Abstract
The necrotrophic fungus Botrytis cinerea causes devastating pre- and post-harvest yield losses in grapevine (Vitis vinifera L.). Although B. cinerea has been well-studied in different plant species, there is limited information related to the resistance and susceptibility mechanisms of Vitis genotypes against B. cinerea infection. In the present study, leaves and berries of twenty four grape genotypes were evaluated against B. cinerea infection. According to the results, one genotype (Ju mei gui) was highly resistant (HR), one genotype (Kyoho) was resistant (R), eight genotypes were susceptible (S), and fourteen genotypes were highly susceptible (HS) against infection of B. cinerea in leaves. Whereas in the case of B. cinerea infection in grape berry, three genotypes were found to be highly resistant, three resistant, eleven genotypes susceptible, and seven were highly susceptible. To further explore the mechanism of disease resistance in grapevine, we evaluated “Ju mei gui” and “Summer black” in terms of B. cinerea progression, reactive oxygen species reactions, jasmonic acid contents, and the activities of antioxidant enzymes in leaf and fruit. We surmise that the resistance of “Ju mei gui” is due to seized fungal growth, minor reactive oxygen species (ROS) production, elevated antioxidant enzyme activity, and more jasmonic acid (JA) contents. This study provides insights into the resistance and susceptibility mechanism of Vitis genotypes against B. cinerea. This will help for the selection of appropriate germplasm to explore the molecular basis of disease resistance mechanisms in grapevine.
1. Introduction
Grape (Vitis vinifera L.) is an extensively cultivated crop that has vast economic importance as it is a source of numerous products [1], though the berry quality and yield of grapevine is restricted by many abiotic and biotic stresses [2]. B. cinerea is a necrotrophic fungus that causes overwhelming grey mold disease. This pathogen is the second most widespread plant pathogen accountable for pre- and post-harvest dwindling and fruit quality worsening [3]. This necrotrophic fungus actively attempts to destroy the living host tissues and naturally senesced plant tissues to use them as nutrients [4] where periods of cold temperatures (18–22 °C) and relative humidity (more than 90%) persist for a long time [5]. The pathogen causes reduction both in quality and yield of wine [6]. Host disease development depends on various traits, such as bunch compactness, morphological, anatomical, and chemical features of the berry skin, which are highly reliant on the grapevine cultivar [7].
The traditional control of B. cinerea includes strong fungicide treatments during the seasonal crop cycle, but the excessive use of fungicides has many negative effects including increase in production cost, development of fungicide resistant strains, and environmental pollution [8,9]. Thus, the development of disease resistant cultivars is the dire need of time. Most cultivated species of V. vinifera are susceptible to many diseases, and the susceptibility differs among the cultivars [10]. In this experiment, the disease signs and symptoms were assessed in a total of 24 grape genotypes at various stages of grape and B. cinerea interactions. Additionally, the contents of reactive oxygen species were calculated, which play important functions in plant physiology, comprising development, cellular signaling, and biotic and abiotic stress tolerance. Reactive oxygen species (ROS) production must be firmly controlled to stabilize the biological functions [11]. Considerable confirmation shows that B. cinerea challenges can initiate the ROS stress on plants [12].
Redox reactions regulate numerous cellular signaling activities and may be directly involved in the cellular redox metabolism [13]. The plant and fungus association is related with ROS production. Oxidative rupture is an initial and universal plant response to pathogen attack. In B. cinerea, plant cell death is favorable to the pathogen and causes susceptibility of the host [14]. Antioxidants avoid and shield the cell from the damage caused by free radicals, which help in sustaining the rate of oxidation reactions in a cell [15] and play a very critical role in mitigating the process of oxidation of other molecules [16]. To avoid the oxidative damage caused by these toxic ROS, the level of the endogenous antioxidant defense system is raised in higher plants [17]. Furthermore, Jasmonic acid (JA) is known to be involved in biotic stress amelioration in plants [18] and plays a key governing function in defense responses to necrotrophs [19], also contributing to reactions to insect and pathogen attacks [20]. Plant hormones like jasmonic acid are involved in biotic stress neutralization [18]. JA plays an important role in the stimulation of induced systemic resistance in plants to pathogen or pest attack and accumulates rapidly in plant tissues after exposure to fungal elicitors [21]. It has also been reported that JA and its methyl ester (MeJA) is involved in plant defense mechanisms against biotic and abiotic stresses [22].
The main objective of this work was to evaluate different grapevine genotypes against B. cinerea by using grape leaves and berries under controlled conditions. Furthermore, we explored the ROS contents, antioxidant enzymes and JA contents in “Ju mei gui” highly resistant (HR) and “Summer black” highly susceptible (HS) genotypes. This study provides information regarding resistance and susceptibility mechanisms of Vitis genotypes that may assist in future breeding programs.
2. Results
2.1. Grape Genotypes and Their Various Levels of Resistance to B. cinerea
Twenty-four grape genotypes were evaluated to investigate the resistance level of leaves against B. cinerea. One genotype was classified as HR, one as resistant, eight as susceptible (S), and fourteen as HS (Table 1). Similarly, Vitis genotypes were evaluated for berry resistance level against B. cinerea infection, three genotypes were HR, three resistant (R), eleven genotypes S, and seven were HS (Table 2). Grapevine genotypes revealed various grades of resistance to B. cinerea. Leaf and berry observations were used to assess B. cinerea infection [23] and the range of leaf and berry lesions caused by B. cinerea were quantified at 72 hpi (hours post inoculation) (Table 1) and 8 dpi (days post inoculation), respectively (Table 2). Few grape genotypes showed substantial variations in B. cinerea resistance (Table 1 and Table 2), and a least significant difference (LSD) test showed resemblance among the replicates, and average disease severity was considerably different (p > 0.05) among the various genotypes (Table 1 and Table 2).

Table 1.
Evaluation of disease severity in 24 Vitis genotypes by using leaves, post B. cinerea inoculation.

Table 2.
Disease severity investigation in 24 Vitis genotypes by using Berries with post B. cinerea inoculation.
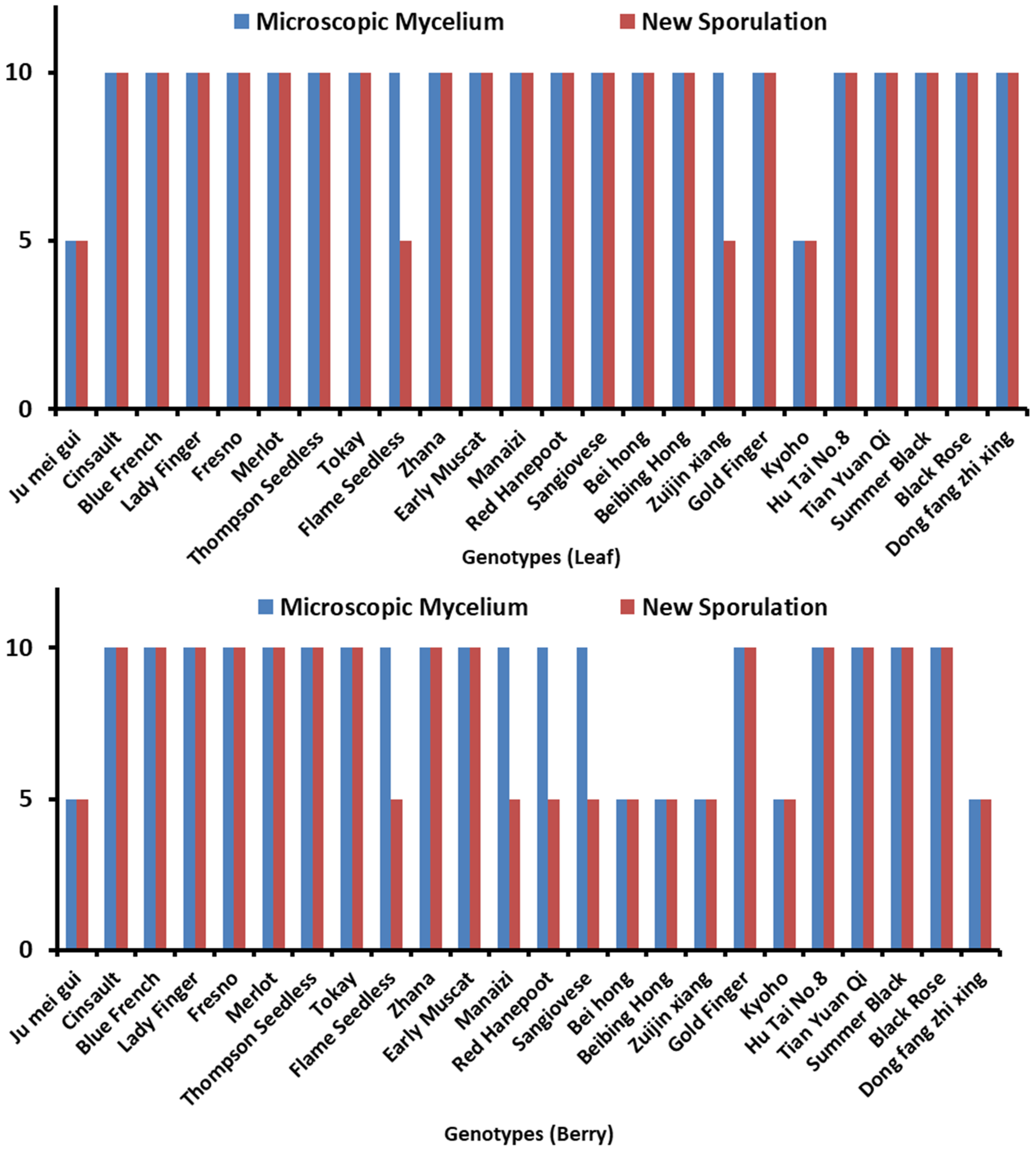
Microscopic mycelium and new sporulation was also observed in inoculated leaves and berries of various genotypes as shown in Figure 1. The leaves and berries of 24 genotypes were assessed to reveal the resistance level against B. cinerea. According to observations, leaves and berries were categorized according to their disease severity index (SI) at 72 hpi and 8 dpi, respectively. Among 24 genotypes, 14 (Table 1) were HS in leaves evaluation while 7 (Table 2) were revealed in berries according to a disease SI of 5.51–7.0. Microscopic mycelium and new sporulation was witnessed on these genotypes. A total of 8 genotypes (Table 1) were S in leaves and 11 (Table 2) in berries evaluation, with mycelium production at 72 hpi, with less/no sporulation (SI of 3.51–5.50).
