Scholar] [CrossRef] [PubMed]
- Beltramello, M.; Williams, K.L.; Simmons, C.P.; Macagno, A.; Simonelli, L.; Than, N.; Quyen, H.; Sukupolvi-Petty, S.; Navarro-Sanchez, E.; Young, P.R.; et al. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010, 8, 271–283. [Google Scholar] [CrossRef] [PubMed]
- de Alwis, R.; Beltramello, M.; Messer, W.B.; Sukupolvi-Petty, S.; Wahala, W.M.P.B.; Kraus, A.; Olivarez, N.P.; Pham, Q.; Brian, J.; Tsai, W.-Y.; et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl. Trop. Dis. 2011, 5, e1188. [Google Scholar] [CrossRef]
- de Alwis, R.; Smith, S.A.; Olivarez, N.P.; Messer, W.B.; Huynh, J.P.; Wahala, W.M.P.B.; White, L.J.; Diamond, M.S.; Baric, R.S.; Crowe, J.E.; et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc. Natl. Acad. Sci. USA 2012, 109, 7439–7444. [Google Scholar] [CrossRef]
- Smith, S.A.; Zhou, Y.; Olivarez, N.P.; Broadwater, A.H.; de Silva, A.M.; Crowe, J.E. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J. Virol. 2012, 86, 2665–2675. [Google Scholar] [CrossRef]
- Smith, S.A.; de Alwis, A.R.; Kose, N.; Harris, E.; Ibarra, K.D.; Kahle, K.M.; Pfaff, J.M.; Xiang, X.; Doranz, B.J.; de Silva, A.M.; et al. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio 2013, 4, e00873-13. [Google Scholar] [CrossRef]
- Smith, S.A.; de Alwis, A.R.; Kose, N.; Jadi, R.S.; de Silva, A.M.; Crowe, J.E. Isolation of dengue virus-specific memory B cells with live virus antigen from human subjects following natural infection reveals the presence of diverse novel functional groups of antibody clones. J. Virol. 2014, 88, 12233–12241. [Google Scholar] [CrossRef]
- Appanna, R.; KG, S.; Xu, M.H.; Toh, Y.-X.; Velumani, S.; Carbajo, D.; Lee, C.Y.; Zuest, R.; Balakrishnan, T.; Xu, W.; et al. Plasmablasts during acute dengue infection represent a small subset of a broader virus-specific memory B cell pool. EBioMedicine 2016, 12, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Cho, A.; Onlamoon, N.; Zheng, N.-Y.; Huang, M.; Kovalenkov, Y.; Chokephaibulkit, K.; Angkasekwinai, N.; Pattanapanyasat, K.; Ahmed, R.; et al. B cell responses during secondary dengue virus infection are dominated by highly cross-reactive, memory-derived plasmablasts. J. Virol. 2016, 90, 5574–5585. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Longo, P.; Miley, M.J.; Montoya, M.; Harris, E.; de Silva, A.M. Dissecting the human serum antibody response to secondary dengue virus infections. PLoS Negl. Trop. Dis. 2017, 11, e0005554. [Google Scholar] [CrossRef] [PubMed]
- Nivarthi, U.K.; Tu, H.A.; Delacruz, M.J.; Swanstrom, J.; Patel, B.; Durbin, A.P.; Whitehead, S.S.; Pierce, K.K.; Kirkpatrick, B.D.; Baric, R.S.; et al. Longitudinal analysis of acute and convalescent B cell responses in a human primary dengue serotype 2 infection model. EBioMedicine 2019, 41, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Narvekar, P.; Montoya, M.; Michlmayr, D.; Balmaseda, A.; Coloma, J.; Harris, E. Primary and secondary dengue virus infections elicit similar memory B cell responses but breadth to other serotypes and cross-reactivity to Zika virus is higher in secondary dengue. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.R.; Zhang, S.L.-X.; Tan, H.C.; Chan, Y.K.; Chow, A.; Lim, A.P.C.; Vasudevan, S.G.; Hanson, B.J.; Ooi, E.E. Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 12479–12484. [Google Scholar] [CrossRef]
- Mathew, A.; West, K.; Kalayanarooj, S.; Gibbons, R.V.; Srikiatkhachorn, A.; Green, S.; Libraty, D.; Jaiswal, S.; Rothman, A.L. B-cell responses during primary and secondary dengue virus infections in humans. J. Infect. Dis. 2011, 204, 1514–1522. [Google Scholar] [CrossRef]
- Zompi, S.; Montoya, M.; Pohl, M.O.; Balmaseda, A.; Harris, E. Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Negl. Trop. Dis. 2012, 6, e1568. [Google Scholar] [CrossRef]
- Xu, M.; Hadinoto, V.; Appanna, R.; Joensson, K.; Toh, Y.X.; Balakrishnan, T.; Ong, S.H.; Warter, L.; Leo, Y.S.; Wang, C.-I.; et al. Plasmablasts generated during repeated dengue infection are virus glycoprotein-specific and bind to multiple virus serotypes. J. Immunol. 2012, 189, 5877–5885. [Google Scholar] [CrossRef]
- Wrammert, J.; Onlamoon, N.; Akondy, R.S.; Perng, G.C.; Polsrila, K.; Chandele, A.; Kwissa, M.; Pulendran, B.; Wilson, P.C.; Wittawatmongkol, O.; et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J. Virol. 2012, 86, 2911–2918. [Google Scholar] [CrossRef]
- Toh, Y.X.; Gan, V.; Balakrishnan, T.; Zuest, R.; Poidinger, M.; Wilson, S.; Appanna, R.; Thein, T.L.; Ong, A.K.-Y.; Ng, L.C.; et al. Dengue serotype cross-reactive, anti-E protein antibodies confound specific immune memory for 1 year after infection. Front. Immunol. 2014, 5, 388. [Google Scholar] [CrossRef] [PubMed]
- Woda, M.; Friberg, H.; Currier, J.R.; Srikiatkhachorn, A.; Macareo, L.R.; Green, S.; Jarman, R.G.; Rothman, A.L.; Mathew, A. Dynamics of dengue virus (DENV)–specific B cells in the response to DENV serotype 1 infections, using flow cytometry with labeled virions. J. Infect. Dis. 2016, 214, 1001–1009. [Google Scholar] [CrossRef]
- Xu, M.; Züst, R.; Toh, Y.X.; Pfaff, J.M.; Kahle, K.M.; Davidson, E.; Doranz, B.J.; Velumani, S.; Tukijan, F.; Wang, C.-I.; et al. Protective capacity of the human anamnestic antibody response during acute dengue virus infection. J. Virol. 2016, 90, 11122–11131. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Williams, K.L.; Wu, Y.-C.; Knight, S.; Balmaseda, A.; Harris, E.; Wang, W.-K. Analysis of cross-reactive antibodies recognizing the fusion loop of envelope protein and correlation with neutralizing antibody titers in Nicaraguan dengue cases. PLoS Negl. Trop. Dis. 2013, 7, e2451. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-Y.; Lai, C.-Y.; Wu, Y.-C.; Lin, H.-E.; Edwards, C.; Jumnainsong, A.; Kliks, S.; Halstead, S.; Mongkolsapaya, J.; Screaton, G.R.; et al. High-avidity and potently neutralizing cross-reactive human monoclonal antibodies derived from secondary dengue virus infection. J. Virol. 2013, 87, 12562–12575. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-Y.; Durbin, A.; Tsai, J.-J.; Hsieh, S.-C.; Whitehead, S.; Wang, W.-K. Complexity of neutralizing antibodies against multiple dengue virus serotypes after heterotypic immunization and secondary infection revealed by in-depth analysis of cross-reactive antibodies. J. Virol. 2015, 89, 7348–7362. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.V.; Kalanarooj, S.; Jarman, R.G.; Nisalak, A.; Vaughn, D.W.; Endy, T.P.; Mammen, M.P.; Srikiatkhachorn, A. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am. J. Trop. Med. Hyg. 2007, 77, 910–913. [Google Scholar] [CrossRef]
- Bhoomiboonchoo, P.; Nisalak, A.; Chansatiporn, N.; Yoon, I.-K.; Kalayanarooj, S.; Thipayamongkolgul, M.; Endy, T.; Rothman, A.L.; Green, S.; Srikiatkhachorn, A.; et al. Sequential dengue virus infections detected in active and passive surveillance programs in Thailand, 1994–2010. BMC Public Health 2010, 15, 250. [Google Scholar] [CrossRef]
- Olkowski, S.; Forshey, B.M.; Morrison, A.C.; Rocha, C.; Vilcarromero, S.; Halsey, E.S.; Kochel, T.J.; Scott, T.W.; Stoddard, S.T. Reduced risk of disease during postsecondary dengue virus infections. J. Infect. Dis. 2013, 208, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Xu, Q.; Nelson, S.; Oliphant, T.; Nybakken, G.E.; Fremont, D.H.; Diamond, M.S. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 2007, 1, 135–145. [Google Scholar] [CrossRef]
- Raut, R.; Corbett, K.S.; Tennekoon, R.N.; Premawansa, S.; Wijewickrama, A.; Premawansa, G.; Mieczkowski, P.; Rückert, C.; Ebel, G.D.; De Silva, A.D.; et al. Dengue type 1 viruses circulating in humans are highly infectious and poorly neutralized by human antibodies. Proc. Natl. Acad. Sci. USA 2019, 116, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.; Jost, C.A.; Xu, Q.; Ess, J.; Martin, J.E.; Oliphant, T.; Whitehead, S.S.; Durbin, A.P.; Graham, B.S.; Dimaond, M.S.; et al. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 2008, 4, e1000060. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.A.; Mukherjee, S.; Kuhn, R.J.; Pierson, T.C. Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. J. Virol. 2014, 88, 11726–11737. [Google Scholar] [CrossRef] [PubMed]
- Fibriansah, G.; Ng, T.-S.; Kostyuchenko, V.A.; Lee, J.; Lee, S.; Wang, J.; Lok, S.-M. Structural changes in dengue virus when exposed to a temperature of 37 °C. J. Virol. 2013, 87, 7585–7592. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, J.; Plevka, P.; Kuhn, R.J.; Diamond, M.S.; Rossmann, M.G. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc. Natl. Acad. Sci. USA 2013, 110, 6795–6799. [Google Scholar] [CrossRef]
- Lim, X.-N.; Shan, C.; Marzinek, J.K.; Dong, H.; Ng, T.S.; Ooi, J.S.G.; Fibriansah, G.; Wang, J.; Verma, C.S.; Bond, P.J.; et al. Molecular basis of dengue virus serotype 2 morphological switch from 29 °C to 37 °C. PLoS Pathog. 2019, 15, e1007996. [Google Scholar] [CrossRef] [PubMed]
- Lok, S.-M.; Kostyuchenko, V.; Nybakken, G.E.; Holdaway, H.A.; Battisti, A.J.; Sukupolvi-Petty, S.; Sedlak, D.; Fremont, D.H.; Chipman, P.R.; Roehrig, J.T.; et al. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat. Struct. Mol. Biol. 2008, 15, 312–317. [Google Scholar] [CrossRef]
- Dowd, K.A.; Jost, C.A.; Durbin, A.P.; Whitehead, S.S.; Pierson, T.C. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog. 2011, 7, e1002111. [Google Scholar] [CrossRef]
- Austin, S.K.; Dowd, K.A.; Shrestha, B.; Nelson, C.A.; Edeling, M.A.; Johnson, S.; Pierson, T.C.; Diamond, M.S.; Fremont, D.H. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog. 2012, 8, e1002930. [Google Scholar] [CrossRef]
