Carp Edema Virus and Cyprinid Herpesvirus-3 Coinfection is Associated with Mass Mortality of Koi (Cyprinus carpio haematopterus) in the Republic of Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Sampling and Necropsy
2.2. DNA Preparation and PCR Amplification
2.3. Cloning and Sequencing of the PCR Products
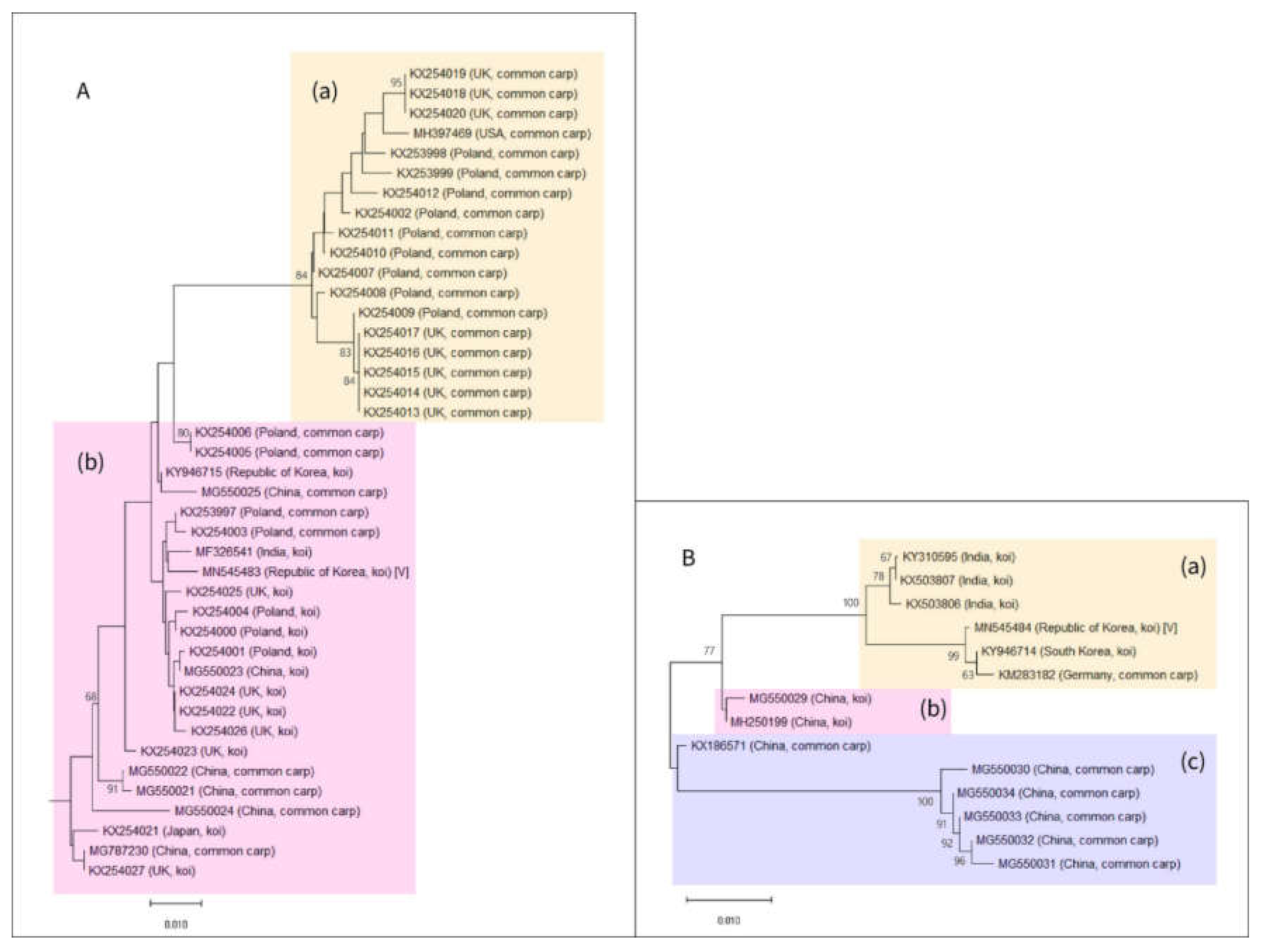
2.4. Phylogenetic Relationship Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (SOFIA); FAO Fisheries and Aquaculture Department: Rome, Italy, 2014; Available online: http://www.fao.org/3/a-i3720e.pdf (accessed on 15 December 2019).