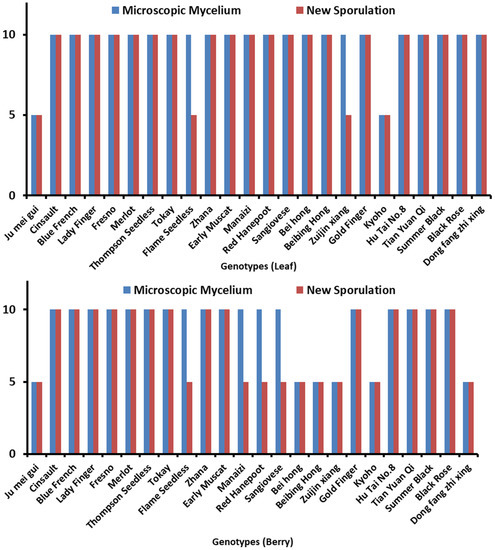
Figure 1.
Demonstrating different Vitis genotypes. Microscopic mycelium and new sporulation in (A) leaves and (B) berries, whereas 5 = absence of microscopic mycelium and new sporulation, while 10 = presence of microscopic mycelium and new sporulation.
2.2. Fungal Growth on Leaves and Berries Post B. cinerea Inoculation
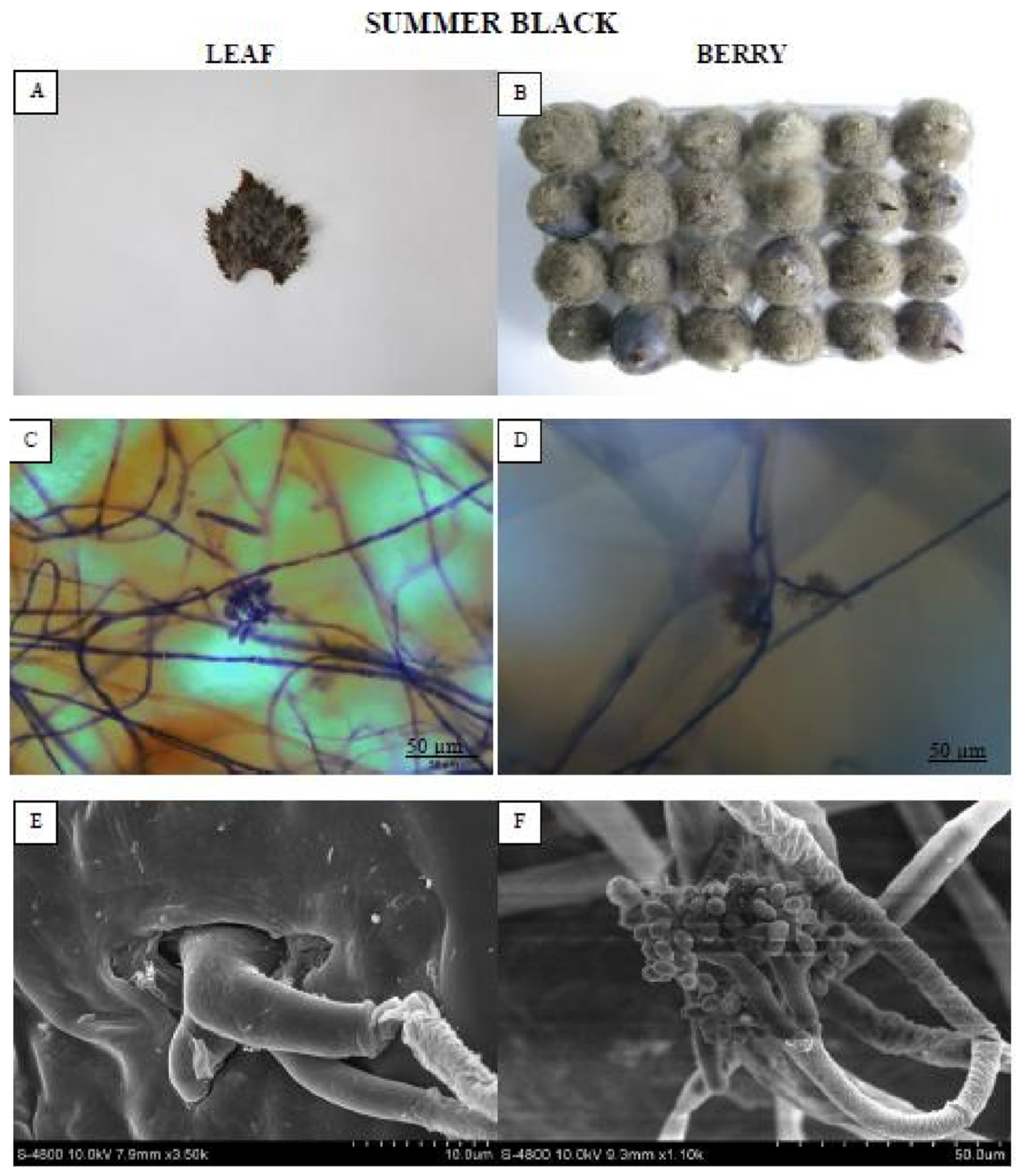
One representative genotype each from the HR and HS categories was selected for macroscopic, microscopic, and SEM evaluation of fungal colonization on leaves and berries at 72 hpi and 8 dpi, respectively. The leaf of “Summer black” (Figure 2) genotype was entirely enclosed in mold and was roofed by mycelium as well as new sporulation.
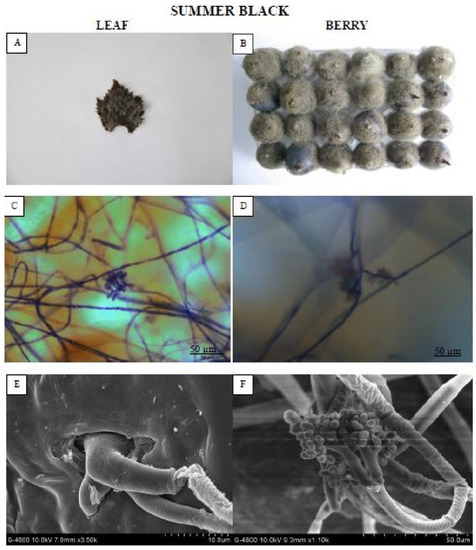
Figure 2.
Showing the highly susceptible (HS) “Summer black” leaf and berry post B. cinerea inoculation comparison by phenotypic (A,B), microscopic (C,D) and electron microscopic (E,F) study. Scale bars (C,D): 50 µm; (E,F): 20 µm. Samples were collected 72 hpi and 8 dpi, respectively.