- Sukupolvi-Petty, S.; Brien, J.D.; Austin, S.K.; Shrestha, B.; Swayne, S.; Kahle, K.; Doranz, B.J.; Johnson, S.; Pierson, T.C.; Fremont, D.H.; et al. Functional analysis of antibodies against dengue virus type 4 reveals strain-dependent epitope exposure that impacts neutralization and protection. J. Virol. 2013, 87, 8826–8842. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.A.; DeMaso, C.R.; Pierson, T.C. Genotypic differences in dengue virus neutralization are explained by a single amino acid mutation that modulates virus breathing. mBio 2015, 6, e01559-15. [Google Scholar] [CrossRef] [PubMed]
- Crill, W.D.; Hughes, H.R.; Delorey, M.J.; Chang, G.-J.J. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS ONE 2009, 4, e4991. [Google Scholar] [CrossRef]
- Wahala, W.M.P.B.; Kraus, A.A.; Haymore, L.B.; Accavitti-Loper, M.A.; De Silva, A.M. Dengue virus neutralization by human immune sera: Role of envelope protein domain III-reactive antibody. Virology 2009, 392, 103–113. [Google Scholar] [CrossRef]
- Lin, H.-E.; Tsai, W.-Y.; Liu, I.-J.; Li, P.-C.; Liao, M.-Y.; Tsai, J.-J.; Wu, Y.-C.; Lai, C.-Y.; Lu, C.-H.; Huang, J.-H.; et al. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high throughput assay. PLoS Negl. Trop. Dis. 2012, 6, e1447. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; de Alwis, R.; Kose, N.; Durbin, A.P.; Whitehead, S.S.; de Silva, A.M.; Crowe, J.E. Human monoclonal antibodies derived from memory B cells following live attenuated dengue virus vaccination or natural infection exhibit similar characteristics. J. Infect. Dis. 2013, 207, 1898–1908. [Google Scholar] [CrossRef]
- de Alwis, R.; Williams, K.L.; Schmid, M.A.; Lai, C.-Y.; Patel, B. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog. 2014, 10, e1004386. [Google Scholar] [CrossRef] [PubMed]
- Crill, W.D.; Chang, G.-J.J. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 2004, 78, 13975–13986. [Google Scholar] [CrossRef]
- Oliphant, T.; Nybakken, G.E.; Engle, M.; Xu, Q.; Nelson, C.A.; Sukupolvi-Petty, S.; Marri, A.; Lachmi, B.-E.; Olshevsky, U.; Fremont, D.H.; et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J. Virol. 2006, 80, 12149–12159. [Google Scholar] [CrossRef] [PubMed]
- Costin, J.M.; Zaitseva, E.; Kahle, K.M.; Nicholson, C.O.; Rowe, D.K.; Graham, A.S.; Bazzone, L.E.; Hogancamp, G.; Sierra, M.F.; Fong, R.H.; et al. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J. Virol. 2013, 87, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Song, J.; Lu, X.; Qin, C.-F.; Qi, J.; Gao, G.F. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1109. [Google Scholar] [CrossRef]
- Chaudhury, S.; Gromowski, G.D.; Ripoll, D.R.; Khavrutskii, I.V.; Desai, V.; Wallqvist, A. Dengue virus antibody database: Systematically linking serotype-specificity with epitope mapping in dengue virus. PLoS Negl. Trop. Dis. 2017, 11, e0005395. [Google Scholar] [CrossRef]
- Gentry, M.K.; Henchal, E.A.; Mccown, J.M.; Brandt, W.E.; Dalrymple, J.M. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 1982, 31, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Serafin, I.L.; Aaskov, J.G. Identification of epitopes on the envelope (E) protein of dengue 2 and dengue 3 viruses using monoclonal antibodies. Arch. Virol. 2001, 146, 2469–2479. [Google Scholar] [CrossRef]
- Tsai, W.-Y.; Chen, H.-L.; Tsai, J.-J.; Dejnirattisai, W.; Jumnainsong, A.; Mongkolsapaya, J.; Screaton, G.; Crowe, J.E.; Wang, W.-K.; Wang, W.-K. Potent neutralizing human monoclonal antibodies preferentially target mature dengue virus particles: Implication for novel strategy for dengue vaccine. J. Virol. 2018, 92, e00056-18. [Google Scholar] [CrossRef]
- Huang, C.Y.-H.; Butrapet, S.; Moss, K.J.; Childers, T.; Erb, S.M.; Calvert, A.E.; Silengo, S.J.; Kinney, R.M.; Blair, C.D.; Roehrig, J.T. The dengue virus type 2 envelope protein fusion peptide is essential for membrane fusion. Virology 2010, 396, 305–315. [Google Scholar] [CrossRef]
- Hughes, H.R.; Crill, W.D.; Chang, G.-J.J. Manipulation of immunodominant dengue virus E protein epitopes reduces potential antibody-dependent enhancement. Virol. J. 2012, 9, 115. [Google Scholar] [CrossRef]
- Crill, W.D.; Hughes, H.R.; Trainor, N.B.; Davis, B.S.; Whitney, M.T.; Chang, G.-J.J. Sculpting humoral immunity through dengue vaccination to enhance protective immunity. Front. Immunol. 2012, 3, 334. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Pierson, T.C.; Ciaramella, G.; Diamond, M.S. Modified mRNA vaccines protect against Zika virus infection. Cell 2017, 168, 1114–1125. [Google Scholar] [CrossRef]
- Huang, K.-J.; Yang, Y.-C.; Lin, Y.-S.; Huang, J.-H.; Liu, H.-S.; Yeh, T.-M.; Chen, S.-H.; Liu, C.-C.; Lei, H.-Y. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J. Immunol. 2006, 176, 2825–2832. [Google Scholar] [CrossRef]
- Luo, Y.-Y.; Feng, J.-J.; Zhou, J.-M.; Yu, Z.-Z.; Fang, D.-Y.; Yan, H.-J.; Zeng, G.-C.; Jiang, L.-F. Identification of a novel infection-enhancing epitope on dengue prM using a dengue cross-reacting monoclonal antibody. BMC Microbiol. 2013, 13, 194. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Pennington, J.G.; Sheng, J.; Yap, M.L.; Plevka, P.; Meng, G.; Sun, L.; Jiang, W.; Rossmann, M.G. Obstruction of dengue virus maturation by Fab fragments of the 2H2 antibody. J. Virol. 2013, 87, 8909–8915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, S.A.; Nivarthi, U.K.; de Alwis, R.; Kose, N.; Sapparapu, G.; Bombardi, R.; Kahle, K.M.; Pfaff, J.M.; Lieberman, S.; Doranz, B.J.; et al. Dengue virus prM-specific human monoclonal antibodies with virus replication-enhancing properties recognize a single immunodominant antigenic site. J. Virol. 2015, 90, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Falconar, A.K.I. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: Design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch. Virol. 1999, 144, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Puttikhunt, C.; Keelapang, P.; Khemnu, N.; Sittisombut, N.; Kasinrerk, W.; Malasit, P. Novel anti-dengue monoclonal antibody recognizing conformational structure of the prM-E heterodimeric complex of dengue virus. J. Med. Virol. 2008, 80, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.Y.; Tan, H.C.; Chow, A.Y.; Lim, A.P.C.; Lok, S.M.; Moreland, N.J.; Vasudevan, S.G.; MacAry, P.A.; Ooi, E.E.; Hanson, B.J. A Human PrM antibody that recognizes a novel cryptic epitope on dengue E glycoprotein. PLoS ONE 2012, 7, e33451. [Google Scholar] [CrossRef]
- Henchal, E.A.; Mccown, J.M.; Burke, D.S.; Seguin, M.C.; Brandt, W.E.; Brandt, W.E. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am. J. Trop. Med. Hyg. 1985, 34, 162–169. [Google Scholar] [CrossRef]
- Vázquez, S.; Guzmán, M.G.; Guillen, G.; Chinea, G.; Pérez, A.B.; Pupo, M.; Rodriguez, R.; Reyes, O.; Garay, H.E.; Delgado, I.; et al. Immune response to synthetic peptides of dengue prM protein. Vaccine 2002, 20, 1823–1830. [Google Scholar] [CrossRef]
- Kaufman, B.M.; Summers, P.L.; Dubois, D.R.; Cohen, W.H.; Gentry, M.K.; Timchak, R.L.; Burke, D.S.; Eckels, K.H. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 1989, 41, 576–580. [Google Scholar] [CrossRef]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Partial maturation: An immune-evasion strategy of dengue virus? Trends Microbiol. 2011, 19, 248–254. [Google Scholar] [CrossRef]
- Rodenhuis-Zybert, I.A.; van der Schaar, H.M.; da Silva Voorham, J.M.; van der Ende-Metselaar, H.; Lei, H.-Y.; Wilschut, J.; Smit, J.M. Immature dengue virus: A veiled pathogen? PLoS Pathog. 2010, 6, e1000718. [Google Scholar] [CrossRef]
- da Silva Voorham, J.M.; Rodenhuis-Zybert, I.A.; Nuñez, N.V.A.; Colpitts, T.M.; van der Ende-Metselaar, H.; Fikrig, E.; Diamond, M.S.; Wilschut, J.; Smit, J.M. Antibodies against the envelope glycoprotein promote infectivity of immature dengue virus serotype 2. PLoS ONE 2012, 7, e29957. [Google Scholar] [CrossRef]
- Wirawan, M.; Fibriansah, G.; Marzinek, J.K.; Lim, X.X.; Ng, T.-S.; Sim, A.Y.L.; Zhang, Q.; Kostyuchenko, V.A.; Shi, J.; Smith, S.A.; et al. Mechanism of enhanced immature dengue virus attachment to endosomal membrane induced by prM antibody. Structure 2019, 27, 253–267.e8. [Google Scholar] [CrossRef] [PubMed]
- Yam-Puc, J.C.; García-Cordero, J.; Calderón-Amador, J.; Donis-Maturano, L.; Cedillo-Barrón, L.; Flores-Romo, L. Germinal center reaction following cutaneous dengue virus infection in immune-competent mice. Front. Immunol. 2015, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sirohi, D.; Dowd, K.A.; Chen, Z.; Diamond, M.S.; Kuhn, R.J.; Pierson, T.C. Enhancing dengue virus maturation using a stable furin over-expressing cell line. Virology 2016, 497, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Keelapang, P.; Nitatpattana, N.; Suphatrakul, A.; Punyahathaikul, S.; Sriburi, R.; Pulmanausahakul, R.; Pichyangkul, S.; Malasit, P.; Yoksan, S.; Sittisombut, N. Generation and preclinical evaluation of a DENV-1/2 prM + E chimeric live attenuated vaccine candidate with enhanced prM cleavage. Vaccine 2013, 31, 5134–5140. [Google Scholar] [CrossRef]
- Oceguera, L.F.; Patiris, P.J.; Chiles, R.E.; Busch, M.P.; Tobler, L.H.; Hanson, C. V Flavivirus serology by Western blot analysis. Am. J. Trop. Med. Hyg. 2007, 77, 159–163. [Google Scholar] [CrossRef]
- Sjatha, F.; Kuwahara, M.; Sudiro, T.M.; Kameoka, M.; Konishi, E. Evaluation of chimeric DNA vaccines consisting of premembrane and envelope genes of Japanese encephalitis and dengue viruses as a strategy for reducing induction of dengue virus infection-enhancing antibody response. Microbiol. Immunol. 2014, 58, 126–134. [Google Scholar] [CrossRef]
- Wang, Y.; Si, L.; Luo, Y.; Guo, X.; Zhou, J.; Fang, D.; Yan, H.; Zeng, G.; Jiang, L. Replacement of pr gene with Japanese encephalitis virus pr using reverse genetics reduces antibody-dependent enhancement of dengue virus 2 infection. Appl. Microbiol. Biotechnol. 2015, 99, 9685–9698. [Google Scholar] [CrossRef]