- FAO. Fishery and Aquaculture Statistics. Global Production by Production Source 1950–2015 (FishstatJ). Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 10 December 2019).
- Kim, D.; Kang, J. Improvement of ornamental fish industry through analysis of recognition and market scale of the ornamental fish. J. Fish. Bus. Adm. 2012, 43, 89–106. [Google Scholar] [CrossRef]
- Haenen, O.L.; Way, M.K.; Gorgoglione, B.; Ito, T.; Paley, R.; Bigarre, L.; Waltzek, T. Novel viral infections threatening Cyprinid fish. Bull. Eur. Assoc. Fish Pathol. 2016, 36, 11–23. [Google Scholar]
- Murakami, Y.; Shitanaka, M.; Toshida, S.; Matsuzato, T. Studies on mass mortality of juvenile carp: About mass mortality showing edema. Bull. Hiroshima Fresh Water Fish Exp. Stn. 1976, 19–33. [Google Scholar]
- Ono, S.; Nagai, A.; Sugai, N. A histopathological study on juvenile colorcarp, Cyprinus carpio, showing edema. Fish Pathol. 1986, 21, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Lewisch, E.; Gorgoglione, B.; Way, K.; El-Matbouli, M. Carp edema virus/Koi sleepy disease: An emerging disease in Central-East Europe. Transbound. Emerg. Dis. 2015, 62, 6–12. [Google Scholar] [CrossRef]
- Kim, S.W.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, H.J.; Kim, S.G.; Kang, J.W.; Park, S.C. First report of carp oedema virus infection of koi (Cyprinus carpio haematopterus) in the Republic of Korea. Transbound. Emerg. Dis. 2018, 65, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Matras, M.; Borzym, E.; Stone, D.; Way, K.; Stachnik, M.; Maj-Paluch, J.; Palusinska, M.; Reichert, M. Carp edema virus in Polish aquaculture—Evidence of significant sequence divergence and a new lineage in common carp Cyprinus carpio (L.). J. Fish Dis. 2017, 40, 319–325. [Google Scholar] [CrossRef]
- Ouyang, P.; Yang, R.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Fang, J.; Chen, Z.; et al. First detection of carp edema virus in association with cyprinid herpesvirus 3 in cultured ornamental koi, Cyprinus carpio L., in China. Aquaculture 2018, 490, 162–168. [Google Scholar] [CrossRef]
- Bretzinger, A.; Fischer-Scherl, T.; Oumouna, M.; Hoffmann, R.; Truyen, U. Mass mortalities in koi carp, Cyprinus carpio, associated with gill and skin disease. Bull. Eur. Assoc. Fish Pathol. 1999, 19, 182–185. [Google Scholar]
- Perelberg, A.; Smirnov, M.; Hutoran, M.; Diamant, A.; Bejerano, Y.; Kotler, M. Epidemilogical Description of a New Viral Disease Afflicting Cultured Cyprinus Carpio In Israel. Isr. J. Aquac. 2003, 55, 5–12. [Google Scholar]
- Haenen, O.L.; Way, M.K.; Bergmann, S.M.; Areil, E. The emergence of koi herpesvirus and its significance to European aquaculture. Bull. Eur. Assoc. Fish Pathol. 2004, 24, 293–307. [Google Scholar]
- Sano, M.; Ito, T.; Kurita, J.; Yanai, T.; Watanabe, N.; Miwa, S.; Iida, T. First detection of koi herpesvirus in cultured common carp Cyprinus carpio in Japan. Fish Pathol. 2004, 39, 165–167. [Google Scholar] [CrossRef]