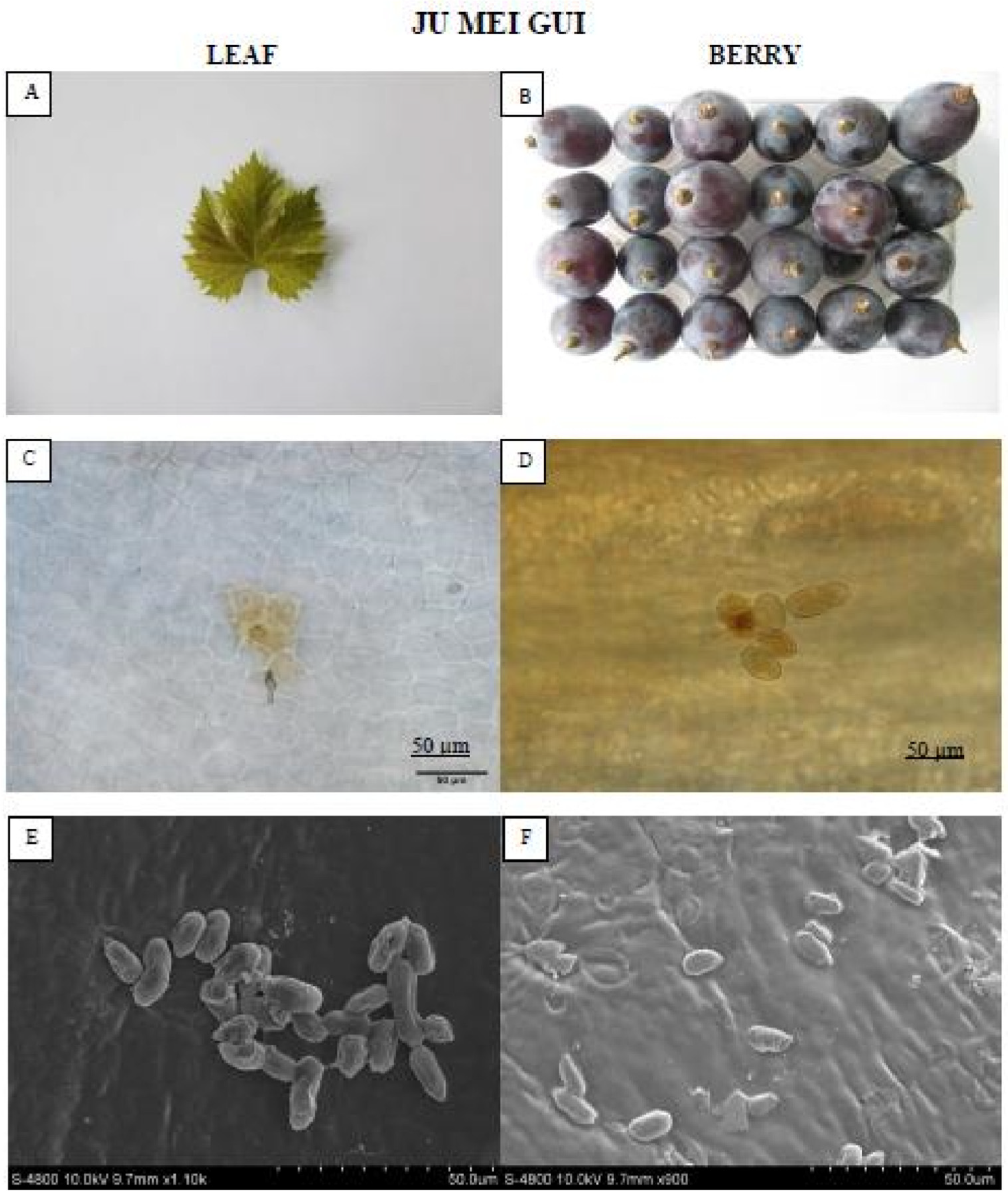
The “Ju mei gui” (Figure 3) formed no necrotic lesions compared to “Summer black.” Moreover, conidia were observed on the leaves and berries of “Ju mei gui” though the subsequent hyphae was absent, representing restrained B. cinerea growth.
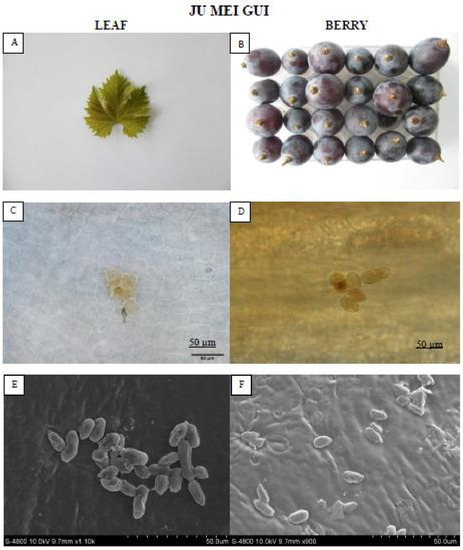
Figure 3.
Showing the HR “Ju mei gui” leaf and berry post B. cinerea inoculation comparison by phenotypic (A,B), microscopic (C,D) and electron microscopic (E,F) study. Scale bars (C,D): 50 µm; (E,F): 20 µm. Samples were collected 72 hpi and 8 dpi respectively.
2.3. Peroxidase and Superoxide Dismutase Activities in ”Ju mei gui” and “Summer black” Post B. cinerea Inoculation
2.3.1. Activities of Superoxide Dismutase (SOD)
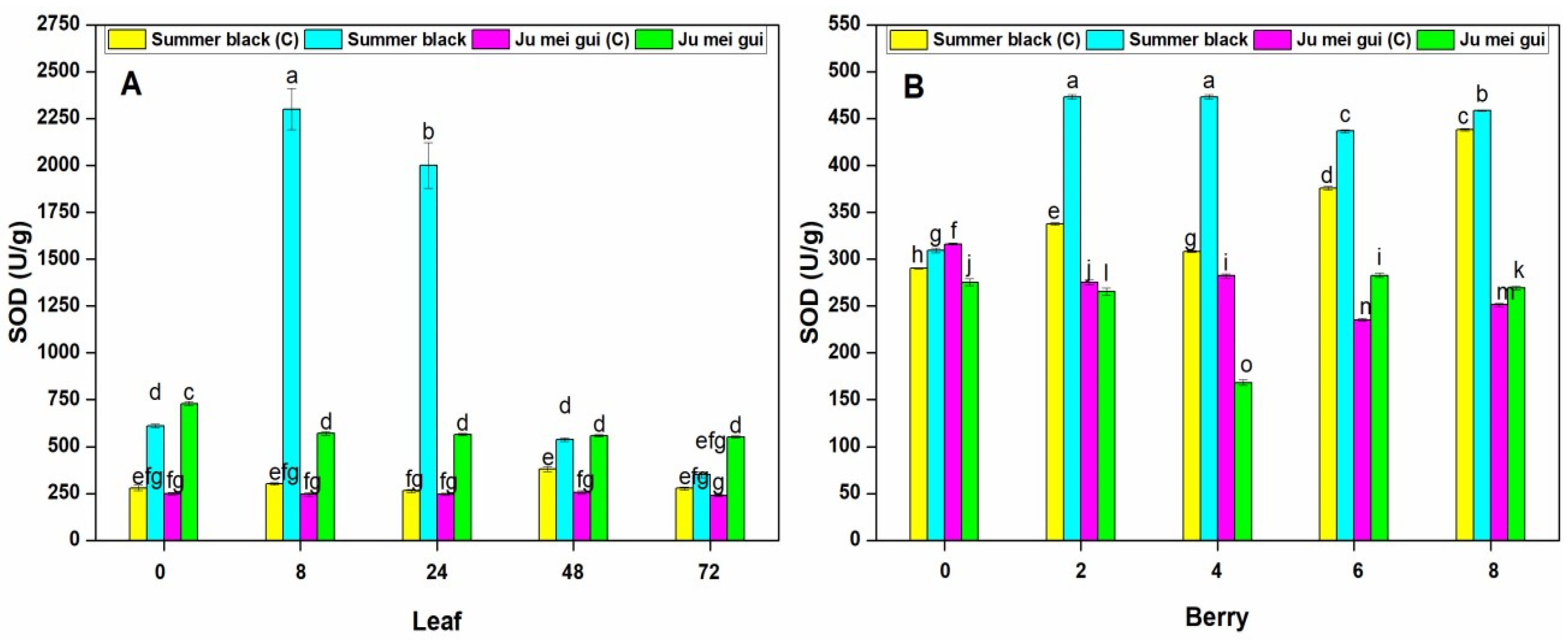
Superoxide dismutase (SOD) and peroxidase (POD) were measured in the infected and control leaves and berries. Stress circumstances interrupt ROS production resulting in plant cell decease, and plants make an arrangement of antioxidant enzymes to hunt destructive ROS and defend cells from oxidative injury [24]. The SOD activities were determined in the infected and control leaves (Figure 4A) of HR “Ju mei gui”and HS “Summer black.” The SOD activities in “Ju mei gui”and “Summer black” inoculated and control was approximately the same at all-time points (0, 8, 24, 48, 72 hpi). However, the highest peak was detected at 8 hpi followed by 24 hpi in “Summer black” inoculated.