- Wang, Y.; Si, L.-L.; Guo, X.-L.; Cui, G.; Fang, D.-Y.; Zhou, J.-M.; Yan, H.-J.; Jiang, L.-F. Substitution of the precursor peptide prevents anti-prM antibody-mediated antibody-dependent enhancement of dengue virus infection. Virus Res. 2017, 229, 57–64. [Google Scholar] [CrossRef]
- Roehrig, J.T.; Bolin, R.A.; Kelly, R.G. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 1998, 246, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Sukupolvi-Petty, S.; Austin, S.K.; Purtha, W.E.; Oliphant, T.; Nybakken, G.E.; Schlesinger, J.J.; Roehrig, J.T.; Gromowski, G.D.; Barrett, A.D.; Fremont, D.H.; et al. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J. Virol. 2007, 81, 12816–12826. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Brien, J.D.; Sukupolvi-Petty, S.; Austin, S.K.; Edeling, M.A.; Kim, T.; O’Brien, K.M.; Nelson, C.A.; Johnson, S.; Fremont, D.H.; et al. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog. 2010, 6, e1000823. [Google Scholar] [CrossRef] [PubMed]
- Sukupolvi-Petty, S.; Austin, S.K.; Engle, M.; Brien, J.D.; Dowd, K.A.; Williams, K.L.; Johnson, S.; Rico-Hesse, R.; Harris, E.; Pierson, T.C.; et al. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J. Virol. 2010, 84, 9227–9239. [Google Scholar] [CrossRef]
- Gromowski, G.D.; Barrett, A.D.T. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 2007, 366, 349–360. [Google Scholar] [CrossRef]
- Brien, J.D.; Austin, S.K.; Sukupolvi-Petty, S.; O’Brien, K.M.; Johnson, S.; Fremont, D.H.; Diamond, M.S. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J. Virol. 2010, 84, 10630–10643. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chou, F.-P.; Wang, Y.-K.; Huang, S.-C.; Cheng, C.-H.; Wu, T.-K. Characterization and epitope mapping of Dengue virus type 1 specific monoclonal antibodies. Virol. J. 2017, 14, 189. [Google Scholar] [CrossRef]
- Renner, M.; Flanagan, A.; Dejnirattisai, W.; Puttikhunt, C.; Kasinrerk, W.; Supasa, P.; Wongwiwat, W.; Chawansuntati, K.; Duangchinda, T.; Cowper, A.; et al. Characterization of a potent and highly unusual minimally enhancing antibody directed against dengue virus. Nat. Immunol. 2018, 19, 1248–1256. [Google Scholar] [CrossRef]
- Thullier, P.; Lafaye, P.; Mégret, F.; Deubel, V.; Jouan, A.; Mazié, J. A recombinant Fab neutralizes dengue virus in vitro. J. Biotechnol. 1999, 69, 183–190. [Google Scholar] [CrossRef]
- Thullier, P.; Demangel, C.; Bedouelle, H.; Me, F.; Jouan, A.; Deubel, V.; Mazie, J.-C.; Lafaye, P. Mapping of a dengue virus neutralizing epitope critical for the infectivity of all serotypes: Insight into the neutralization mechanism. J. Gen. Virol. 2001, 82, 1885–1892. [Google Scholar] [CrossRef]
- Lisova, O.; Hardy, F.; Petit, V.; Bedouelle, H. Mapping to completeness and transplantation of a group-specific, discontinuous, neutralizing epitope in the envelope protein of dengue virus. J. Gen. Virol. 2007, 88, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Rajamanonmani, R.; Nkenfou, C.; Clancy, P.; Yau, Y.H.; Shochat, S.G.; Sukupolvi-Petty, S.; Schul, W.; Diamond, M.S.; Vasudevan, S.G.; Lescar, J. On a mouse monoclonal antibody that neutralizes all four dengue virus serotypes. J. Gen. Virol. 2009, 90, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Gromowski, G.D.; Roehrig, J.T.; Diamond, M.S.; Lee, J.C.; Pitcher, T.J.; Barrett, A.D.T. Mutations of an antibody binding energy hot spot on domain III of the dengue 2 envelope glycoprotein exploited for neutralization escape. Virology 2010, 407, 237–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Megret, F.; Hugnot, J.P.; Falconar, A.; Gentry, M.K.; Morens, D.M.; Murray, J.M.; Schlesinger, J.I.; Wright, P.J.; Young, P.; Van Regenmortel, M.H.V.; et al. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue virus envelope glycoprotein. Virology 1992, 187, 480–491. [Google Scholar] [CrossRef]
- Cockburn, J.J.B.; Sanchez, M.E.N.; Fretes, N.; Urvoas, A.; Staropoli, I.; Kikuti, C.M.; Coffey, L.L.; Seisdedos, F.A.; Bedouelle, H.; Rey, F.A. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure 2012, 20, 303–314. [Google Scholar] [CrossRef]
- Pierson, T.C.; Kuhn, R.J. Capturing a virus while it catches its breath. Structure 2012, 20, 200–202. [Google Scholar] [CrossRef][Green Version]
- Tharakaraman, K.; Robinson, L.N.; Hatas, A.; Chen, Y.L.; Siyue, L.; Raguram, S.; Sasisekharan, V.; Wogan, G.N.; Sasisekharan, R. Redesign of a cross-reactive antibody to dengue virus with broad-spectrum activity and increased in vivo potency. Proc. Natl. Acad. Sci. USA 2013, 110, E1555–E1564. [Google Scholar] [CrossRef]
- Robinson, L.N.; Tharakaraman, K.; Rowley, K.J.; Costa, V.V.; Chan, K.R.; Wong, Y.H.; Ong, L.C.; Tan, H.C.; Koch, T.; Cain, D.; et al. Structure-guided design of an anti-dengue antibody directed to a non-immunodominant epitope. Cell 2015, 162, 493–504. [Google Scholar] [CrossRef]
- Midgley, C.M.; Flanagan, A.; Tran, H.B.; Dejnirattisai, W.; Chawansuntati, K.; Jumnainsong, A.; Wongwiwat, W.; Duangchinda, T.; Mongkolsapaya, J.; Grimes, J.M.; et al. Structural analysis of a dengue cross-reactive antibody complexed with envelope domain III reveals the molecular basis of cross-reactivity. J. Immunol. 2012, 188, 4971–4979. [Google Scholar] [CrossRef]
- Li, X.-Q.; Qiu, L.-W.; Chen, Y.; Wen, K.; Cai, J.-P.; Chen, J.; Pan, Y.-X.; Li, J.; Hu, D.-M.; Huang, Y.-F.; et al. Dengue virus envelope domain III immunization elicits predominantly cross-reactive, poorly neutralizing antibodies localized to the AB loop: Implications for dengue vaccine design. J. Gen. Virol. 2013, 94, 2191–2201. [Google Scholar] [CrossRef]
- Li, J.; Watterson, D.; Chang, C.-W.; Che, X.-Y.; Li, X.-Q.; Ericsson, D.J.; Qiu, L.-W.; Cai, J.-P.; Chen, J.; Fry, S.R.; et al. Structural and functional characterization of a cross-reactive dengue virus neutralizing antibody that recognizes a cryptic epitope. Structure 2018, 26, 51–59. [Google Scholar] [CrossRef]
- Wahala, W.M.P.B.; Huang, C.; Butrapet, S.; White, L.J.; de Silva, A.M. Recombinant dengue type 2 viruses with altered E protein domain III epitopes are efficiently neutralized by human immune sera. J. Virol. 2012, 86, 4019–4023. [Google Scholar] [CrossRef]
- Midgley, C.M.; Bajwa-Joseph, M.; Vasanawathana, S.; Limpitikul, W.; Wills, B.; Flanagan, A.; Waiyaiya, E.; Tran, H.B.; Cowper, A.E.; Chotiyarnwong, P.; et al. An in-depth analysis of original antigenic sin in dengue virus infection. J. Virol. 2011, 85, 410–421. [Google Scholar] [CrossRef]
- Williams, K.L.; Wahala, W.M.P.B.; Orozco, S.; de Silva, A.M.; Harris, E. Antibodies targeting dengue virus envelope domain III are not required for serotype-specific protection or prevention of enhancement in vivo. Virology 2012, 429, 12–20. [Google Scholar] [CrossRef]
- Guzman, M.G.; Hermida, L.; Bernardo, L.; Ramirez, R.; Guillén, G. Domain III of the envelope protein as a dengue vaccine target. Expert Rev. Vaccines 2010, 9, 137–147. [Google Scholar] [CrossRef]
- Fonseca, B.A.L.; Khoshnood, K.; Shope, R.E.; Mason, P.W. Flavivirus type-specific antigens produced from fusions of a portion of the E protein gene with the Escherichia coli trpe gene. Am. J. Trop. Med. Hyg. 1991, 44, 500–508. [Google Scholar] [CrossRef]
- Simmons, M.; Hayes, C.G.; Wu, S.J.; Nelson, W.M. Evaluation of the protective efficacy of a recombinant dengue envelope B domain fusion protein against dengue 2 virus infection in mice. Am. J. Trop. Med. Hyg. 1998, 58, 655–662. [Google Scholar] [CrossRef]
- Hermida, L.; Rodríguez, R.; Lazo, L.; Silva, R.; Zulueta, A.; Chinea, G.; López, C.; Guzmán, M.G.; Guillén, G. A dengue-2 Envelope fragment inserted within the structure of the P64k meningococcal protein carrier enables a functional immune response against the virus in mice. J. Virol. Methods 2004, 115, 41–49. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Liu, S.-J.; Tsai, J.-P.; Li, Y.-S.; Chen, M.-Y.; Liu, H.-H.; Chong, P.; Leng, C.-H.; Chen, H.-W. A Novel single-dose dengue subunit vaccine induces memory immune responses. PLoS ONE 2011, 6, e23319. [Google Scholar] [CrossRef]
- Mota, J.; Acosta, M.; Argotte, R.; Figueroa, R.; Méndez, A.; Ramos, C. Induction of protective antibodies against dengue virus by tetravalent DNA immunization of mice with domain III of the envelope protein. Vaccine 2005, 23, 3469–3476. [Google Scholar] [CrossRef]
- Azevedo, A.S.; Yamamura, A.M.Y.; Freire, M.S.; Trindade, G.F.; Bonaldo, M.; Galler, R.; Alves, A.M.B. DNA vaccines against dengue virus type 2 based on truncate envelope protein or its domain III. PLoS ONE 2011, 6, e20528. [Google Scholar] [CrossRef] [PubMed]
- Poggianella, M.; Campos, J.L.S.; Chan, K.R.; Tan, H.C.; Bestagno, M.; Ooi, E.E.; Burrone, O.R. Dengue E protein domain III-based DNA immunisation induces strong antibody responses to all four viral serotypes. PLoS Negl. Trop. Dis. 2015, 9, e0003947. [Google Scholar] [CrossRef] [PubMed]
- Khanam, S.; Khanna, N.; Swaminathan, S. Induction of neutralizing antibodies and T cell responses by dengue virus type 2 envelope domain III encoded by plasmid and adenoviral vectors. Vaccine 2006, 24, 6513–6525. [Google Scholar] [CrossRef] [PubMed]
- Brandler, S.; Lucas-Hourani, M.; Moris, A.; Frenkiel, M.-P.; Combredet, C.; Février, M.; Bedouelle, H.; Schwartz, O.; Desprès, P.; Tangy, F. Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl. Trop. Dis. 2007, 1, e96. [Google Scholar] [CrossRef]
- Arora, U.; Tyagi, P.; Swaminathan, S.; Khanna, N. Virus-like particles displaying envelope domain III of dengue virus type 2 induce virus-specific antibody response in mice. Vaccine 2013, 31, 873–878. [Google Scholar] [CrossRef]
- Chua, A.J.; Vituret, C.; Tan, M.L.; Gonzalez, G.; Boulanger, P.; Ng, M.-L.; Hong, S.-S. A novel platform for virus-like particle-display of flaviviral envelope domain III: Induction of Dengue and West Nile virus neutralizing antibodies. Virol. J. 2013, 10, 129. [Google Scholar] [CrossRef]