- Kurita, J.; Yuasa, K.; Ito, T.; Sano, M.; Hedrick, R.P.; Engelsma, M.Y.; Haenen, O.L.M.; Sunarto, A.; Kholidin, E.B.; Chou, H.; et al. Molecular epidemiology of koi herpesvirus. Fish Pathol. 2009, 44, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Taylor, N.G.H.; Dixon, P.F.; Jeffery, K.R.; Peeler, E.J.; Denham, K.L.; Way, K. Koi herpesvirus: Distribution and prospects for control in England and Wales. J. Fish Dis. 2010, 33, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.K.; Joh, S.J.; Jang, H.; Shin, S.P.; Choresca, C.H., Jr.; Han, J.E.; Kim, J.H.; Jun, J.W.; Park, S.C. Detection of koi herpesvirus (KHV) from koi (Cyprinus carpio koi) broodstock in South Korea. Aquaculture 2011, 311, 42–47. [Google Scholar] [CrossRef]
- Cho, M.Y.; Won, K.M.; Han, H.; Kim, H.J.; Jee, B.; Kim, S.; Lee, S.J.; Kim, J.W.; Park, M.A. Current status of detection of aquatic animal pathogens in cultured juveniles for stock enhancement from 2009 to 2012. J. Fish Pathol. 2013, 26, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Hedrick, R.P.; Gilad, O.; Yun, S.C.; Mcdowell, T.S.; Waltzek, T.B.; Kelley, G.O.; Adkison, M.A. Initial isolation and characterization of a herpes-like virus (KHV) from koi and common carp. Bull. Fish. Res. Agency 2005, 2, 1–7. [Google Scholar]
- Zhou, J.; Wang, Z.; YE, Y.; Wu, Q. PCR-RFLP analysis of mitochondrial DNA ND5/6 region among 3 subspecies of common carp (Cyprinus carpio L.) and its application to genetic discrimination of subspecies. Chin. Sci. Bull. 2003, 48, 465–468. [Google Scholar] [CrossRef]
- Oyamatsu, T.; Matoyama, H.; Yamamoto, K.; Fukuda, H. A trial for the Detection of Carp Edema Virus by Using Polymerase Chain Reaction. Aquac. Sci. 1997, 45, 247–251. [Google Scholar]
- Bercovier, H.; Fishman, Y.; Nahary, R.; Sinai, S.; Zlotkin, A.; Eyngor, M.; Gilad, O.; Eldar, A.; Hedrick, R.P. Cloning of the koi herpesvirus (KHV) gene encoding thymidine kinase and its use for a highly sensitive PCR based diagnosis. BMC Microbiol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Gilad, O.; Yun, S.; Andree, K.B.; Adkison, M.A.; Zlotkin, A.; Bercovier, H.; Eldar, A.; Hedrick, R.P. Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi. Dis. Aquat. Org. 2002, 48, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigarre, L.; Baud, M.; Cabon, J.; Antychowicz, J.; Bergmann, S.M.; Engelsma, M.; Pozet, F.; Reichert, M.; Castric, J. Differentiation between Cyprinid herpesvirus type-3 lineages using duplex PCR. J. Virol. Methods 2009, 158, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Adamek, M.; Jung-Schroers, V.; Hellmann, J.; Teitge, F.; Bergmann, S.M.; Runge, M.; Kleingeld, D.W.; Way, K.; Stone, D.M.; Steinhagen, D. Concentration of carp edema virus (CEV) DNA in koi tissues affected by koi sleepy disease (KSD). Dis. Aquat. Org. 2016, 119, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilad, O.; Yun, S.; Zagmutt-Vergara, F.J.; Leutenegger, C.M.; Bercovier, H.; Hedrick, R.P. Concentrations of a Koi herpesvirus (KHV) in tissues of experimentally infected Cyprinus carpio koi as assessed by real-time TaqMan PCR. Dis. Aquat. Org. 2004, 60, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Holopainen, R.; Honkanen, J.; Jensen, B.B.; Ariel, E.; Tapiovaara, H. Quantitation of ranaviruses in cell culture and tissue samples. J. Virol. Methods 2011, 171, 225–233. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [Green Version]