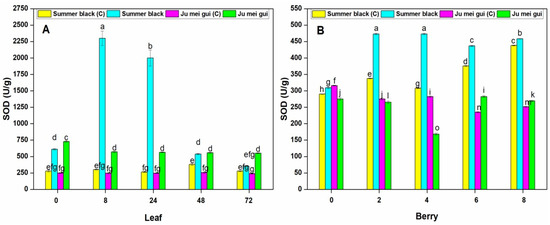
Figure 4.
Superoxide dismutase (SOD) activities of “Ju mei gui” and “Summer black” leaves (A) and berries (B) at 0, 8, 24, 48, 72 h post-inoculation (hpi) and 0, 2, 4, 6, 8 days post-inoculation (dpi), respectively, with Botrytis suspension and using sterile water as the control. The letter “C” with variety name stands for control. Small letters indicate significant differences according to an LSD test (p < 0.05) between “Ju mei gui” and “Summer black.”
Similarly, we observed the SOD activities at different time points (0, 2, 4, 6, 8 dpi) in berries (Figure 4B) for “Ju mei gui”and “Summer black.” Elevated levels were observed in “Summer black” inoculated, followed by “Summer black” control at 2 dpi, followed by 4 dpi and prolonged until 8 dpi. According to our observations, SOD levels were higher in inoculated leaves (Figure 2A) as related to inoculated berries (Figure 2B) at 8 hpi and 2 dpi, respectively.
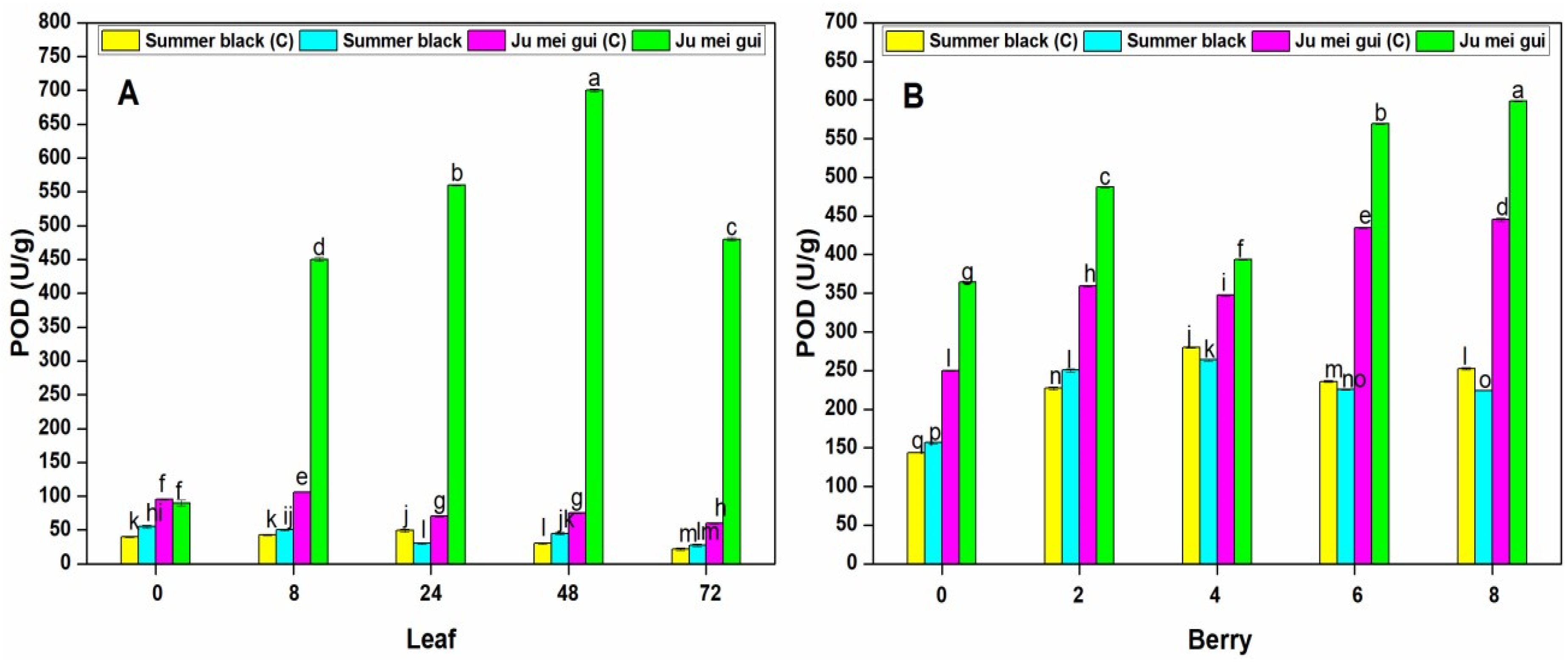
2.3.2. Activities of Peroxidase (POD)
The POD activities were observed in the infected and control leaves (Figure 5A) at various time points (0, 8, 24, 48, 72 hpi) of “Ju mei gui” and “Summer black.” The POD activities in the “Ju mei gui”and “Summer black” inoculated and control were approximately the same at 0 hpi. However, a gradual increase was observed in “Ju mei gui” inoculated at 8 hpi, followed by 24 hpi and 48 hpi, and then slightly decreased at 72 hpi. Similarly, we observed the SOD activities in berries (Figure 5B) for the HR and HS genotypes, and the elevated levels were observed in “Ju mei gui” inoculated, followed by “Ju mei gui” control at all-time points (0, 2, 4, 6, 8 dpi). By comparing the leaves and berries POD activities, POD levels were higher in inoculated leaves (Figure 3A) compared to inoculated berries (Figure 3B) at 8 hpi and 2 dpi, respectively.
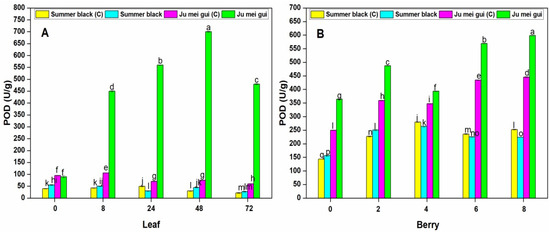
Figure 5.
Peroxidase (POD) activities of “Ju mei gui” and “Summer black” leaves (A) and berries (B) at 0, 8, 24, 48, 72 h post-inoculation (hpi) and 0, 2, 4, 6, 8 days post-inoculation (dpi), respectively, with Botrytis suspension and using sterile water as the control. The letter “C” with variety name stands for control. Small letters indicate significant differences according to an LSD test (p < 0.05) between “Ju mei gui” and “Summer black.”