- Lazo, L.; Izquierdo, A.; Suzarte, E.; Gil, L.; Valdés, I.; Marcos, E.; Álvarez, M.; Romero, Y.; Guzmán, M.G.; Guillén, G.; et al. Evaluation in mice of the immunogenicity and protective efficacy of a tetravalent subunit vaccine candidate against dengue virus. Microbiol. Immunol. 2014, 58, 219–226. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, T.; Zhou, X.-Z.; Deng, Y.-Q.; Li, X.-F.; Chen, S.-P.; Zhu, S.-Y.; Zhou, X.; Qin, E.-D.; Qin, C.-F. Induction of neutralizing antibodies against four serotypes of dengue viruses by MixBiEDIII, a tetravalent dengue vaccine. PLoS ONE 2014, 9, e86573. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, V.; Arora, U.; Shukla, R.; Poddar, A.; Shanmugam, R.K.; White, L.J.; Mattocks, M.M.; Raut, R.; Perween, A.; Tyagi, P.; et al. A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice. PLoS Negl. Trop. Dis. 2018, 12, e0006191. [Google Scholar] [CrossRef]
- Hermida, L.; Bernardo, L.; Martín, J.; Alvarez, M.; Prado, I.; López, C.; Sierra, B.D.L.C.; Martínez, R.; Rodríguez, R.; Zulueta, A.; et al. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 2006, 24, 3165–3171. [Google Scholar] [CrossRef]
- Valdés, I.; Hermida, L.; Martín, J.; Menéndez, T.; Gil, L.; Lazo, L.; Castro, J.; Niebla, O.; López, C.; Bernardo, L.; et al. Immunological evaluation in nonhuman primates of formulations based on the chimeric protein P64k-domain III of dengue 2 and two components of Neisseria meningitidis. Vaccine 2009, 27, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- McBurney, S.P.; Sunshine, J.E.; Gabriel, S.; Huynh, J.P.; Sutton, W.F.; Fuller, D.H.; Haigwood, N.L.; Messer, W.B. Evaluation of protection induced by a dengue virus serotype 2 envelope domain III protein scaffold/DNA vaccine in non-human primates. Vaccine 2016, 34, 3500–3507. [Google Scholar] [CrossRef] [PubMed]
- Block, O.K.T.; Shanaka, W.W.; Rodrigo, I.; Quinn, M.; Jin, X.; Rose, R.C.; Schlesinger, J.J. A tetravalent recombinant dengue domain III protein vaccine stimulates neutralizing and enhancing antibodies in mice. Vaccine 2010, 28, 8085–8094. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-Y.; Pan, C.-H.; Hsieh, C.-H.; Tsai, J.-P.; Chen, M.-Y.; Liu, H.-H.; Liu, S.-J.; Chong, P.; Leng, C.-H.; Chen, H.-W. Lipidated dengue-2 envelope protein domain III independently stimulates long-lasting neutralizing antibodies and reduces the risk of antibody-dependent enhancement. PLoS Negl. Trop. Dis. 2013, 7, e2432. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, R.K.; Shukla, R.; Arora, U.; Swaminathan, S.; Khanna, N. Dengue envelope-based ‘four-in-one’ virus-like particles produced using Pichia pastoris induce enhancement-lacking, domain III-directed tetravalent neutralising antibodies in mice. Sci. Rep. 2018, 8, 8643. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Rajpoot, R.K.; Arora, U.; Poddar, A.; Swaminathan, S.; Khanna, N. Pichia pastoris-expressed bivalent virus-like particulate vaccine induces domain III-focused bivalent neutralizing antibodies without antibody-dependent enhancement in vivo. Front. Microbiol. 2018, 8, 2644. [Google Scholar] [CrossRef]
- Leng, C.-H.; Liu, S.-J.; Tsai, J.-P.; Li, Y.-S.; Chen, M.-Y.; Liu, H.-H.; Lien, S.-P.; Yueh, A.; Hsiao, K.-N.; Lai, L.-W.; et al. A novel dengue vaccine candidate that induces cross-neutralizing antibodies and memory immunity. Microbes Infect. 2009, 11, 288–295. [Google Scholar] [CrossRef]
- Frei, J.C.; Wirchnianski, A.S.; Govero, J.; Vergnolle, O.; Dowd, K.A.; Pierson, T.C.; Kielian, M.; Girvin, M.E.; Diamond, M.S.; Lai, J.R. Engineered dengue virus domain III proteins elicit cross-neutralizing antibody responses in mice. J. Virol. 2018, 92, e01023-18. [Google Scholar] [CrossRef]
- Valdés, I.; Hermida, L.; Gil, L.; Lazo, L.; Castro, J.; Martín, J.; Bernardo, L.; López, C.; Niebla, O.; Menéndez, T.; et al. Heterologous prime-boost strategy in non-human primates combining the infective dengue virus and a recombinant protein in a formulation suitable for human use. Int. J. Infect. Dis. 2010, 14, e377–e383. [Google Scholar] [CrossRef][Green Version]
- Zlatkovic, J.; Stiasny, K.; Heinz, F.X. Immunodominance and functional activities of antibody responses to inactivated West Nile virus and recombinant subunit vaccines in mice. J. Virol. 2011, 85, 1994–2003. [Google Scholar] [CrossRef]
- Teoh, E.P.; Kukkaro, P.; Teo, E.W.; Lim, A.P.C.; Tan, T.T.; Yip, A.; Schul, W.; Aung, M.; Kostyuchenko, V.A.; Leo, Y.S.; et al. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci. Transl. Med. 2012, 4, 139ra83. [Google Scholar] [CrossRef]
- Fibriansah, G.; Tan, J.L.; Smith, S.A.; Alwis, A.R.; Ng, T.; Kostyuchenko, V.A.; Ibarra, K.D.; Wang, J.; Harris, E.; Silva, A.; et al. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol. Med. 2014, 6, 358–371. [Google Scholar] [CrossRef]
- Fibriansah, G.; Ibarra, K.D.; Ng, T.-S.; Smith, S.A.; Tan, J.L.; Lim, X.-N.; Ooi, J.S.G.; Kostyuchenko, V.A.; Wang, J.; de Silva, A.M.; et al. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 2015, 349, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Baric, T.J.; Yount, B.L.; Widman, D.G.; Durbin, A.; Whitehead, S.; Baric, R.S.; de Silva, A.M. Human dengue virus serotype 2 neutralizing antibodies target two distinct quaternary epitopes. PLoS Pathog. 2018, 14, e1006934. [Google Scholar] [CrossRef] [PubMed]
- Fibriansah, G.; Tan, J.L.; Smith, S.A.; de Alwis, R.; Ng, T.-S.; Kostyuchenko, V.A.; Jadi, R.S.; Kukkaro, P.; de Silva, A.M.; Crowe, J.E.; et al. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat. Commun. 2015, 6, 6341. [Google Scholar] [CrossRef] [PubMed]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.-C.; Kikuti, C.M.; Sanchez, M.E.N.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520, 109–113. [Google Scholar] [CrossRef]
- Nivarthi, U.K.; Kose, N.; Sapparapu, G.; Widman, D.; Gallichotte, E.; Pfaff, J.M.; Doranz, B.J.; Weiskopf, D.; Sette, A.; Durbin, A.P.; et al. Mapping the human memory B cell and serum neutralizing antibody responses to dengue virus serotype 4 infection and vaccination. J. Virol. 2017, 91, e02041-16. [Google Scholar] [CrossRef]
- Andrade, D.V.; Warnes, C.; Young, E.; Katzelnick, L.C.; Balmaseda, A.; de Silva, A.M.; Baric, R.S.; Harris, E. Tracking the polyclonal neutralizing antibody response to a dengue virus serotype 1 type-specific epitope across two populations in Asia and the Americas. Sci. Rep. 2019, 9, 16258. [Google Scholar] [CrossRef]
- Swanstrom, J.A.; Nivarthi, U.K.; Patel, B.; Delacruz, M.J.; Yount, B.; Widman, D.G.; Durbin, A.P.; Whitehead, S.S.; De Silva, A.M.; Baric, R.S. Beyond neutralizing antibody levels: The epitope specificity of antibodies induced by national institutes of health monovalent dengue virus vaccines. J. Infect. Dis. 2019, 220, 219–227. [Google Scholar] [CrossRef]
- Gallichotte, E.N.; Widman, D.G.; Yount, B.L.; Wahala, W.M.; Durbin, A.; Whitehead, S.; Sariol, C.A.; Crowe, J.E.; de Silva, A.M.; Baric, R.S. A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. mBio 2015, 6, e01461-15. [Google Scholar] [CrossRef]
- Messer, W.B.; Yount, B.L.; Royal, S.R.; de Alwis, R.; Widman, D.G.; Smith, S.A.; Crowe, J.E.; Pfaff, J.M.; Kahle, K.M.; Doranz, B.J.; et al. Functional transplant of a dengue virus serotype 3 (DENV3)-specific human monoclonal antibody epitope into DENV1. J. Virol. 2016, 90, 5090–5097. [Google Scholar] [CrossRef]
- Andrade, D.V.; Katzelnick, L.C.; Widman, D.G.; Balmaseda, A.; de Silva, A.M.; Baric, R.S.; Harris, E. Analysis of individuals from a dengue-endemic region helps define the footprint and repertoire of antibodies targeting dengue virus 3 type-specific epitopes. mBio 2017, 8, e01205-17. [Google Scholar] [CrossRef]
- Widman, D.G.; Young, E.; Nivarthi, U.; Swanstrom, J.A.; Royal, S.R.; Yount, B.L.; Debbink, K.; Begley, M.; Marcet, S.; Durbin, A.; et al. Transplantation of a quaternary structure neutralizing antibody epitope from dengue virus serotype 3 into serotype 4. Sci. Rep. 2017, 7, 17169. [Google Scholar] [CrossRef]
- Young, E.; Carnahan, R.H.; Andrade, D.V.; Kose, N.; Nargi, R.S.; Fritch, E.J.; Munt, J.E.; Doyle, M.P.; White, L.; Baric, T.J.; et al. Identification of dengue virus serotype 3 specific antigenic sites targeted by neutralizing human antibodies. Cell Host Microbe 2020, 27, 710–724.e7. [Google Scholar] [CrossRef]
- Rouvinski, A.; Dejnirattisai, W.; Guardado-Calvo, P.; Vaney, M.-C.; Sharma, A.; Duquerroy, S.; Supasa, P.; Wongwiwat, W.; Haouz, A.; Barba-Spaeth, G.; et al. Covalently linked dengue virus envelope glycoprotein dimers reduce exposure of the immunodominant fusion loop epitope. Nat. Commun. 2017, 8, 15411. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.-C.; Medits, I.; Sharma, A.; Simon-Lorière, E.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Haouz, A.; et al. Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature 2016, 536, 48–53. [Google Scholar] [CrossRef]
- Swanstrom, J.A.; Plante, J.A.; Plante, K.S.; Young, E.F.; McGowan, E.; Gallichotte, E.N.; Widman, D.G.; Heise, M.T.; de Silva, A.M.; Baric, R.S. Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. mBio 2016, 7, e0112316. [Google Scholar] [CrossRef]
- Fernandez, E.; Dejnirattisai, W.; Cao, B.; Scheaffer, S.M.; Supasa, P.; Wongwiwat, W.; Esakky, P.; Drury, A.; Mongkolsapaya, J.; Moley, K.H.; et al. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat. Immunol. 2017, 18, 1261–1269. [Google Scholar] [CrossRef]
- Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Peterson, R.; Nkolola, J.P.; Borducchi, E.N.; Supasa, P.; Mongkolsapaya, J.; Screaton, G.R.; Barouch, D.H. Therapeutic and protective efficacy of a dengue antibody against Zika infection in rhesus monkeys. Nat. Med. 2018, 24, 721–723. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent developments in recombinant protein-based dengue vaccines. Front. Immunol. 2018, 9, 1919. [Google Scholar] [CrossRef]
- Metz, S.W.; Gallichotte, E.N.; Brackbill, A.; Premkumar, L.; Miley, M.J.; Baric, R.; de Silva, A.M. In vitro assembly and stabilization of dengue and Zika virus envelope protein homo-dimers. Sci. Rep. 2017, 7, 4524. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.L.C.; Marchese, S.; Rana, J.; Mossenta, M.; Poggianella, M.; Bestagno, M.; Burrone, O.R. Temperature-dependent folding allows stable dimerization of secretory and virus-associated E proteins of Dengue and Zika viruses in mammalian cells. Sci. Rep. 2017, 7, 966. [Google Scholar] [CrossRef] [PubMed]
- Glasner, D.R.; Puerta-Guardo, H.; Beatty, P.R.; Harris, E. The good, the bad, and the shocking: The multiple roles of dengue virus nonstructural protein 1 in protection and pathogenesis. Annu. Rev. Virol. 2018, 5, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Winkler, G.; Randolph, V.B.; Cleaves, G.R.; Ryan, T.E.; Stollar, V. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology 1988, 162, 187–196. [Google Scholar] [CrossRef]
- Akey, D.L.; Brown, W.C.; Dutta, S.; Konwerski, J.; Jose, J.; Jurkiw, T.J.; Delproposto, J.; Ogata, C.M.; Skiniotis, G.; Kuhn, R.J.; et al. Flavivirus NS1 crystal structures reveal a surface for membrane association and regions of interaction with the immune system. Science 2014, 343, 881–885. [Google Scholar] [CrossRef]
- Winkler, G.; Maxwell, S.E.; Ruemmler, C.; Stollar, V. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology 1989, 171, 302–305. [Google Scholar] [CrossRef]
- Jacobs, M.G.; Robinson, P.J.; Bletchly, C.; Mackenzie, J.M.; Young, P.R. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J. 2000, 14, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Flamand, M.; Megret, F.; Mathieu, M.; Lepault, J.; Rey, F.A.; Deubel, V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J. Virol. 1999, 73, 6104–6110. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Hilditch, P.A.; Bletchly, C.; Halloran, W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000, 38, 1053–1057. [Google Scholar] [CrossRef]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef]
- Wang, C.; Puerta-Guardo, H.; Biering, S.B.; Glasner, D.R.; Tran, E.B.; Patana, M.; Gomberg, T.A.; Malvar, C.; Lo, N.T.; Espinosa, D.A.; et al. Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathog. 2019, 15, e1007938. [Google Scholar] [CrossRef]
- Glasner, D.R.; Ratnasiri, K.; Puerta-Guardo, H.; Espinosa, D.A.; Beatty, P.R.; Harris, E. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017, 13, e1006673. [Google Scholar] [CrossRef]
- Lin, S.W.; Chuang, Y.C.; Lin, Y.S.; Lei, H.Y.; Liu, H.S.; Yeh, T.M. Dengue virus nonstructural protein NS1 binds to prothrombin/thrombin and inhibits prothrombin activation. J. Infect. 2012, 64, 325–334. [Google Scholar] [CrossRef]
- Chao, C.-H.; Wu, W.-C.; Lai, Y.-C.; Tsai, P.-J.; Perng, G.-C.; Lin, Y.-S.; Yeh, T.-M. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019, 15, e1007625. [Google Scholar] [CrossRef]
- Kuno, G.; Vorndam, A.V.; Gubler, D.J.; Gómez, I. Study of anti-dengue NS1 antibody by Western blot. J. Med. Virol. 1990, 32, 102–108. [Google Scholar] [CrossRef]
- Churdboonchart, V.; Bhamarapravati, N.; Peampramprecha, S.; Sirinavin, S. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1991, 44, 481–493. [Google Scholar] [CrossRef]
- Shu, P.Y.; Chen, L.K.; Chang, S.F.; Yueh, Y.Y.; Chow, L.; Chien, L.J.; Chin, C.; Lin, T.H.; Huang, J.H. Dengue NS1-specific antibody responses: Isotype distribution and serotyping in patients with Dengue fever and Dengue hemorrhagic fever. J. Med. Virol. 2000, 62, 224–232. [Google Scholar] [CrossRef]
- Valdés, K.; Alvarez, M.; Pupo, M.; Vázquez, S.; Rodríguez, R.; Guzmán, M.G. Human Dengue antibodies against structural and nonstructural proteins. Clin. Diagn. Lab. Immunol. 2000, 7, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Hertz, T.; Beatty, P.R.; MacMillen, Z.; Killingbeck, S.S.; Wang, C.; Harris, E. Antibody epitopes identified in critical regions of dengue virus nonstructural 1 protein in mouse vaccination and natural human infections. J. Immunol. 2017, 198, 4025–4035. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, D.; Gomes, L.; Jeewandara, C.; Jayarathna, G.S.B.; Herath, D.; Perera, P.A.; Fernando, S.; Wijewickrama, A.; Hardman, C.S.; Ogg, G.S.; et al. Role of NS1 antibodies in the pathogenesis of acute secondary dengue infection. Nat. Commun. 2018, 9, 5242. [Google Scholar] [CrossRef]
- Falkler, W.A.; Diwan, A.R.; Halstead, S.B. Human antibody to dengue soluble complement-fixing (SCF) antigens. J. Immunol. 1973, 111, 1804–1809. [Google Scholar]
- Twiddy, S.S.; Woelk, C.H.; Holmes, E.C. Phylogenetic evidence for adaptive evolution of dengue viruses in nature. J. Gen. Virol. 2002, 83, 1679–1689. [Google Scholar] [CrossRef]
- Falconar, A.K.I.; Young, P.R.; Miles, M.A. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch. Virol. 1994, 137, 315–326. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Vaughn, D.W.; Del Angel, R.M. Recognition of synthetic oligopeptides from nonstructural proteins NS1 and NS3 of Dengue-4 virus by sera from Dengue virus—Infected children. Am. J. Trop. Med. Hyg. 1997, 56, 466–470. [Google Scholar] [CrossRef]
- Falconar, A.K.I. Antibody responses are generated to immunodominant ELK/KLE-type motifs on the nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: Implications for diagnosis, pathogenesis, and vaccine design. Clin. Vaccine Immunol. 2007, 14, 493–504. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, J.-M.; Yin, Y.; Fang, D.-Y.; Tang, Y.-X.; Jiang, L.-F. Selection and identification of B-cell epitope on NS1 protein of dengue virus type 2. Virus Res. 2010, 150, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, Y.; Guo, Y.; Qiu, L.; Ding, X.; Che, X. Comprehensive mapping of immunodominant and conserved serotype- and group-specific B-cell epitopes of nonstructural protein 1 from dengue virus type 1. Virology 2010, 398, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Henriques, H.R.; Rampazo, E.V.; Gonçalves, A.J.S.; Vicentin, E.C.M.; Amorim, J.H.; Panatieri, R.H.; Amorim, K.N.S.; Yamamoto, M.M.; Ferreira, L.C.S.; Alves, A.M.B.; et al. Targeting the non-structural protein 1 from dengue virus to a dendritic cell population confers protective immunity to lethal virus challenge. PLoS Negl. Trop. Dis. 2013, 7, e2330. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.M.; Paes, M.V.; Barreto, D.F.; Pinhão, A.T.; Barth, O.M.; Queiroz, J.L.S.; Armôa, G.R.G.; Freire, M.S.; Alves, A.M.B. Protection against dengue type 2 virus induced in mice immunized with a DNA plasmid encoding the non-structural 1 (NS1) gene fused to the tissue plasminogen activator signal sequence. Vaccine 2006, 24, 195–205. [Google Scholar] [CrossRef]
- Amorim, J.H.; Diniz, M.O.; Cariri, F.A.M.O.; Rodrigues, J.F.; Bizerra, R.S.P.; Gonçalves, A.J.S.; de Barcelos Alves, A.M.; de Souza Ferreira, L.C. Protective immunity to DENV2 after immunization with a recombinant NS1 protein using a genetically detoxified heat-labile toxin as an adjuvant. Vaccine 2012, 30, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.; Alves, R.; Caetano, B.; Pereira, L.; Mitsunari, T.; Amorim, J.; Polatto, J.; Botosso, V.; Gallina, N.; Palacios, R.; et al. Epitope sequences in Dengue virus NS1 protein identified by monoclonal antibodies. Antibodies 2017, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Henchal, E.A.; Henchal, L.S.; Schlesinger, J.J. Synergistic interactions of Anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with Dengue 2 virus. J. Gen. Virol. 1988, 69, 2101–2107. [Google Scholar] [CrossRef]
- Schlesinger, J.J.; Brandriss, M.W.; Walsh, E.E. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J. Immunol. 1985, 135, 2805–2809. [Google Scholar]
- García, G.; Arango, M.; Pérez, A.B.; Fonte, L.; Sierra, B.; Rodríguez-Roche, R.; Aguirre, E.; Fiterre, I.; Guzmán, M.G. Antibodies from patients with dengue viral infection mediate cellular cytotoxicity. J. Clin. Virol. 2006, 37, 53–57. [Google Scholar] [CrossRef]
- Wan, S.-W.; Chen, P.-W.; Chen, C.-Y.; Lai, Y.-C.; Chu, Y.-T.; Hung, C.-Y.; Lee, H.; Wu, H.F.; Chuang, Y.-C.; Lin, J.; et al. Therapeutic effects of monoclonal antibody against Dengue virus NS1 in a STAT1 knockout mouse model of dengue infection. J. Immunol. 2017, 199, 2834–2844. [Google Scholar] [CrossRef]
- Chung, K.M.; Thompson, B.S.; Fremont, D.H.; Diamond, M.S. Antibody recognition of cell surface-associated NS1 triggers Fc-gamma receptor-mediated phagocytosis and clearance of West Nile Virus-infected cells. J. Virol. 2007, 81, 9551–9555. [Google Scholar] [CrossRef] [PubMed]
- Falgout, B.; Bray, M.; Schlesinger, J.J.; Lai, C.-J. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J. Virol. 1990, 64, 4356–4363. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-F.; Liao, C.-L.; Lin, Y.-L.; Yeh, C.-T.; Chen, L.-K.; Huang, Y.-F.; Chou, H.-Y.; Huang, J.-L.; Shaio, M.-F.; Sytwu, H.-K. Evaluation of protective efficacy and immune mechanisms of using a non-structural protein NS1 in DNA vaccine against dengue 2 virus in mice. Vaccine 2003, 21, 3919–3929. [Google Scholar] [CrossRef]
- Costa, S.M.; Azevedo, A.S.; Paes, M.V.; Sarges, F.S.; Freire, M.S.; Alves, A.M.B. DNA vaccines against dengue virus based on the ns1 gene: The influence of different signal sequences on the protein expression and its correlation to the immune response elicited in mice. Virology 2007, 358, 413–423. [Google Scholar] [CrossRef]