- Tempero, G.W.; Ling, N.; Hicks, B.J.; Osborne, M.W. Age composition, growth, and reproduction of koi carp (Cyprinus carpio) in the lower Waikato region, New Zealand. N. Zeal. J. Mar. Fresh. 2010, 40, 571–583. [Google Scholar] [CrossRef]
- Adamek, M.; Oschilewski, A.; Wohlsein, P.; Jung-Schroers, V.; Teitge, F.; Dawson, A.; Gela, D.; Piackova, V.; Kocour, M.; Adamek, J.; et al. Experimental infections of different carp strains with the carp edema virus (CEV) give insights into the infection biology of the virus and indicate possible solutions to problems caused by koi sleepy disease (KSD) in carp aquaculture. Vet. Res. 2017, 48, 12. [Google Scholar] [CrossRef] [Green Version]
- World Organisation for Animal Health (OIE). Chapter 2.3.7. Infection with Koi Herpesvirus. In Manual of Diagnostic Tests for Aquatic Animals; World Organisation for Animal Health: Paris, France, 2019; Available online: https://www.oie.int/index.php?id=2439&L=0&htmfile=chapitre_koi_herpesvirus.htm (accessed on 10 February 2020).
- Aoki, T.; Hirono, I.; Kurokawa, K.; Fukuda, H.; Nahary, R.; Eldar, A.; Davison, A.J.; Waltzek, T.B.; Bercovier, H.; Hedrick, R.P. Genome sequences of three koi herpesvirus isolates representing the expanding distribution of an emerging disease threatening koi and common carp worldwide. Am. Soc. Microbiol. 2007, 81, 5058–5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Kwon, S.R. Evidence for two koi herpesvirus (KHV) genotypes in South Korea. Dis. Aquat. Org. 2013, 104, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.W.; Giri, S.S.; Kim, S.G.; Kwon, J.; Oh, W.T.; Park, S.C. Carp Edema Virus and Cyprinid Herpesvirus-3 Coinfection is Associated with Mass Mortality of Koi (Cyprinus carpio haematopterus) in the Republic of Korea. Pathogens 2020, 9, 222. https://doi.org/10.3390/pathogens9030222
Kim SW, Giri SS, Kim SG, Kwon J, Oh WT, Park SC. Carp Edema Virus and Cyprinid Herpesvirus-3 Coinfection is Associated with Mass Mortality of Koi (Cyprinus carpio haematopterus) in the Republic of Korea. Pathogens. 2020; 9(3):222. https://doi.org/10.3390/pathogens9030222
Chicago/Turabian StyleKim, Sang Wha, Sib Sankar Giri, Sang Guen Kim, Jun Kwon, Woo Taek Oh, and Se Chang Park. 2020. "Carp Edema Virus and Cyprinid Herpesvirus-3 Coinfection is Associated with Mass Mortality of Koi (Cyprinus carpio haematopterus) in the Republic of Korea" Pathogens 9, no. 3: 222. https://doi.org/10.3390/pathogens9030222
APA StyleKim, S. W., Giri, S. S., Kim, S. G., Kwon, J., Oh, W. T., & Park, S. C. (2020). Carp Edema Virus and Cyprinid Herpesvirus-3 Coinfection is Associated with Mass Mortality of Koi (Cyprinus carpio haematopterus) in the Republic of Korea. Pathogens, 9(3), 222. https://doi.org/10.3390/pathogens9030222