2.4. Hydrogen Peroxide (H2O2) Accumulation in HR ”Ju mei gui” and HS “Summer black”leaves and berries in Response to Infection with B. cinerea
2.4.1. H2O2 Activities in “Ju mei gui” and “Summer black” Following Inoculation with B. cinerea
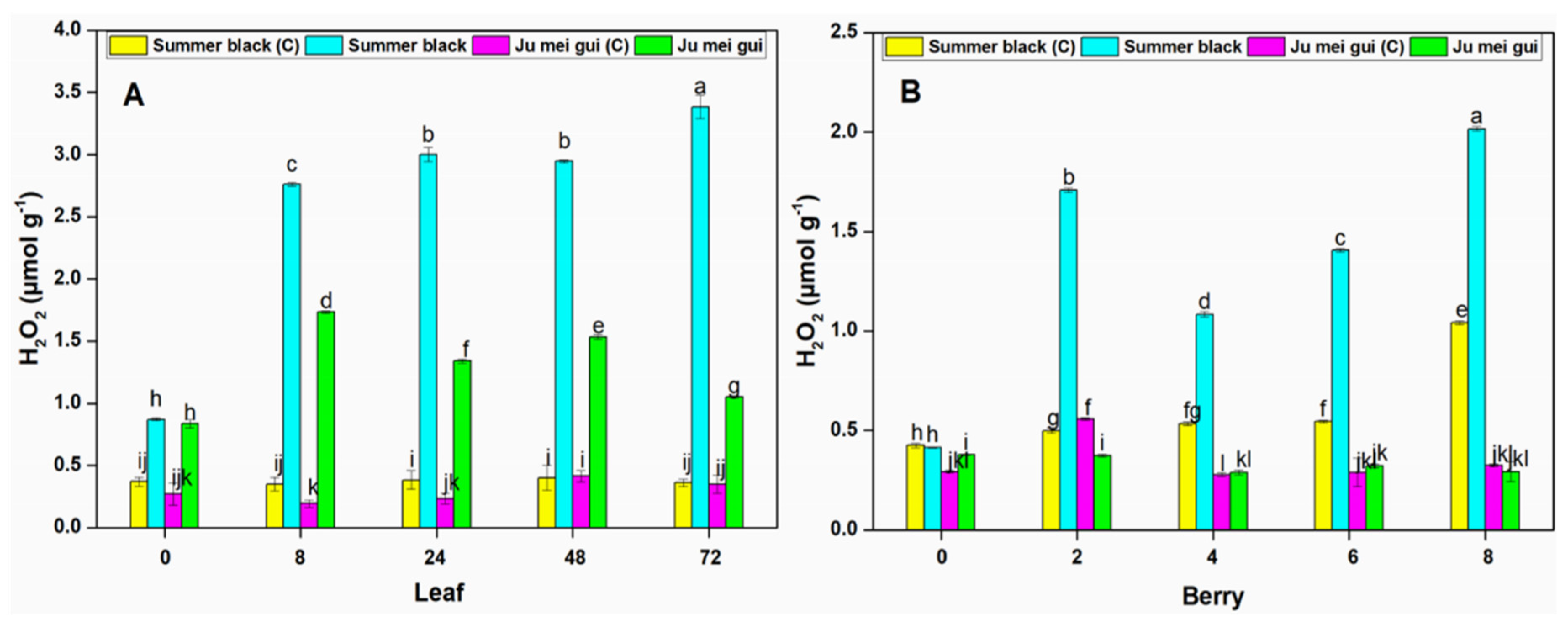
The H2O2 activities were measured at various time points (0, 8, 24, 48, 72 hpi) in the infected and control leaves (Figure 6A) of HR “Ju mei gui”and HS “Summer black.” The H2O2 activies in the “Ju mei gui”and “Summer black” inoculated and control were approximately the same at 0 hpi. However, a gradual elevation was observed at 8 to 72 hpi followed by “‘Ju mei gui” inoculated (Figure 6A). Likewise, we observed the H2O2 activities at different time points (0, 2, 4, 6, 8 dpi) in berries (Figure 6B) for the “Ju mei gui”and “Summer black” and found that there was no difference between all treatments at 0 hpi while a sudden increase was observed at 2 dpi and dropdown at 4 dpi but again a gradual increase was observed at 6 dpi followed by 8 dpi (Figure 6B).
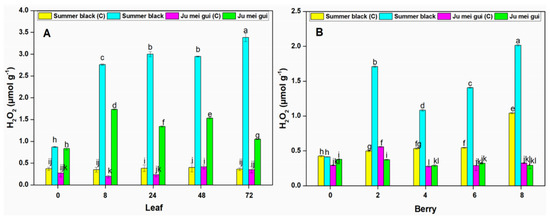
Figure 6.
Hydrogen peroxide (H2O2) contents of “Ju mei gui” and “Summer black” leaves (A) and berries (B) at 0, 8, 24, 48, 72 h post-inoculation (hpi) and 0, 2, 4, 6, 8 days post-inoculation (dpi), respectively, with Botrytis suspension and using sterile water as the control. The letter “C” with variety name stands for control. Small letters indicate significant differences according to an LSD test (p < 0.05) between “Ju mei gui” and “Summer black.”
2.4.2. Superoxide Radicals (O2-) Activities in “Ju mei gui” and “Summer black” Post-B. cinerea Inoculation
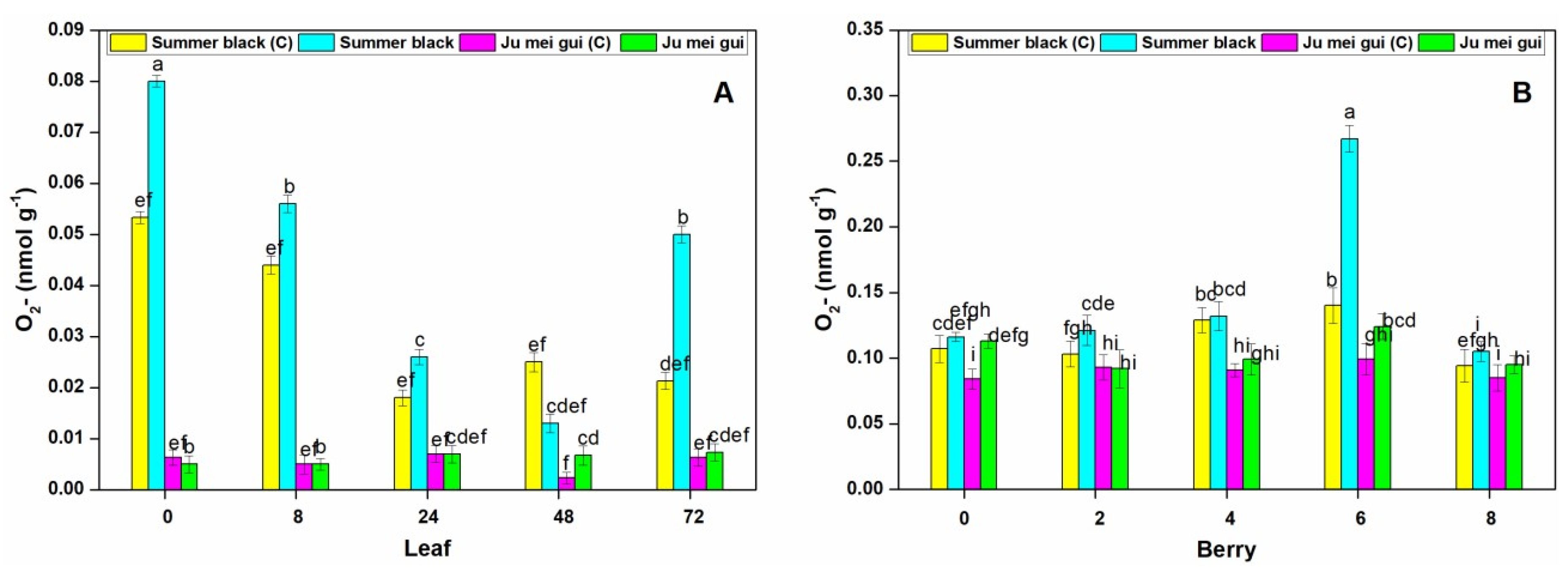
The O2- activities were measured at various time points (0, 8, 24, 48, 72 hpi) in inoculated and control leaves (Figure 7A) of HR “Ju mei gui” and HS “Summer black”. The O2- activities in the “Summer black” inoculated and control were highest at 0 hpi, followed by 8 hpi. However, a sudden decrease was observed at the rest of time points (Figure 7A). We also observed the O2- activities at different time points (0, 2, 4, 6, 8 dpi) in berries (Figure 7B) for the HR and HS genotypes and no significant differences were found at various time points, except a sudden increase observed in “Summer black” inoculated at 6 dpi (Figure 7B).
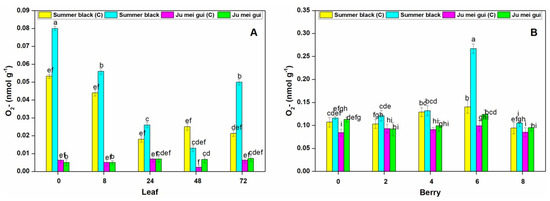
Figure 7.
Superoxide radical (O2-) contents of “Ju mei gui” and “Summer black” leaves (A) and berries (B) at 0, 8, 24, 48, 72 h post-inoculation (hpi) and 0, 2, 4, 6, 8 days post-inoculation (dpi), respectively, with Botrytis suspension and using sterile water as the control. The letter “C”with variety name stands for control. Small letters indicate significant differences according to an LSD test (p < 0.05) between “Ju mei gui”and “Summer black.”