- Falgout, B.; Chanock, R.; Lai, C.-J. Proper processing of dengue virus nonstructural glycoprotein NS1 Requires the N-terminal hydrophobic signal sequence and the downstream nonstructural protein NS2a. J. Virol. 1989, 63, 1852–1860. [Google Scholar] [CrossRef]
- Costa, S.M.; Freire, M.S.; Alves, A.M.B. DNA vaccine against the non-structural 1 protein (NS1) of dengue 2 virus. Vaccine 2006, 24, 4562–4564. [Google Scholar] [CrossRef]
- Espinosa, D.A.; Beatty, P.R.; Reiner, G.L.; Sivick, K.E.; Glickman, L.H.; Dubensky, T.W.; Harris, E. Cyclic dinucleotide-adjuvanted dengue virus nonstructural protein 1 induces protective antibody and T cell responses. J. Immunol. 2019, 202, 1153–1162. [Google Scholar] [CrossRef]
- Ambuel, Y.; Young, G.; Brewoo, J.N.; Paykel, J.; Weisgrau, K.L.; Rakasz, E.G.; Haller, A.A.; Royals, M.; Huang, C.Y.-H.; Capuano, S.; et al. A rapid immunization strategy with a live-attenuated tetravalent dengue vaccine elicits protective neutralizing antibody responses in non-human primates. Front. Immunol. 2014, 5, 263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Glasner, D.R.; Watkins, H.; Puerta-Guardo, H.; Kassa, Y.; Egan, M.A.; Dean, H.; Harris, E. Magnitude and functionality of the NS1-specific antibody response elicited by a live-attenuated tetravalent dengue vaccine candidate. J. Infect. Dis. 2020, 221, 867–877. [Google Scholar] [CrossRef]
- Nascimento, E.J.M.; George, J.K.; Velasco, M.; Bonaparte, M.I.; Zheng, L.; DiazGranados, C.A.; Marques, E.T.A.; Huleatt, J.W. Development of an anti-dengue NS1 IgG ELISA to evaluate exposure to dengue virus. J. Virol. Methods 2018, 257, 48–57. [Google Scholar] [CrossRef]
- Falconar, A.K.I. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: Potential implications in haemorrhagic fever pathogenesis. Arch. Virol. 1997, 142, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Lei, H.-Y.; Shiau, A.-L.; Liu, C.-C.; Liu, H.-S.; Yeh, T.-M.; Chen, S.-H.; Lin, Y.-S. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J. Med. Virol. 2003, 69, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Lei, H.-Y.; Shiau, A.-L.; Liu, H.-S.; Yeh, T.-M.; Chen, S.-H.; Liu, C.-C.; Chiu, S.-C.; Lin, Y.-S. Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J. Immunol. 2002, 169, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Chiu, S.-C.; Hsiao, Y.-L.; Wan, S.-W.; Lei, H.-Y.; Shiau, A.-L.; Liu, H.-S.; Yeh, T.-M.; Chen, S.-H.; Liu, C.-C.; et al. Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. J. Immunol. 2005, 174, 395–403. [Google Scholar] [CrossRef]
- Wan, S.W.; Yang, Y.W.; Chu, Y.T.; Lin, C.F.; Chang, C.P.; Yeh, T.M.; Anderson, R.; Lin, Y.S. Anti-dengue virus nonstructural protein 1 antibodies contribute to platelet phagocytosis by macrophages. Thromb. Haemost. 2016, 115, 646–656. [Google Scholar]
- Cheng, H.-J.; Lei, H.-Y.; Lin, C.-F.; Luo, Y.-H.; Wan, S.-W.; Liu, H.-S.; Yeh, T.-M.; Lin, Y.-S. Anti-dengue virus nonstructural protein 1 antibodies recognize protein disulfide isomerase on platelets and inhibit platelet aggregation. Mol. Immunol. 2009, 47, 398–406. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lin, C.-F.; Lei, H.-Y.; Lin, S.-C.; Liu, H.-S.; Yeh, T.-M.; Anderson, R.; Lin, Y.-S. Deletion of the C-terminal region of dengue virus nonstructural protein 1 (NS1) abolishes anti-NS1-mediated platelet dysfunction and bleeding tendency. J. Immunol. 2009, 183, 1797–1803. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lei, H.-Y.; Lin, Y.-S.; Liu, H.-S.; Wu, H.-L.; Yeh, T.-M. Dengue virus-induced autoantibodies bind to plasminogen and enhance its activation. J. Immunol. 2011, 187, 6483–6490. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lin, Y.-S.; Liu, H.-S.; Yeh, T.-M. Molecular mimicry between dengue virus and coagulation factors induces antibodies to inhibit thrombin activity and enhance fibrinolysis. J. Virol. 2014, 88, 13759–13768. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lin, J.; Lin, Y.-S.; Wang, S.; Yeh, T.-M. Dengue virus nonstructural protein 1-induced antibodies cross-react with human plasminogen and enhance its activation. J. Immunol. 2016, 196, 1218–1226. [Google Scholar] [CrossRef]
- Lin, C.-F.; Wan, S.-W.; Chen, M.-C.; Lin, S.-C.; Cheng, C.-C.; Chiu, S.-C.; Hsiao, Y.-L.; Lei, H.-Y.; Liu, H.-S.; Yeh, T.-M.; et al. Liver injury caused by antibodies against dengue virus nonstructural protein 1 in a murine model. Lab. Investig. 2008, 88, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Lei, H.-Y.; Liu, C.-C.; Liu, H.-S.; Yeh, T.-M.; Wang, S.-T.; Yang, T.-I.; Sheu, F.-C.; Kuo, C.-F.; Lin, Y.-S. Generation of IgM anti-platelet autoantibody in dengue patients. J. Med. Virol. 2001, 63, 143–149. [Google Scholar] [CrossRef]
- Saito, M.; Oishi, K.; Inoue, S.; Dimaano, E.M.; Alera, M.T.P.; Robles, A.M.P.; Estrella, B.D.; Kumatori, A.; Moji, K.; Alonzo, M.T.; et al. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin. Exp. Immunol. 2004, 138, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.-W.; Lin, C.-F.; Chen, M.-C.; Lei, H.-Y.; Liu, H.-S.; Yeh, T.-M.; Liu, C.-C.; Lin, Y.-S.; Wan, S.-W.; Lin, C.-F.; et al. C-terminal region of dengue virus nonstructural protein 1 is involved in endothelial cell cross-reactivity via molecular mimicry. Am. J. Infect. Dis. 2008, 4, 85–91. [Google Scholar] [CrossRef]
- Cheng, H.-J.; Lin, C.-F.; Lei, H.-Y.; Liu, H.-S.; Yeh, T.-M.; Luo, Y.-H.; Lin, Y.-S. Proteomic analysis of endothelial cell autoantigens recognized by anti-dengue virus nonstructural protein 1 antibodies. Exp. Biol. Med. 2009, 234, 63–73. [Google Scholar] [CrossRef]
- Liu, I.-J.; Chiu, C.-Y.; Chen, Y.-C.; Wu, H.-C. Molecular mimicry of human endothelial cell antigen by autoantibodies to nonstructural protein 1 of dengue virus. J. Biol. Chem. 2011, 286, 9726–9736. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Wan, S.-W.; Lu, Y.-T.; Huang, C.-H.; Lin, C.-F.; Anderson, R.; Liu, H.-S.; Yeh, T.-M.; Yen, Y.-T.; Wu-Hsieh, B.A.; Lin, Y.-S. Protection against dengue virus infection in mice by administration of antibodies against modified nonstructural protein 1. PLoS ONE 2014, 9, e92495. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Nie, K.; Du, S.; Qiu, J.; Pang, X.; Wang, P.; Cheng, G. Flavivirus NS1 protein in infected host sera enhances viral acquisition by mosquitoes. Nat. Microbiol. 2016, 1, 16087. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Chuang, Y.-C.; Liu, C.-C.; Ho, T.-S.; Lin, Y.-S.; Anderson, R.; Yeh, T.-M. Antibodies against modified NS1 wing domain peptide protect against dengue virus infection. Sci. Rep. 2017, 7, 6975. [Google Scholar] [CrossRef]
- Zivny, J.; DeFronzo, M.; Jarry, W.; Jameson, J.; Cruz, J.; Ennis, F.A.; Rothman, A.L. Partial agonist effect influences the CTL response to a heterologous dengue virus serotype. J. Immunol. 1999, 163, 2754–2760. [Google Scholar] [PubMed]
- Zivny, J.; Kurane, I.; Leporati, A.M.; Ibe, M.; Takiguchi, M.; Zeng, L.L.; Brinton, M.A.; Ennis, F.A. A single nine-amino acid peptide induces virus-specific, CD8+ human cytotoxic T lymphocyte clones of heterogeneous serotype specificities. J. Exp. Med. 1995, 182, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Zivna, I.; Green, S.; Vaughn, D.W.; Kalayanarooj, S.; Stephens, H.A.F.; Chandanayingyong, D.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J. Immunol. 2002, 168, 5959–5965. [Google Scholar] [CrossRef] [PubMed]
- Friberg, H.; Bashyam, H.; Toyosaki-Maeda, T.; Potts, J.A.; Greenough, T.; Kalayanarooj, S.; Gibbons, R.V.; Nisalak, A.; Srikiatkhachorn, A.; Green, S.; et al. Cross-reactivity and expansion of dengue-specific T cells during acute primary and secondary infections in humans. Sci. Rep. 2011, 1, 51. [Google Scholar] [CrossRef] [PubMed]
- Friberg, H.; Burns, L.; Woda, M.; Kalayanarooj, S.; Endy, T.P.; Stephens, H.A.; Green, S.; Rothman, A.L.; Mathew, A. Memory CD8+ T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunol. Cell Biol. 2011, 89, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Kurane, I.; Okamoto, Y.; Ennis, F.A.; Brinton, M.A. Identification of amino acids involved in recognition by dengue virus NS3-specific, HLA-DR15-restricted cytotoxic CD4+ T-cell clones. J. Virol. 1996, 70, 3108–3117. [Google Scholar] [CrossRef]
- Livingston, P.G.; Kurane, I.; Dai, L.C.; Okamoto, Y.; Lai, C.J.; Men, R.; Karaki, S.; Takiguchi, M.; Ennis, F.A. Dengue virus-specific, HLA-B35-restricted, human CD8+ cytotoxic T lymphocyte (CTL) clones. Recognition of NS3 amino acids 500 to 508 by CTL clones of two different serotype specificities. J. Immunol. 1995, 154, 1287–1295. [Google Scholar] [PubMed]
- Mongkolsapaya, J.; Duangchinda, T.; Dejnirattisai, W.; Vasanawathana, S.; Avirutnan, P.; Jairungsri, A.; Khemnu, N.; Tangthawornchaikul, N.; Chotiyarnwong, P.; Sae-Jang, K.; et al. T cell responses in dengue hemorrhagic fever: Are cross-reactive T cells suboptimal? J. Immunol. 2006, 176, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Zeng, L.; Brinton, M.A.; Ennis, F.A. Definition of an epitope on NS3 recognized by human CD4+Cytotoxic T lymphocyte clones cross-reactive for dengue virus types 2, 3, and 4. Virology 1998, 240, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L.; Kumaran, E.A.; Thein, T.-L.; Too, C.T.; Gan, V.C.H.; Hanson, B.J.; Wilder-Smith, A.; Bertoletti, A.; Gascoigne, N.R.J.; Lye, D.C.; et al. Virus-specific T lymphocytes home to the skin during natural dengue infection. Sci. Transl. Med. 2015, 7, 278ra35. [Google Scholar] [CrossRef]