2.5. Jasmonic Acid Contents in Leaves and Berries of “Ju mei gui” and “Summer black” with Post-B. cinerea Inoculation
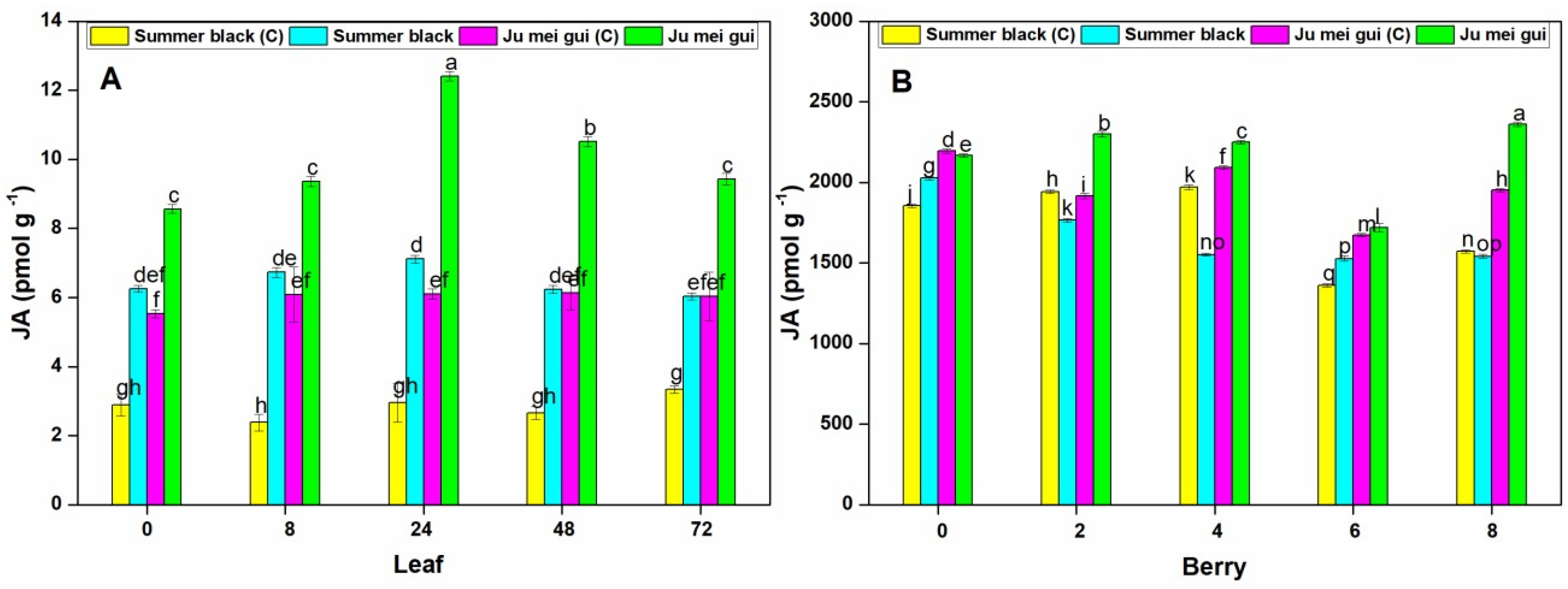
The JA activities were measured at various time points (0, 8, 24, 48, 72 hpi) in the inoculated and control leaves (Figure 8A) of “Ju mei gui” and “Summer black.” The JA activities in the “Ju mei gui” and “Summer black” inoculated were higher than that of control treatments. An increase was observed at all-time points with the highest peak at 24 hpi in “Ju mei gui” followed by “Summer black” inoculated and “Ju mei gui”control (Figure 8A). Moreover, the “Summer black” inoculated and “Ju mei gui”control were approximately the same at all-time points. Likewise, we observed the JA activities at different time points (0, 2, 4, 6, 8 dpi) in berries (Figure 8B) for “Ju mei gui”and “Summer black”, and found that there was no difference between almost all treatments, except for higher levels of “Ju mei gui”inoculated at all-time points (Figure 8B).
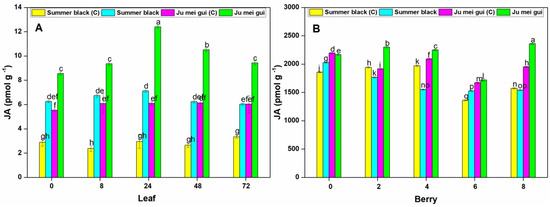
Figure 8.
Jasmonic acid (JA) activities of protein extracts from “Ju mei gui” and “Summer black” leaves (A) and berries (B) at 0, 8, 24, 48, 72 h post-inoculation (hpi) and 0, 2, 4, 6, 8 days post-inoculation (dpi), respectively, with Botrytis suspension and using sterile water as the control. The letter “C” with variety name stands for control. Three independent experiments were used for the means and standard errors. Small letters indicate significant differences according to an LSD test (p < 0.05) between “Ju mei gui” and “Summer black.”
3. Discussion
B. cinerea is particularly challenging as it not only devastates green tissue, decreasing yield potential, but also can infect fruit [25]. The world’s population is predicted to rise to more than 9.7 billion by 2050, and worldwide crop production should be doubled in order to meet the increased demand for food. Reducing yield losses to pests and diseases will be a key step in the direction of achieving this challenge [26]. Thus, in light of previous studies, it is necessary to protect crops from pests and diseases to ensure food safety and to develop disease resistant cultivars to reduce the problem of yield and quality losses. In this study, grape genotypes were evaluated against B. cinerea infection by using grape leaves and berries. Vitis genotypes differ in expressions of their resistance to infection, score of fungal growth and disease severity to B. cinerea [27]. Here, 24 different Vitis genotypes were evaluated for disease resistance in leaves and berries. According to the results, cultivars were divided into highly resistant, resistant, susceptible, and highly susceptible (Table 1 and Table 2).
Distinct growth of B. cinerea on grape leaves (72 hpi) and berries (8 dpi) was studied phenotypically, microscopically, and via scanning electron microscopy (SEM). In “Summer black,” the pathogen infection feasted significantly and showed signs of sporulation on 72 hpi, whereas in “Ju mei gui” leaves, fungal growth was considerably stuck as revealed by the lesser germination and infection rates. On the “Ju mei gui” leaves, maximum B. cinerea conidia failed to develop into infection pegs, which are in line with previous reports [28]. Similarly, sporulation masses on Langao-5 (V. davidii) and Baihe-35-1 (V. pseudoreticulata) were considerably lesser as compared to the HS cultivar Pinot noir (V. vinifera) [11]. ROS production is conjoint in response to pathogen occurrence and elevated contents of ROS were detected in “Summer black.” This is in accordance with previous studies where host–pathogen interactions ROS accretions endorse pathogen growth, disease development, and assist colonization on leaves. In genotype “Ju mei gui”, little amounts of ROS post-inoculation were detected, signifying that the antioxidant enzymes uphold redox equilibrium and shield cells from ROS destruction [17,26].
Oxidative stress interrupts the redox equilibrium in diseased tissues, thereby facilitating disease progression [29]. In this study, elevated ROS accumulation after inoculation was noticed in leaves and berries of “Summer black.” We determined that “Summer black” was extensively affected from the constant presence of ROS, and that “Ju mei gui” was not capable of experiencing significant oxidative stress because of a timely initiated antioxidative system. H2O2 production is induced in plant cells and accompanied by O2- generation, which can encourage programmed cell death and disease lesion expansion, thus increasing B. cinerea infection [30]. H2O2 in higher or lower contents increase either the susceptibility or resistance, respectively, to B. cinerea, while O2- serves as a first substrate for H2O2 formation [14,29,31] and supports B. cinerea attack [32]. “Summer black” leaves showed minor differences in POD activity with lesion development post-inoculation. Conversely, they showed elevated SOD activity, which is related with H2O2 production and O2- decline. The increased levels of POD activity were found in “Ju mei gui” and no substantial alteration was detected in SOD activity. Small amounts of ROS production are necessary for the antioxidative system to maintain redox equilibrium [33], and we also detected that the infected “Summer black” exhibited an inadequate antioxidative system, causing constantly higher ROS production.
Previously, it has been reported that the resistant genotype “Pingli-5” produced low ROS production and activated its antioxidant enzymes when interacting with B. cinerea, which is correlated with its pathogen resistance. Meanwhile, susceptible Red globe experienced severe pathogen infection and continuously produced ROS and was found to have relatively inactive antioxidative responses [28]. On the other hand, “Ju mei gui” displayed comparatively rapid changes in antioxidative capacity, particularly POD activity, and less ROS-induced stress. Significantly higher levels of ROS were detected in “Summer black” but not in “Ju mei gui” which may be a main feature in the capability of genotype “Ju mei gui” to protect itself against B. cinerea. These findings are in line with the previous studies in that the coordination between ROS production and scavenging mechanisms are related to the antioxidative system during biotic stress [34].
JA contents were measured in both leaves and berries of “Ju mei gui” and “Summer black” genotypes, and higher contents were found in “Ju mei gui.” We noted high JA contents in the “Ju mei gui” control, which were nearly equivalent to the JA contents observed in inoculated “Summer black” (Figure 8), which suggests that the presence of more contents of JA in “Ju mei gui” may act to control B. cinerea. These results are in accordance with the studies reported that high JA contents block B. cinerea infection and reinforce grape resistance to B. cinerea [35]. Furthermore, JA is a vital component in the plant defense responses against insects and microbial pathogens [36], is a major hormone involved in plant defense responses [37] and its production occurs relatively rapidly in plant tissues after interactions with fungal elicitors [21,38].
Finally, the resistance of “Ju mei gui” can attribute to minor fungal growth, less ROS production, elevated antioxidant enzyme activities, and more JA contents. Furthermore, serious fungal infection of “Summer black” and persistent ROS detection coincide with rather unaffected antioxidant functions and minute JA contents. This study provides an understanding into B. cinerea infection in grapevine, which can be a precious source for researchers by providing information for choosing appropriate grapevine genotypes.
4. Materials and Methods
4.1. Grape and Fungal Resources
Grape (leaves & berries) were acquired from the Grapevine garden (340 12’N, 1080 07’E) of Northwest Agriculture and Forestry University, Yangling, Shaanxi, China. The area is located 520 m above sea level. Annual mean temperature and rainfall are 12.9 °C and 660 mm, respectively. Maximum rainfall occurs between July and September. Twenty plants for each genotype were used for leaf and berry assessments. Leaves and berries were collected on the dates in the year 2019, i.e., June 5, (genotypes 1 to 10), June 11, (genotypes 11 to 20), and June 16, (genotypes 21 to 24). Before starting the experiment, B. cinerea spores were isolated from “Flame seedless” cultivar (V. vinifera) grown in a greenhouse located on the North campus of the Northwest A&F University, Shaanxi, China. Spores were cultured on a potato dextrose agar (PDA) medium at 22 °C. B. cinerea developed white to gray colonies on potato dextrose agar (PDA) culture medium and produced filamentous, hyaline, branched and septate mycelia with prominent cell walls. The conidia were unicellular, hyaline to slightly colored, smooth, ovoid to ellipsoid. Furthermore, the conidia were produced on short sterigmata on the swollen tips of aerial, branched conidiophores. Black, melanized, elongated or spherical sclerotia were produced under unfavorable conditions in vitro, which play an important role in pathogen survival, dispersal and multiplication. As far as the growth of the pathogen is concerned, the following developments were observed: at 4 hpi, there was no significant change; at 8 hpi, appressoria development was initiated and became visible at 12 hpi under microscope. The multiplication rate increased after 18 hpi with development of infection pegs; the hyphae were developed after 36 hpi and mycelium growth was seen after 48 hpi; the mycelium was increased on the subsequent time points till the sporulation; sporulation started at 120 hpi and covered the whole surface on day 21 having new sporulation. After this, the conidia were removed, and an optimum concentration of 1.5 x 106 spores·mL−1 for leaf assessment was prepared in sterilized water as earlier described by [28,39]. Similarly, for berry evaluation an optimum concentration of 1 × 105 spores·mL−1 was prepared [39,40]. The conidia suspension was assured to have a conidia/spore germination percentage of 95% or more before all experimentations.
4.2. Detached Leaf and Berry Evaluation
Detached leaf evaluation post-inoculation was done according to previous methods [28,39]. Leaves of the same size and age (from the shoot at nodes 3 and 4) were arbitrarily selected from the grape plants. The detached leaves were washed with distilled water. For laboratory assessment, 48 leaves from each of three replicates of each genotype were evaluated. The leaves were quickly transferred to trays with 0.8% agar and sprayed evenly with the conidial suspension. Control leaves were sprayed with distilled water. The trays were placed in an incubator with a relative humidity of 90–100%, for the first 24 h in the dark and then 12/12 h light/dark at 22 °C.
Berry evaluation was performed using grape berries of the same size and age, E-L 39 stage, as it is the optimum stage to study B. cinerea disease reactions [39,40,41]. Berries were randomly selected and harvested from the grape plants and washed several times with distilled water for research evaluation. One hundred and five berries from three replicates of individual genotypes were assessed. The berries were sprayed uniformly with the conidial suspension. Control berries were sprayed with distilled water and kept in an incubator with a relative humidity of more than 90% at 22 °C for 8 days duration.
4.3. Disease Severity Assessment
Disease severity was evaluated and rated as previously described [42] with slight modifications. The disease symptoms observed on the leaves were ranked from 1 to 7 (Rank 1 = 0.1–5.0%, 2 = 5.1–15.0%, 3 = 15.1–30.0%, 4 = 30.1–45.0%, 5 = 45.1–65%, 6 = 65.1–85.0% and 7 = 85.1–100.0%) on the basis of the estimated percentage of lesions over the entire leaf and berry surface. The ranking was then converted into a severity index (SI) according to the formula:
The resistance level was rated into four classes on the basis of the SI values. Disease resistance levels of the different genotypes were categorized as highly resistant (SI: 0–1.50), resistant (SI: 1.51–3.50), susceptible (SI: 3.51–5.50), and highly susceptible (SI: 5.51–7.0). Susceptibility data for the disease were collected in 2019. The average SI values of the data were used to evaluate the resistance level.
4.4. Microscopic Assessment of B. cinerea Development
One representative genotype from each category was used to characterize the colonization of the grape leaves and berries by B. cinerea using light microscopy. The following genotypes were used for each category: HR for “Ju mei gui,” and HS for “Summer black.” The leaf and berry skins were cut into 2–3 cm2 segments, and fixed and decolorized in 100% ethanol and in saturated chloral hydrate. The samples were stored in 50% glycerol and stained with aniline blue solution at the time of observation with an Olympus BX-51 microscope (Olympus, Tokyo, Japan) [43].
4.5. Scanning Electron Microscopy (SEM)
The structural growth of B. cinerea on leaves and berries of one representative genotype, “Ju mei gui” or “Summer black,” was observed using SEM (JEOL FESEM S-4800 scanning electron microscope, JEOL, Tokyo, Japan). Infected leaf and berry skins were cut into 1.0–1.5 cm2 pieces and immersed in 4% glutaraldehyde. After vacuum infiltration for 30 min, the infected leaf and berry skins were rinsed five times for 5, 10, 15, 20, 20 min, respectively, with 0.1 M sodium phosphate buffer (PBS) (pH 6.8). The segments were dehydrated in an ethanol gradient: 30%, 50%, and 70% for 15 min each; 80% and 90% for 20 min each; and 100% alcohols twice for 30 min. Finally, the samples were incubated in acetone for 30 min and isoamyl acetate twice for 15 and 30 min in three biological replicates. The segments were desiccated by CO2, coated with gold in a sputter coater, and then observed under a scanning electron microscope at 15 kV [43].
4.6. Reactive Oxygen Species
4.6.1. H2O2 Determination
H2O2 levels in “Ju mei gui” and “Summer black” leaves and berries were measured at various time points (0, 8, 24, 48, 72 hpi, and 0, 2, 4, 6, 8 dpi, respectively) as previously described [44].
4.6.2. O2- Determination
The “Ju mei gui” and “Summer black” leaf and berry O2- production rates were determined at time points 0, 8, 24, 48, 72 hpi, and 0, 2, 4, 6, 8 dpi, respectively, as previously described [45].
4.7. Antioxidant Enzymes Analyses
SOD activity was determined in extracts from “Ju mei gui” and “Summer black” leaves and berries at various time points (0, 8, 24, 48, 72 hpi, and 0, 2, 4, 6, 8 dpi, respectively) as previously described [26]. Similarly, POD activity was measured [46].
4.8. JA Measurements in “Ju mei gui” and “Summer black”
JA levels were measured in inoculated and control “Ju mei gui” and “Summer black” leaves and berries that were sampled at time points 0, 8, 24, 48, 72 hpi, and (0, 2, 4, 6, 8 dpi, respectively, and instantly frozen in liquid nitrogen. Further analysis was carried out as previously described [47] and JA was computed with a competitive enzyme-linked immunosorbent assay (ELISA) assay [48].
4.9. Statistical Analysis
For experimentation, three biological replicates were used with completely randomized design (CRD). Means and standard errors were calculated from independent replicates using SPSS 13.0. Least significant difference (LSD) 0.05 was used to calculate substantial variations in data 2019. All images were combined with the help of Adobe Photoshop. All graphs were prepared using the Origin Pro 2016 32-bit.