- Malavige, G.N.; Jeewandara, C.; Alles, K.M.L.; Salimi, M.; Gomes, L.; Kamaladasa, A.; Jayaratne, S.D.; Ogg, G.S. Suppression of virus specific immune responses by IL-10 in acute dengue infection. PLoS Negl. Trop. Dis. 2013, 7, e2409. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, S.J.; Zeng, W.; Kurane, I.; Ennis, F.A. Identification of two epitopes on the dengue 4 virus capsid protein recognized by a serotype-specific and a panel of serotype-cross-reactive human CD4+ cytotoxic T-lymphocyte clones. J. Virol. 1996, 70, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Loke, H.; Bethell, D.B.; Phuong, C.X.T.; Dung, M.; Schneider, J.; White, N.J.; Day, N.P.; Farrar, J.; Hill, A.V.S. Strong HLA class I–restricted T Cell responses in dengue hemorrhagic fever: A double-edged sword? J. Infect. Dis. 2001, 184, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.-L.; Li, Q.; Wang, Z.-B.; Xia, K.-D.; Guo, J.-L.; Liu, W.-Q.; Wen, J.-S. HLA-A*0201-restricted CD8+ T-cell epitopes identified in dengue viruses. Virol. J. 2012, 9, 259. [Google Scholar] [CrossRef]
- Nascimento, E.J.M.; Mailliard, R.B.; Khan, A.M.; Sidney, J.; Sette, A.; Guzman, N.; Paulaitis, M.; de Melo, A.B.; Cordeiro, M.T.; Gil, L.V.G.; et al. Identification of conserved and HLA promiscuous DENV3 T-cell epitopes. PLoS Negl. Trop. Dis. 2013, 7, e2497. [Google Scholar] [CrossRef]
- Bashyam, H.S.; Green, S.; Rothman, A.L. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J. Immunol. 2006, 176, 2817–2824. [Google Scholar] [CrossRef]
- Imrie, A.; Meeks, J.; Gurary, A.; Sukhbataar, M.; Kitsutani, P.; Effler, P.; Zhao, Z. Differential functional avidity of dengue virus-specific T-cell clones for variant peptides representing heterologous and previously encountered serotypes. J. Virol. 2007, 81, 10081–10091. [Google Scholar] [CrossRef]
- Simmons, C.P.; Dong, T.; Chau, N.V.; Thi, N.; Dung, P.; Nguyen, T.; Chau, B.; Thi, L.; Thao, T.; Dung, N.T.; et al. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J. Virol. 2005, 79, 5665–5675. [Google Scholar] [CrossRef]
- Duan, Z.; Guo, J.; Huang, X.; Liu, H.; Chen, X.; Jiang, M.; Wen, J. Identification of cytotoxic T lymphocyte epitopes in dengue virus serotype 1. J. Med. Virol. 2015, 87, 1077–1089. [Google Scholar] [CrossRef]
- Rivino, L.; Kumaran, E.A.P.; Jovanovic, V.; Nadua, K.; Teo, E.W.; Pang, S.W.; Teo, G.H.; Gan, V.C.H.; Lye, D.C.; Leo, Y.S.; et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J. Virol. 2013, 87, 2693–2706. [Google Scholar] [CrossRef]
- Weiskopf, D.; Cerpas, C.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; Sanches, F.P.; Silvera, C.G.T.; Costa, P.R.; et al. Human CD8+ T-cell responses against the 4 dengue virus serotypes are associated with distinct patterns of protein targets. J. Infect. Dis. 2015, 212, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Sidney, J.; Peters, B.; Shresta, S.; Sette, A. Immunodominance changes as a function of the infecting dengue virus serotype and primary versus secondary infection. J. Virol. 2014, 88, 11383–11394. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; De Silva, A.D.; Phillips, E.; Mallal, S.; Premawansa, S.; et al. HLA-DRB1 alleles are associated with different magnitudes of dengue virus–specific CD4 + T-cell responses. J. Infect. Dis. 2016, 214, 1117–1124. [Google Scholar] [CrossRef]
- Grifoni, A.; Angelo, M.A.; Lopez, B.; O’Rourke, P.H.; Sidney, J.; Cerpas, C.; Balmaseda, A.; Silveira, C.G.T.; Maestri, A.; Costa, P.R.; et al. Global assessment of dengue virus-specific CD4+ T cell responses in dengue-endemic areas. Front. Immunol. 2017, 8, 1309. [Google Scholar] [CrossRef]
- Kurane, I.; Innis, B.L.; Nimmannitya, S.; Nisalak, A.; Meager, A.; Janus, J.; Ennis, F.A. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J. Clin. Invest. 1991, 88, 1473–1480. [Google Scholar] [CrossRef]
- Green, S.; Vaughn, D.W.; Kalayanarooj, S.; Nimmannitya, S.; Suntayakorn, S.; Nisalak, A.; Lew, R.; Innis, B.L.; Kurane, I.; Rothman, A.L.; et al. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 1999, 179, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Endy, T.P.; Houng, H.H.; Green, S.; Kalayanarooj, S.; Suntayakorn, S.; Chansiriwongs, W.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 2002, 185, 1213–1221. [Google Scholar] [CrossRef]
- Green, S.; Pichyangkul, S.; Vaughn, D.W.; Kalayanarooj, S.; Nimmannitya, S.; Nisalak, A.; Kurane, I.; Rothman, A.L.; Ennis, F.A. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J. Infect. Dis. 1999, 180, 1429–1435. [Google Scholar] [CrossRef]
- Lan, N.T.P.; Kikuchi, M.; Huong, V.T.Q.; Ha, D.Q.; Thuy, T.T.; Tham, V.D.; Tuan, H.M.; Tuong, V.V.; Nga, C.T.P.; Van Dat, T.; et al. Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS Negl. Trop. Dis. 2008, 2, e304. [Google Scholar] [CrossRef]
- Falcón-Lezama, J.A.; Ramos, C.; Zuñiga, J.; Juárez-Palma, L.; Rangel-Flores, H.; García-Trejo, A.R.; Acunha-Alonzo, V.; Granados, J.; Vargas-Alarcón, G. HLA class I and II polymorphisms in Mexican Mestizo patients with dengue fever. Acta Trop. 2009, 112, 193–197. [Google Scholar] [CrossRef]
- Malavige, G.N.; Rostron, T.; Rohanachandra, L.T.; Jayaratne, S.D.; Fernando, N.; de Silva, A.D.; Liyanage, M.; Ogg, G. HLA class I and class II associations in Dengue viral infections in a Sri Lankan population. PLoS ONE 2011, 6, e20581. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.P.; Brasil, P.E.A.A.D.; Cabello, G.M.K.; Souza, R.V.B.; Brasil, P.; Georg, I.; Cabello, P.H.; De Castro, L. HLA-A*01 allele: A risk factor for dengue haemorrhagic fever in Brazil’s population. Mem. Inst. Oswaldo Cruz 2012, 107, 224–230. [Google Scholar] [CrossRef]
- de Alencar, L.X.E.; de Mendonça Braga-Neto, U.; do Nascimento, E.J.M.; Cordeiro, M.T.; Silva, A.M.; de Brito, C.A.A.; da Silva, M.d.P.C.; Gil, L.H.V.G.; Montenegro, S.M.L.; Marques, E.T.D.A. HLA-B*44 is associated with dengue severity caused by DENV-3 in a brazilian population. J. Trop. Med. 2013, 2013, 648475. [Google Scholar]
- LaFleur, C.; Granados, J.; Vargas-Alarcon, G.; Ruíz-Morales, J.; Villarreal-Garza, C.; Higuera, L.; Hernández-Pacheco, G.; Cutiño-Moguel, T.; Rangel, H.; Figueroa, R.; et al. HLA-DR antigen frequencies in Mexican patients with dengue virus infection: HLA-DR4 as a possible genetic resistance factor for dengue hemorrhagic fever. Hum. Immunol. 2002, 63, 1039–1044. [Google Scholar] [CrossRef]
- Gagnon, S.J.; Ennis, F.A.; Rothman, A.L. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4+ cytotoxic T-lymphocyte clones. J. Virol. 1999, 73, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- Mangada, M.M.; Endy, T.P.; Nisalak, A.; Chunsuttiwat, S.; Vaughn, D.W.; Libraty, D.H.; Green, S.; Ennis, F.A.; Rothman, A.L. Dengue-Specific T Cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J. Infect. Dis. 2002, 185, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Mangada, M.M.; Rothman, A.L. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J. Immunol. 2005, 175, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhou, D.-S.; Zhang, J.-L.; Morida, H.; Wang, J.-L.; Yasui, K. Dengue-specific CD8+ T cells have both protective and pathogenic roles in dengue virus infection. Immunol. Lett. 2004, 95, 167–174. [Google Scholar] [CrossRef]
- Talarico, L.B.; Batalle, J.P.; Byrne, A.B.; Brahamian, J.M.; Ferretti, A.; García, A.G.; Mauri, A.; Simonetto, C.; Hijano, D.R.; Lawrence, A.; et al. The role of heterotypic DENV-specific CD8+ T lymphocytes in an immunocompetent mouse model of secondary dengue virus infection. EBioMedicine 2017, 20, 202–216. [Google Scholar] [CrossRef]
- Hsieh, M.-F.; Lai, S.-L.; Chen, J.-P.; Sung, J.-M.; Lin, Y.-L.; Wu-Hsieh, B.A.; Gerard, C.; Luster, A.; Liao, F. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J. Immunol. 2006, 177, 1855–1863. [Google Scholar] [CrossRef]
- Amorim, J.H.; dos Santos Alves, R.P.; Bizerra, R.; Pereira, S.A.; Pereira, L.R.; Fabris, D.L.N.; Santos, R.A.; Romano, C.M.; de Souza Ferreira, L.C. Antibodies are not required to a protective immune response against dengue virus elicited in a mouse encephalitis model. Virology 2016, 487, 41–49. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Eddy, W.E.; Tang, W.W.; Miller, R.; Shresta, S. CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J. Immunol. 2014, 193, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Zompi, S.; Santich, B.H.; Beatty, P.R.; Harris, E. Protection from secondary dengue virus infection in a mouse model reveals the role of serotype cross-reactive B and T cells. J. Immunol. 2012, 188, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Tang, W.W.; Eddy, W.E.; King, K.; Sanchez, M.C.; Shresta, S. CD8+ T cells can mediate short-term protection against heterotypic dengue virus reinfection in mice. J. Virol. 2015, 89, 6494–6505. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.-H.; Zhang, S.L.; Tan, H.C.; Kwek, S.S.; Sessions, O.M.; Chan, C.-Y.; Liu, I.D.; Lee, C.K.; Tambyah, P.A.; Ooi, E.E.; et al. Persistent dengue infection in an immunosuppressed patient reveals the roles of humoral and cellular immune responses in virus clearance. Cell Host Microbe 2019, 26, 601–605.e3. [Google Scholar] [CrossRef]
- Simon-Lorière, E.; Duong, V.; Tawfik, A.; Ung, S.; Ly, S.; Casadémont, I.; Prot, M.; Courtejoie, N.; Bleakley, K.; Buchy, P.; et al. Increased adaptive immune responses and proper feedback regulation protect against clinical dengue. Sci. Transl. Med. 2017, 9, eaal5088. [Google Scholar] [CrossRef]
- de Matos, A.M.; Carvalho, K.I.; Rosa, D.S.; Villas-Boas, L.S.; da Silva, W.C.; de Lima Rodrigues, C.L.; Oliveira, O.M.N.P.F.; Levi, J.E.; Araújo, E.S.A.; Pannuti, C.S.; et al. CD8+ T lymphocyte expansion, proliferation and activation in dengue fever. PLoS Negl. Trop. Dis. 2015, 9, e0003520. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, D.T.; Fernando, S.; Gomes, L.; Jeewandara, C.; Ginneliya, A.; Samarasekara, S.; Wijewickrama, A.; Hardman, C.S.; Ogg, G.S.; Malavige, G.N. Quantification of dengue virus specific T cell responses and correlation with viral load and clinical disease severity in acute dengue infection. PLoS Negl. Trop. Dis. 2018, 12, e0006540. [Google Scholar] [CrossRef]