5. Conclusions
In this study, twenty-four grapevine genotypes were evaluated for leaf and berry resistance against B. cinerea, which revealed that one genotype was HR, one was R, eight genotypes were S, and fourteen were HS genotypes. Similarly, of the Vitis genotypes evaluated for grape berry: three genotypes were found to be HR, three resistant, eleven genotypes S, and seven were HS. Moreover, in the experimental results, it was indicated that the resistance of “Ju mei gui” can attribute to minor fungal growth, less ROS production, elevated antioxidant enzymes activity, and more JA contents. Furthermore, serious fungal infection of “Summer black” and persistent ROS detection coincide with rather unaffected antioxidant functions and minute JA contents. This study provides a basis for the understanding of B. cinerea infection and resistance mechanisms in resistant and susceptible cultivars of grapevine.
Author Contributions
Y.Z. and M.U.R. designed the experiments. M.U.R. and M.H. conducted the experiments. M.U.R. wrote the manuscript. Q.M. and B.A. helped with the experiments. Y.Z. critically reviewed the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Xiping Wang of Northwest A&F University for providing the plant materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tu, M.X.; Wang, X.H.; Feng, T.Y.; Sun, X.M.; Wang, Y.Q.; Huang, L.; Gao, M.; Wang, X.P. Expression of a grape (Vitis vinifera) bZIP transcription factor, VIbZIP36, in Arabidopsis thaliana confers tolerance of drought stress during seed germination and seedling establishment. Plant Sci. 2016, 252, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H. Grapevine breeding and genetics in China: History, current status and the future. Acta Hortic. 2015, 1082, 165–176. [Google Scholar]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D. The top fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Mengiste, T. Plant immunity to necrotrophs. Annu. Rev. Phytopathol. 2012, 50, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Cadle-davidson, L. Monitoring pathogenesis of natural. Vitis 2008, 4, 387–395. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Traité d’Oenologie Microbiologie du Vin et Vinifications; Dunod: Paris, France, 1998. [Google Scholar]
- Latorre, B.; Elfar, K.; Ferrada, E. Gray mold caused by Botrytis cinerea, limits grape production in Chile. Crop Protect. 2015, 42, 305–330. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 4, 133–141. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health. 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Peros, J.P.; Nguyen, T.H.; Troulet, C.; Michel-Romitti, C.; Notteghem, J.L. Assessment of powdery mildew resistance of grape and Erysiphe necator pathogenicity using laboratory assay. Vitis 2006, 45, 29–36. [Google Scholar]
- Liu, Y.; He, C. Regulation of plant reactive oxygen species (ROS) in stress responses: Learning from AtRBOHD. Plant Cell Rep. 2016, 35, 995–1007. [Google Scholar] [CrossRef]
- Temme, N.; Tudzynski, P. Does Botrytis cinerea ignore H2O2 -induced oxidative stress during infection? Characterization of botrytis activator protein 1. APS J. 2009, 22, 987–998. [Google Scholar]
- Dietz, K.J. Thiol-based peroxidases and ascorbate peroxidases: Why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar] [PubMed]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ahamadi, S.A.K.; Ebadi, A.; Jahanbakhsh, S. Changes in enzymatic and nonenzymatic antioxidant defense mechanisms of canola seedlings at different drought stress and nitrogen levels. Turkish J. Agric. 2015, 39, 601–612. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S. Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In Handbook of Plant and Crop Stress, 3rd ed.; Pessarakli, M., Ed.; CRC Press, Taylor and Francis Publishing Company: Boca Raton, FL, USA, 2010; pp. 89–138. [Google Scholar]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Kang, J.H.; Wang, L.; Giri, A.; Baldwin, I.T. Silencing threonine deaminase and JAR4 in nicotiana attenuate impairs jasmonic acid-isoleucine-mediated defenses against manduca sexta. Plant Cell 2006, 18, 3303–3320. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonate in plants. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef]
- Poolsawat, O.; Tharapreuksapong, A.; Wongkaew, S.; Chaowiset, W.; Tantasawat, P. Laboratory and field evaluations of resistance to Sphaceloma ampelinum causing anthracnose in grapevine. Australas. Plant Pathol. 2012, 41, 263–269. [Google Scholar]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- FAO. Prevention of Post-Harvest Food Losses: Fruits, Vegetables and Root Crops: A Training Manual; FAO: Rome, Italy, 1989. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Gabler, F.M.; Smilanick, J.L.; Mansour, M.; Ramming, D.W.; Mackey, B.E. Correlations of morphological, anatomical, and chemical features of grape berries with resistance to Botrytis cinerea. Phytopathology 2003, 93, 1263–1273. [Google Scholar] [CrossRef]
- Wan, R.; Hou, X.; Wang, X.; Qu, J.; Singer, S.D.; Wang, Y.; Wang, X. Resistance evaluation of Chinese wild Vitis genotypes against Botrytis cinerea and different responses of resistant and susceptible hosts to the infection. Front. Plant Sci. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.F.; Gabler, F.M.; Margosan, D.A.; Hashim-Buckey, J. Control of post-harvest gray mold of table grapes in the San Joaquin Valley of California by fungicides applied during the growing season. Plant Dis. 2010, 94, 250–257. [Google Scholar] [CrossRef]
- Asai, S.; Yoshioka, H. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen Botrytis cinerea in nicotiana benthamiana. APS J. 2009, 22, 619–629. [Google Scholar] [CrossRef]
- Asselbergh, B.; Curvers, K.; Franca, S.C.; Audenaert, K.; Vuylsteke, M.; Van Breusegem, F. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 2007, 144, 1863–1877. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Li, X.; Ouyang, Z.; Huang, L.; Hong, Y. The denovo biosynthesis of vitamin B6 is required for disease resistance against Botrytis cinerea in tomato. Mol. Plant-Microbe Interact. 2014, 27, 688–699. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. In Posidonia oceanica cadmium induces changes in DNA methylation and chromatin patterning. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar]
- Jia, H.; Zhang, C.; Pervaiz, T.; Zhao, P.; Liu, Z.; Wang, B. Jasmonic acid involves in grape fruit ripening and resistant against Botrytis cinerea. Funct. Integr. Genom. 2016, 16, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, M.; Van Dam, N.M.; Van Loon, J.J.A.; Dicke, M. Jasmonic acid-induced changes in brassica oleracea affect oviposition preference of two specialist herbivores. J. Chem. Ecol. 2007, 33, 655–668. [Google Scholar] [CrossRef]
- Li, C.; Schilmiller, A.L.; Liu, G.; Lee, G.I.; Jayanty, S.; Sageman, C. Role of beta-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell 2005, 17, 971–986. [Google Scholar] [CrossRef]
- Surya, S.; Li-Ling, C.; Kathleen, S.; Hui, G.; Chin-Feng, H. A phenotypic study of Botrytis bunch rot resistance in Vitis aestivalis-derived ‘Norton’ grape. Trop. Plant Pathol. 2015, 40, 279–282. [Google Scholar]
- Rahman, M.U.; Hanif, M.; Wan, R.; Hou, X.; Ahmad, B.; Wang, X. Screening Vitis genotypes for responses to Botrytis cinerea and evaluation of antioxidant enzymes, reactive oxygen species and jasmonic acid in resistant and susceptible hosts. Molecules 2019, 24, 5. [Google Scholar] [CrossRef]
- Coombe, B.G. Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Li, D.; Wan, Y.; Wang, Y.; He, P. Relatedness of resistance to anthracnose and to white rot in Chinese wild grapes. Vit. J. Grapevine Res. 2008, 47, 213–215. [Google Scholar]
- Rahman, M.U.; Hanif, M.; Shah, K.; Ahmad, B.; Wang, X. In vitro evaluation of berries of various Vitis genotypes for disease resistance to Botrytis cinerea. Vitis 2019, 58, 123–130. [Google Scholar]
- Moloi, M.J.; Westhuizen, A.J. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J. Plant Physiol. 2006, 163, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Elstner, E.F.; Heupel, A. Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib). Planta 1976, 320, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. Biol. Roles Copp. 1977, 59, 125–142. [Google Scholar]
- Royo, J.; Leon, J.; Vancanneyt, G.; Albar, J.P.; Rosahl, S.; Ortego, F. Antisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insect pests. Proc. Natl. Acad. Sci. USA 1999, 96, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.; Kehlen, A.; Stahl, K.; Knöfel, H.D.; Sembdner, G.; Weiler, E.W. Quantification of rapid, transient increases in jasmonic acid in wounded plants using a monoclonal antibody. Planta 1993, 191, 86–94. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).