- Hatch, S.; Endy, T.P.; Thomas, S.; Mathew, A.; Potts, J.; Pazoles, P.; Libraty, D.H.; Gibbons, R.; Rothman, A.L. Intracellular cytokine production by dengue virus–specific T cells correlates with subclinical secondary infection. J. Infect. Dis. 2011, 203, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Jeewandara, C.; Adikari, T.N.; Gomes, L.; Fernando, S.; Fernando, R.H.; Perera, M.K.T.; Ariyaratne, D.; Kamaladasa, A.; Salimi, M.; Prathapan, S.; et al. Functionality of dengue virus specific memory T cell responses in individuals who were hospitalized or who had mild or subclinical dengue infection. PLoS Negl. Trop. Dis. 2015, 9, e0003673. [Google Scholar] [CrossRef]
- Wijeratne, D.T.; Fernando, S.; Gomes, L.; Jeewandara, C.; Jayarathna, G.; Perera, Y.; Wickramanayake, S.; Wijewickrama, A.; Ogg, G.S.; Malavige, G.N. Association of dengue virus-specific polyfunctional T-cell responses with clinical disease severity in acute dengue infection. Immun. Inflamm. Dis. 2019, 7, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Dung, N.T.P.; Le Duyen, H.T.; Van Thuy, N.T.; Van Ngoc, T.; Chau, N.V.V.; Hien, T.T.; Rowland-Jones, S.L.; Dong, T.; Farrar, J.; Wills, B.; et al. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J. Immunol. 2010, 184, 7281–7287. [Google Scholar] [CrossRef] [PubMed]
- de Alwis, R.; Bangs, D.J.; Angelo, M.A.; Cerpas, C.; Fernando, A.; Sidney, J.; Peters, B.; Gresh, L.; Balmaseda, A.; de Silva, A.D.; et al. Immunodominant dengue virus-specific CD8+ T cell responses are associated with a memory PD-1+ phenotype. J. Virol. 2016, 90, 4771–4779. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Bangs, D.J.; Sidney, J.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; Crotty, S.; Peters, B.; Sette, A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc. Natl. Acad. Sci. USA 2015, 112, E4256–E4263. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Babor, M.; Lane, J.; Schulten, V.; Patil, V.S.; Seumois, G.; Rosales, S.L.; Fu, Z.; Picarda, G.; Burel, J.; et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat. Commun. 2017, 8, 1473. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Madrigal, A.; Schmiedel, B.J.; Clarke, J.; O’Rourke, P.; de Silva, A.D.; Harris, E.; Peters, B.; Seumois, G.; Weiskopf, D.; et al. Precursors of human CD4+ cytotoxic T lymphocytes identified by single-cell transcriptome analysis. Sci. Immunol. 2018, 3, eaan8664. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, T.; Wakim, L.M.; Eidsmo, L.; Reading, P.C.; Heath, W.R.; Carbone, F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009, 10, 524–530. [Google Scholar] [CrossRef]
- Guy, B.; Nougarede, N.; Begue, S.; Sanchez, V.; Souag, N.; Carre, M.; Chambonneau, L.; Morrisson, D.N.; Shaw, D.; Qiao, M.; et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008, 26, 5712–5721. [Google Scholar] [CrossRef]
- Harenberg, A.; Begue, S.; Mamessier, A.; Gimenez-Fourage, S.; Ching Seah, C.; Wei Liang, A.; Li Ng, J.; Yun Toh, X.; Archuleta, S.; Wilder-Smith, A.; et al. Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hum. Vaccin. Immunother. 2013, 9, 2317–2325. [Google Scholar] [CrossRef]
- Grifoni, A.; Voic, H.; Dhanda, S.K.; Kidd, C.K.; Brien, J.D.; Buus, S.; Stryhn, A.; Durbin, A.P.; Whitehead, S.; Diehl, S.A.; et al. T cell responses induced by attenuated flavivirus vaccination are specific and show limited cross-reactivity with other flavivirus species. J. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Waickman, A.T.; Friberg, H.; Gargulak, M.; Kong, A.; Polhemus, M.; Endy, T.; Thomas, S.J.; Jarman, R.G.; Currier, J.R. Assessing the diversity and stability of cellular immunity generated in response to the candidate live-attenuated dengue virus vaccine TAK-003. Front. Immunol. 2019, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; George, S.L.; Stinchcomb, D.T.; Osorio, J.E.; Partidos, C.D. CD8+ T-cell responses in flavivirus-naive individuals following immunization with a live-attenuated tetravalent dengue vaccine candidate. J. Infect. Dis. 2015, 212, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S.; et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J. Virol. 2015, 89, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Phillips, E.; Mallal, S.; Diehl, S.A.; et al. Human CD4+ T cell responses to an attenuated tetravalent dengue vaccine parallel those induced by natural infection in magnitude, HLA restriction, and antigen specificity. J. Virol. 2017, 91, e02147-16. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.; Eisenhauer, P.; Diehl, S.A.; Pierce, K.K.; Whitehead, S.S.; Durbin, A.P.; Kirkpatrick, B.D.; Sette, A.; Weiskopf, D.; Boyson, J.E.; et al. Rapid induction and maintenance of virus-specific CD8+ TEMRA and CD4+ TEM cells following protective vaccination against dengue virus challenge in humans. Front. Immunol. 2020, 11, 479. [Google Scholar] [CrossRef]
- Yauch, L.E.; Prestwood, T.R.; May, M.M.; Morar, M.M.; Zellweger, R.M.; Peters, B.; Sette, A.; Shresta, S. CD4 + T Cells Are not required for the induction of dengue virus-specific CD8 + T cell or antibody responses but contribute to protection after vaccination. J. Immunol. 2010, 185, 5405–5416. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Miller, R.; Eddy, W.E.; White, L.J.; Johnston, R.E.; Shresta, S. Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog. 2013, 9, e1003723. [Google Scholar] [CrossRef]
- Gil, L.; Izquierdo, A.; Lazo, L.; Valdés, I.; Ambala, P.; Ochola, L.; Marcos, E.; Suzarte, E.; Kariuki, T.; Guzmán, G.; et al. Capsid protein: Evidences about the partial protective role of neutralizing antibody-independent immunity against dengue in monkeys. Virology 2014, 456–457, 70–76. [Google Scholar] [CrossRef][Green Version]
- Costa, S.M.; Yorio, A.P.; Gonçalves, A.J.S.; Vidale, M.M.; Costa, E.C.B.; Mohana-Borges, R.; Motta, M.A.; Freire, M.S.; Alves, A.M.B. Induction of a protective response in mice by the dengue virus NS3 protein using DNA vaccines. PLoS ONE 2011, 6, e25685. [Google Scholar] [CrossRef]
- Simmons, M.; Sun, P.; Putnak, R. Recombinant dengue 2 virus NS3 helicase protein enhances antibody and T-cell response of purified inactivated vaccine. PLoS ONE 2016, 11, e0152811. [Google Scholar] [CrossRef]
- Kao, Y.-S.; Yu, C.-Y.; Huang, H.-J.; Tien, S.-M.; Wang, W.-Y.; Yang, M.; Anderson, R.; Yeh, T.-M.; Lin, Y.-S.; Wan, S.-W. Combination of modified NS1 and NS3 as a novel vaccine strategy against dengue virus infection. J. Immunol. 2019, 203, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.J.S.; Oliveira, E.R.A.; Costa, S.M.; Paes, M.V.; Silva, J.F.A.; Azevedo, A.S.; Mantuano-Barradas, M.; Nogueira, A.C.M.A.; Almeida, C.J.; Alves, A.M.B. Cooperation between CD4+ T cells and humoral immunity is critical for protection against dengue using a DNA vaccine based on the NS1 antigen. PLoS Negl. Trop. Dis. 2015, 9, e0004277. [Google Scholar] [CrossRef]
- Pinto, P.B.A.; Assis, M.L.; Vallochi, A.L.; Pacheco, A.R.; Lima, L.M.; Quaresma, K.R.L.; Pereira, B.A.S.; Costa, S.M.; Alves, A.M.B. T cell responses induced by DNA vaccines based on the DENV2 E and NS1 proteins in mice: Importance in protection and immunodominant epitope identification. Front. Immunol. 2019, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Alves, R.P.; Pereira, L.R.; Fabris, D.L.N.; Salvador, F.S.; Santos, R.A.; de Andrade Zanotto, P.M.; Romano, C.M.; Amorim, J.H.; de Souza Ferreira, L.C. Production of a recombinant dengue virus 2 NS5 protein and potential use as a vaccine antigen. Clin. Vaccine Immunol. 2016, 23, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Cantaert, T.; Colas, C.; Prot, M.; Casadémont, I.; Levillayer, L.; Thalmensi, J.; Langlade-Demoyen, P.; Gerke, C.; Bahl, K.; et al. A modified mRNA vaccine targeting immunodominant NS Epitopes protects against dengue virus infection in HLA Class I transgenic mice. Front. Immunol. 2019, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Ngono, A.E.; Chen, H.-W.; Tang, W.W.; Joo, Y.; King, K.; Weiskopf, D.; Sidney, J.; Sette, A.; Shresta, S. Protective role of cross-reactive CD8 T cells against dengue virus infection. EBioMedicine 2016, 13, 284–293. [Google Scholar] [CrossRef]
- Wang, P.-G.; Kudelko, M.; Lo, J.; Siu, L.Y.L.; Kwok, K.T.H.; Sachse, M.; Nicholls, J.M.; Bruzzone, R.; Altmeyer, R.M.; Nal, B. Efficient assembly and secretion of recombinant subviral particles of the four dengue serotypes using native prM and E proteins. PLoS ONE 2009, 4, e8325. [Google Scholar] [CrossRef]
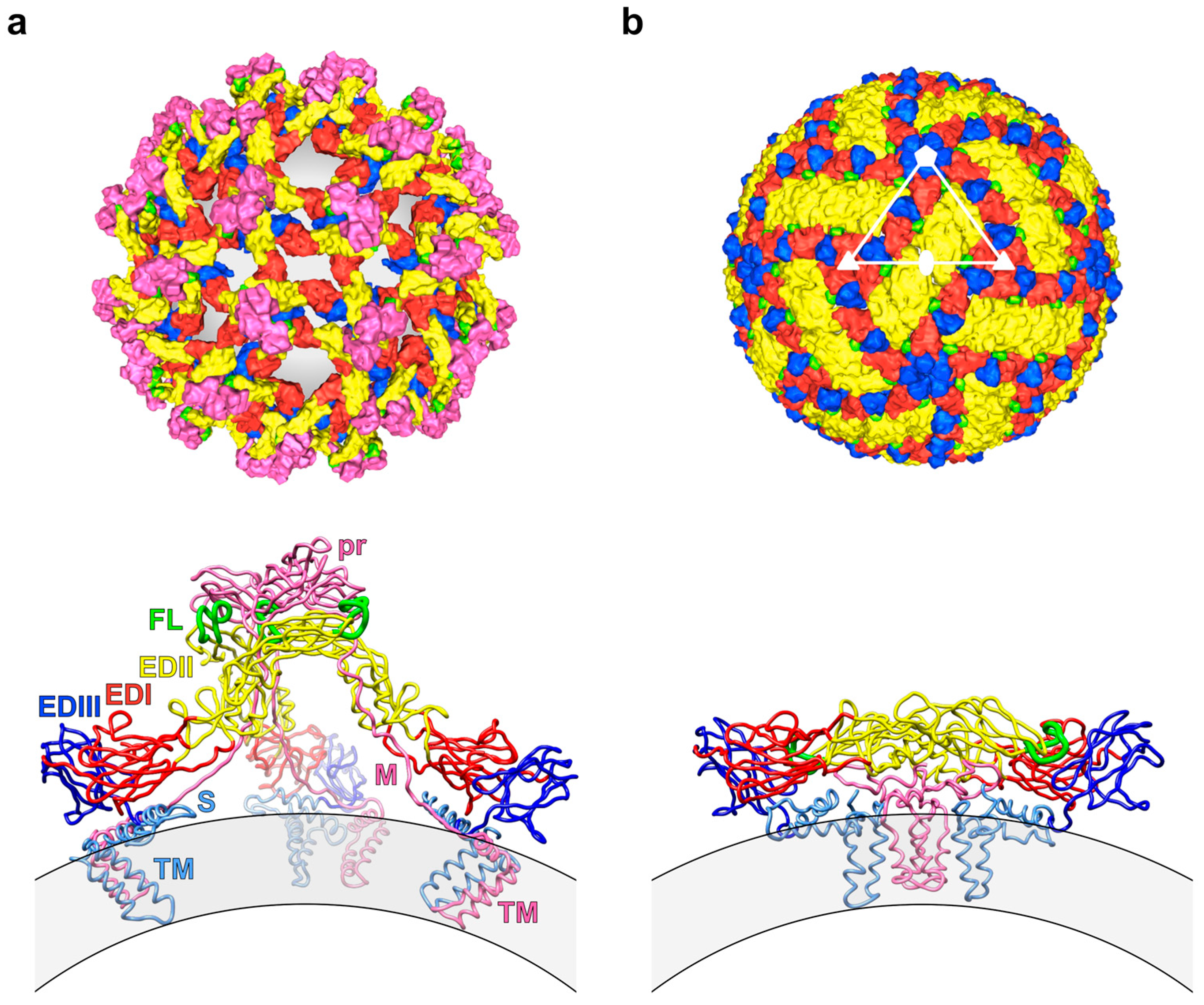
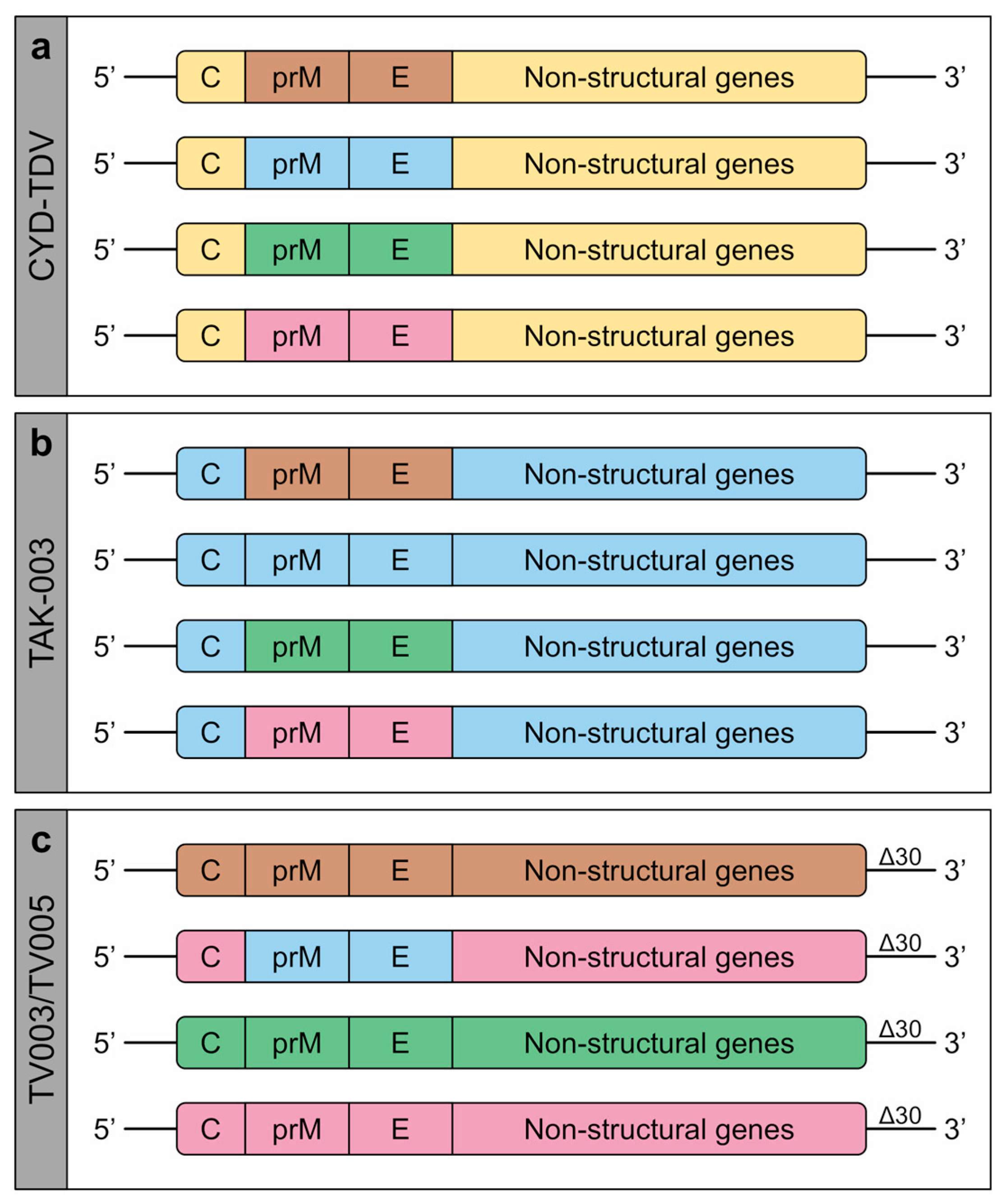
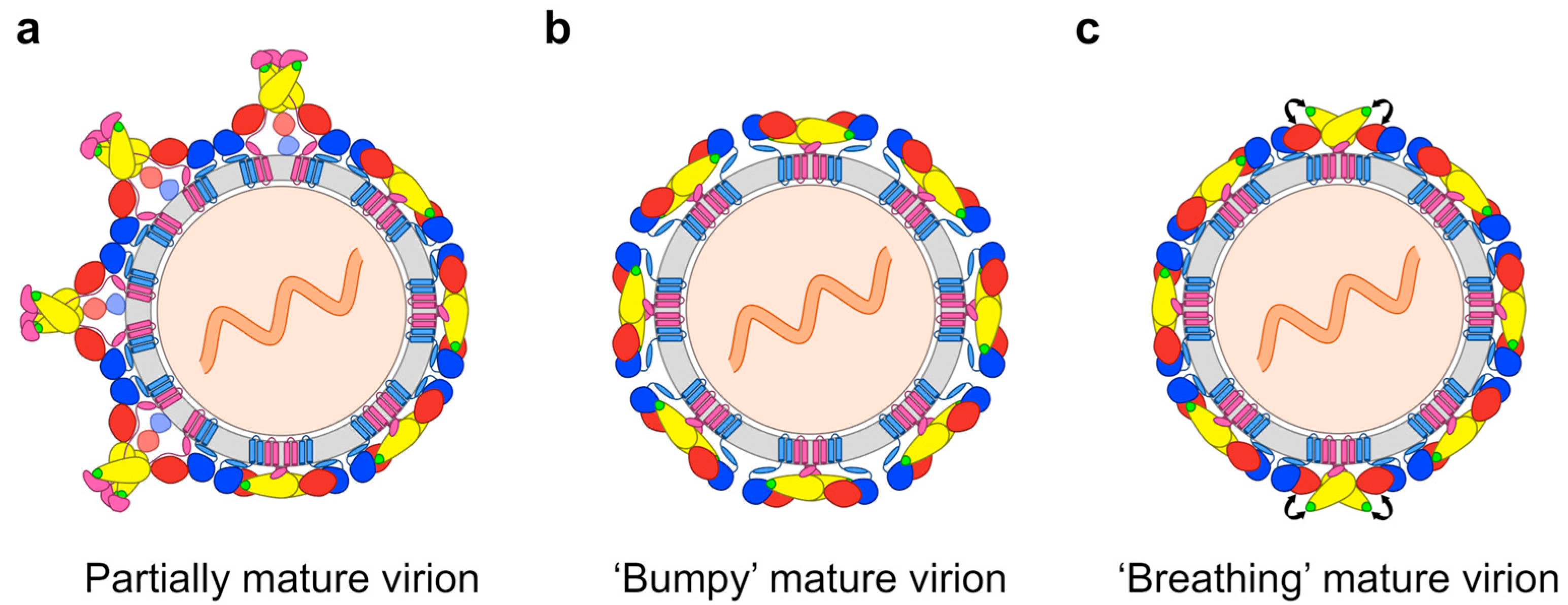
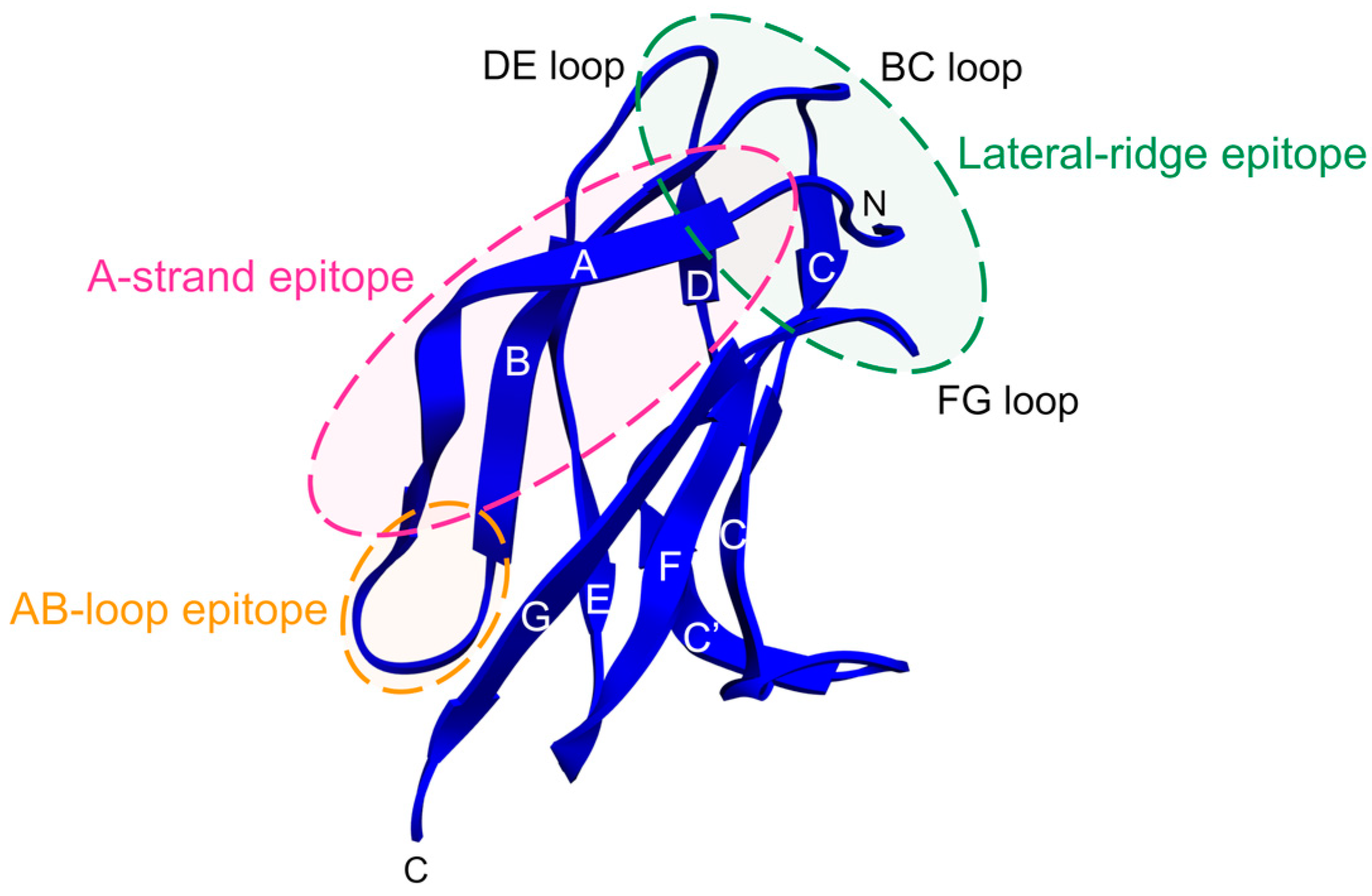
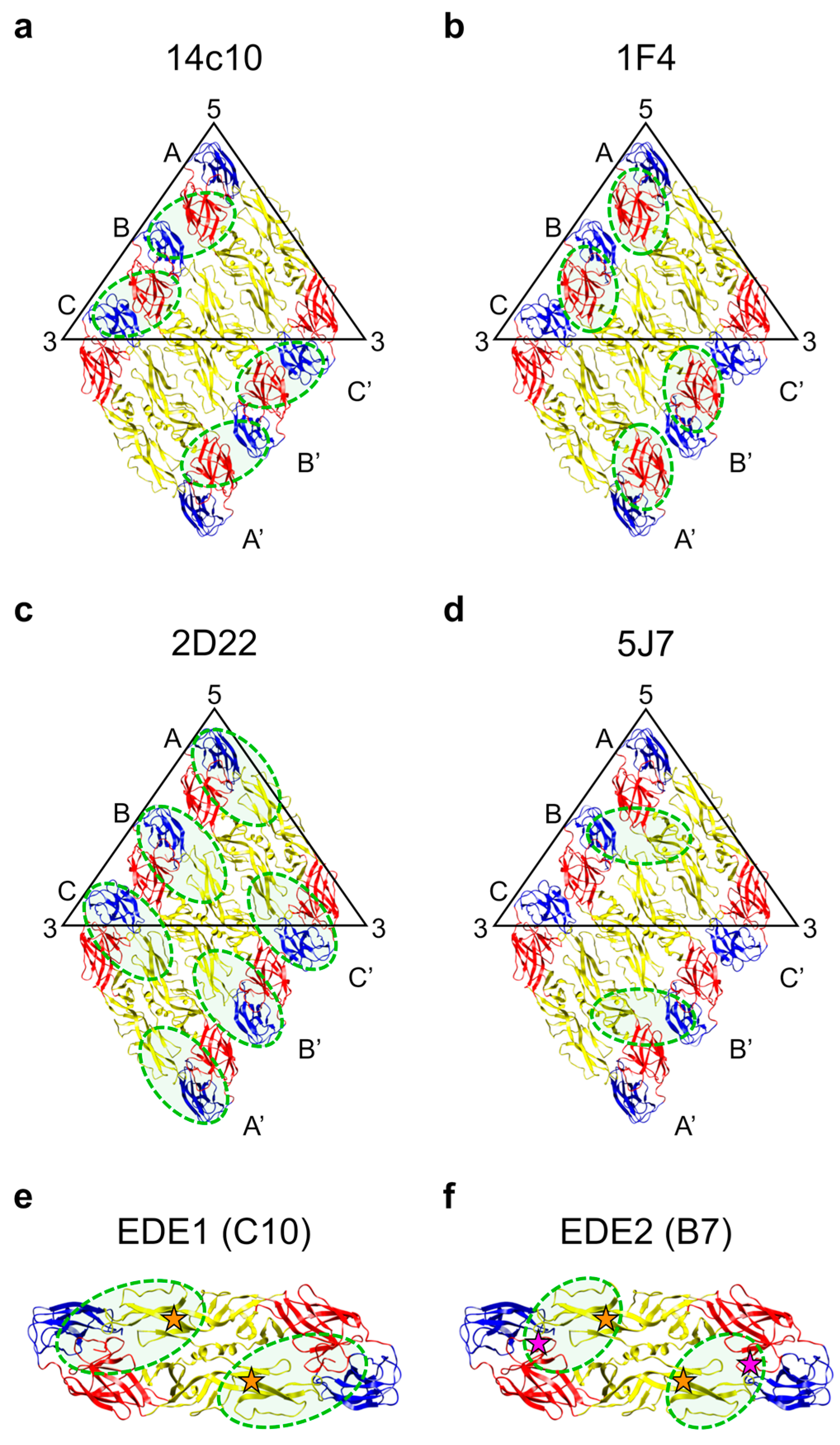
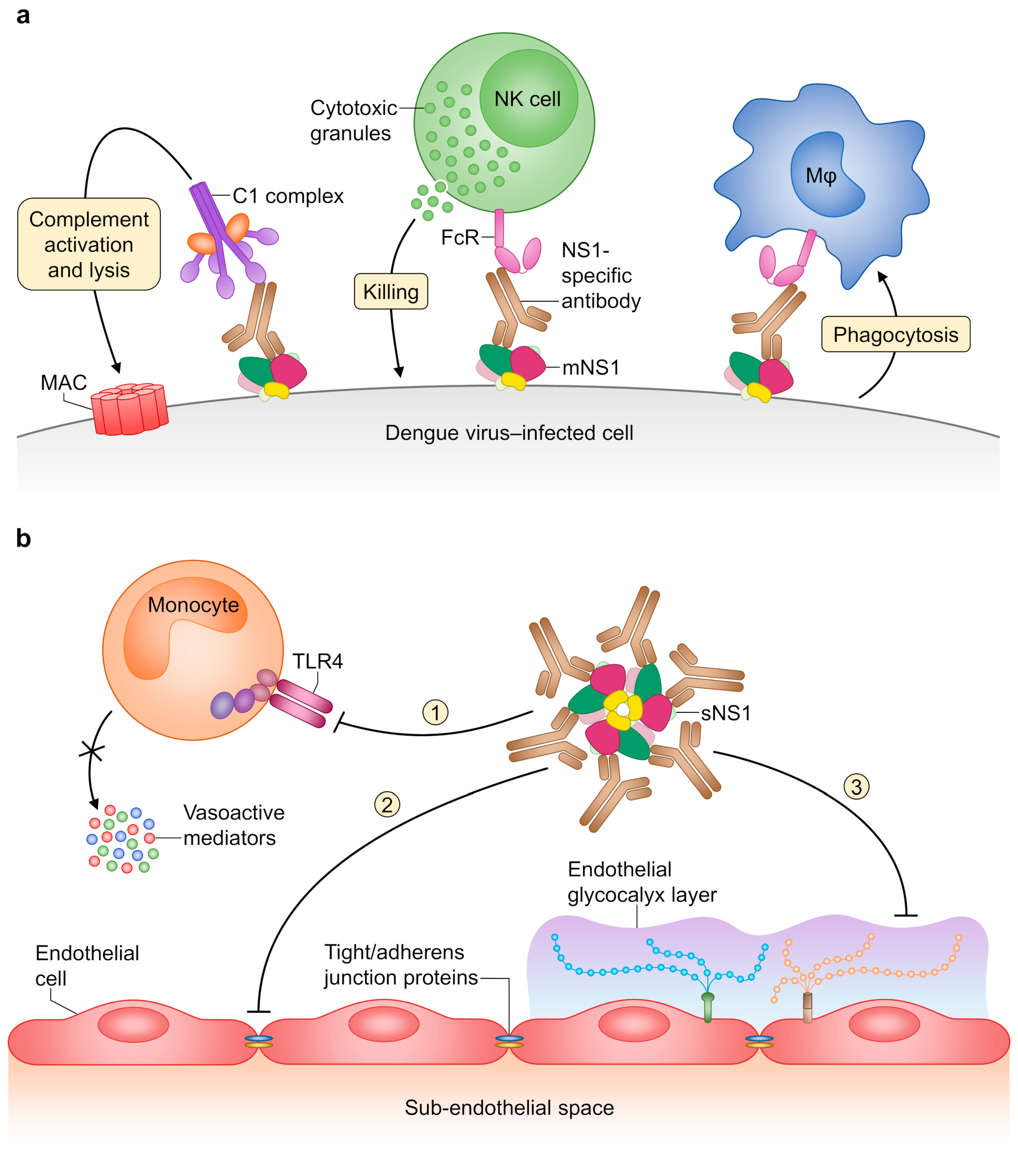
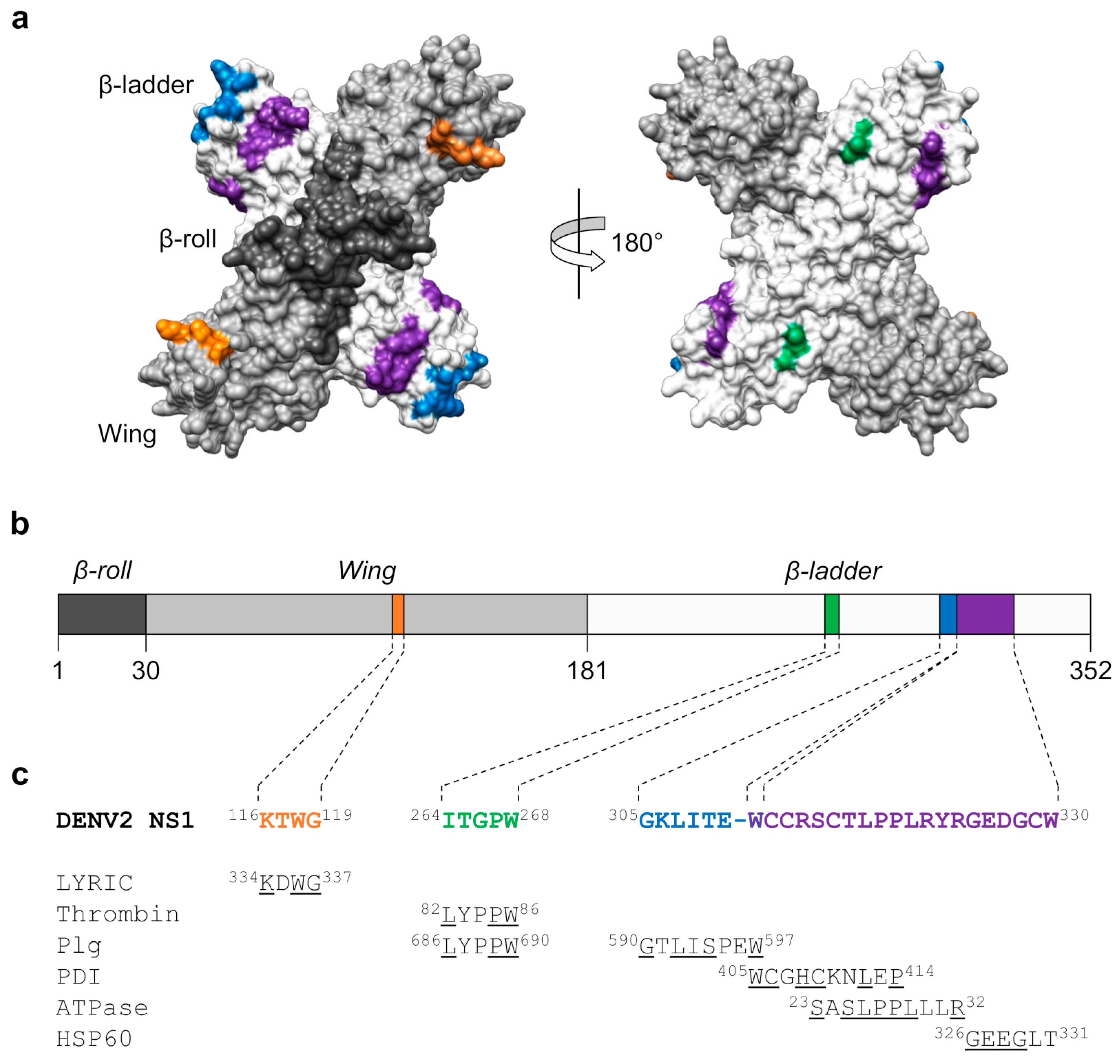
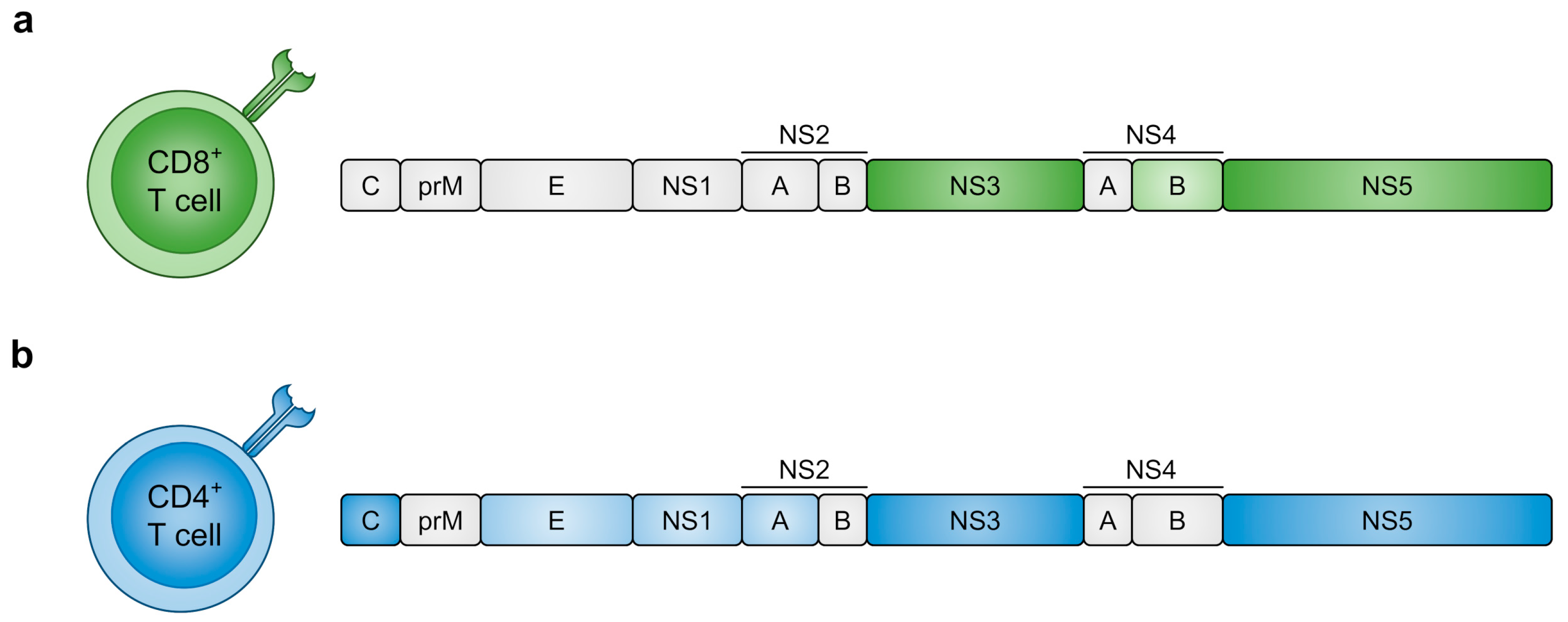
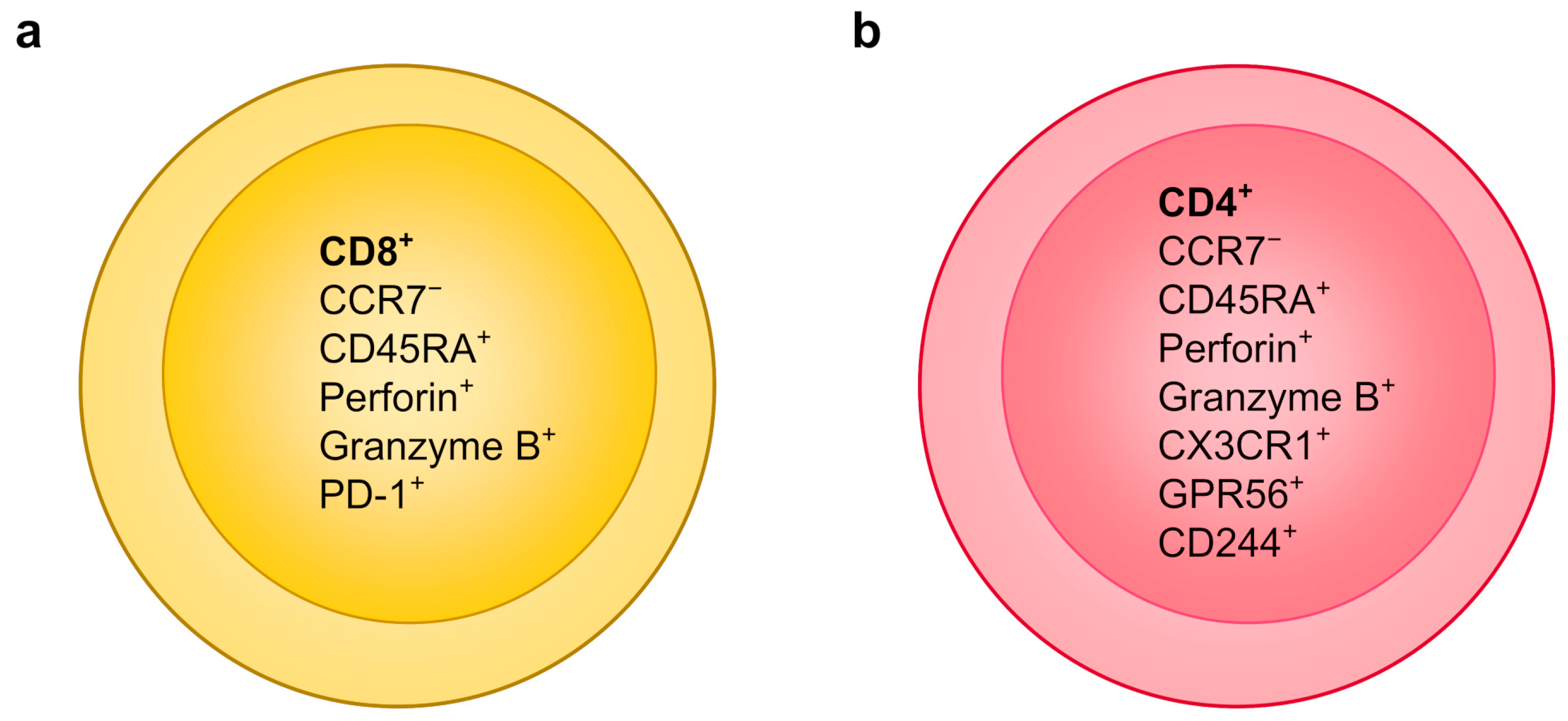
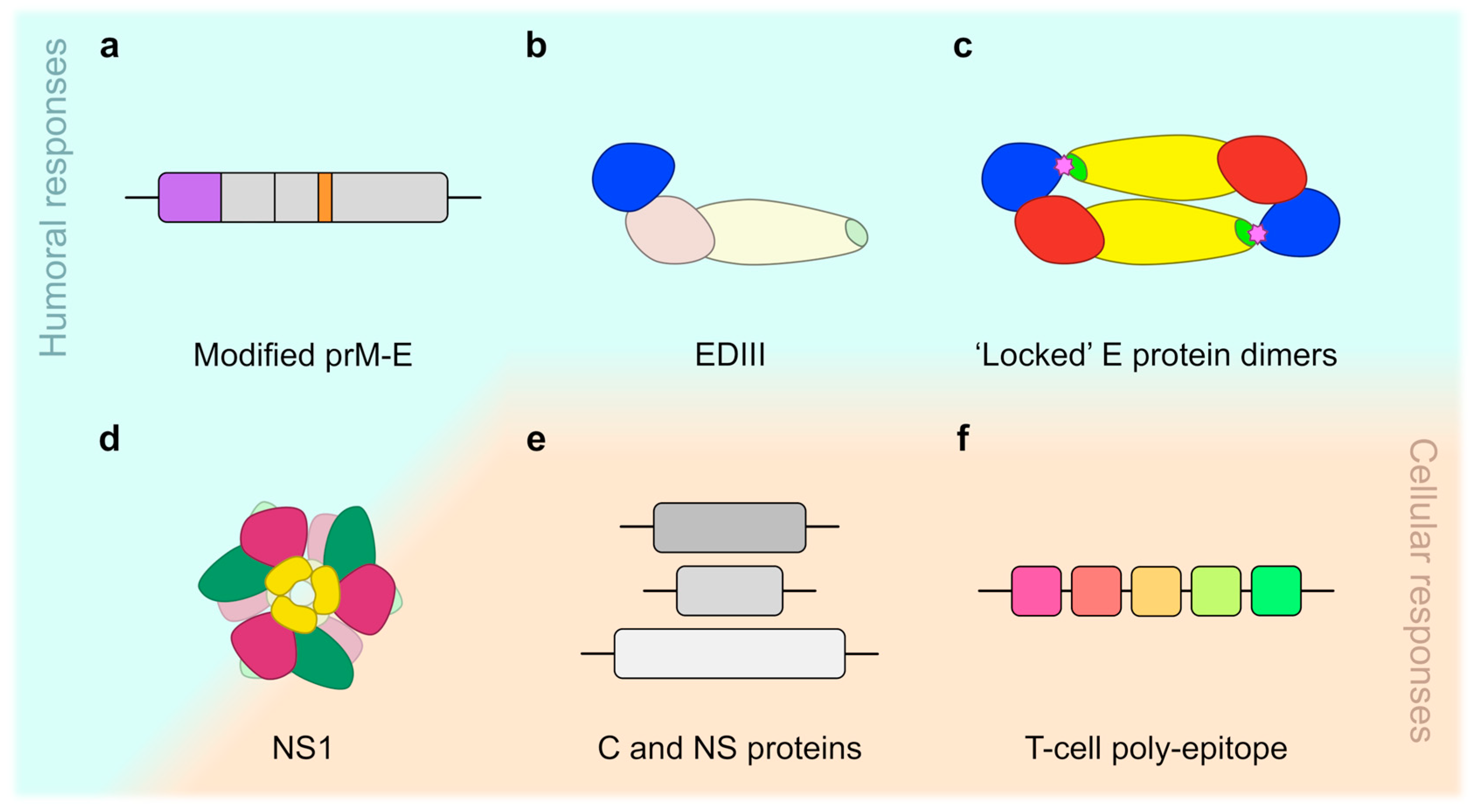
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilken, L.; Rimmelzwaan, G.F. Adaptive Immunity to Dengue Virus: Slippery Slope or Solid Ground for Rational Vaccine Design? Pathogens 2020, 9, 470. https://doi.org/10.3390/pathogens9060470
Wilken L, Rimmelzwaan GF. Adaptive Immunity to Dengue Virus: Slippery Slope or Solid Ground for Rational Vaccine Design? Pathogens. 2020; 9(6):470. https://doi.org/10.3390/pathogens9060470
Chicago/Turabian StyleWilken, Lucas, and Guus F. Rimmelzwaan. 2020. "Adaptive Immunity to Dengue Virus: Slippery Slope or Solid Ground for Rational Vaccine Design?" Pathogens 9, no. 6: 470. https://doi.org/10.3390/pathogens9060470
APA StyleWilken, L., & Rimmelzwaan, G. F. (2020). Adaptive Immunity to Dengue Virus: Slippery Slope or Solid Ground for Rational Vaccine Design? Pathogens, 9(6), 470. https://doi.org/10.3390/pathogens9060470




