Development of Monoclonal Antibody Specific to Foot-and-Mouth Disease Virus Type A for Serodiagnosis
Abstract
1. Introduction
2. Results
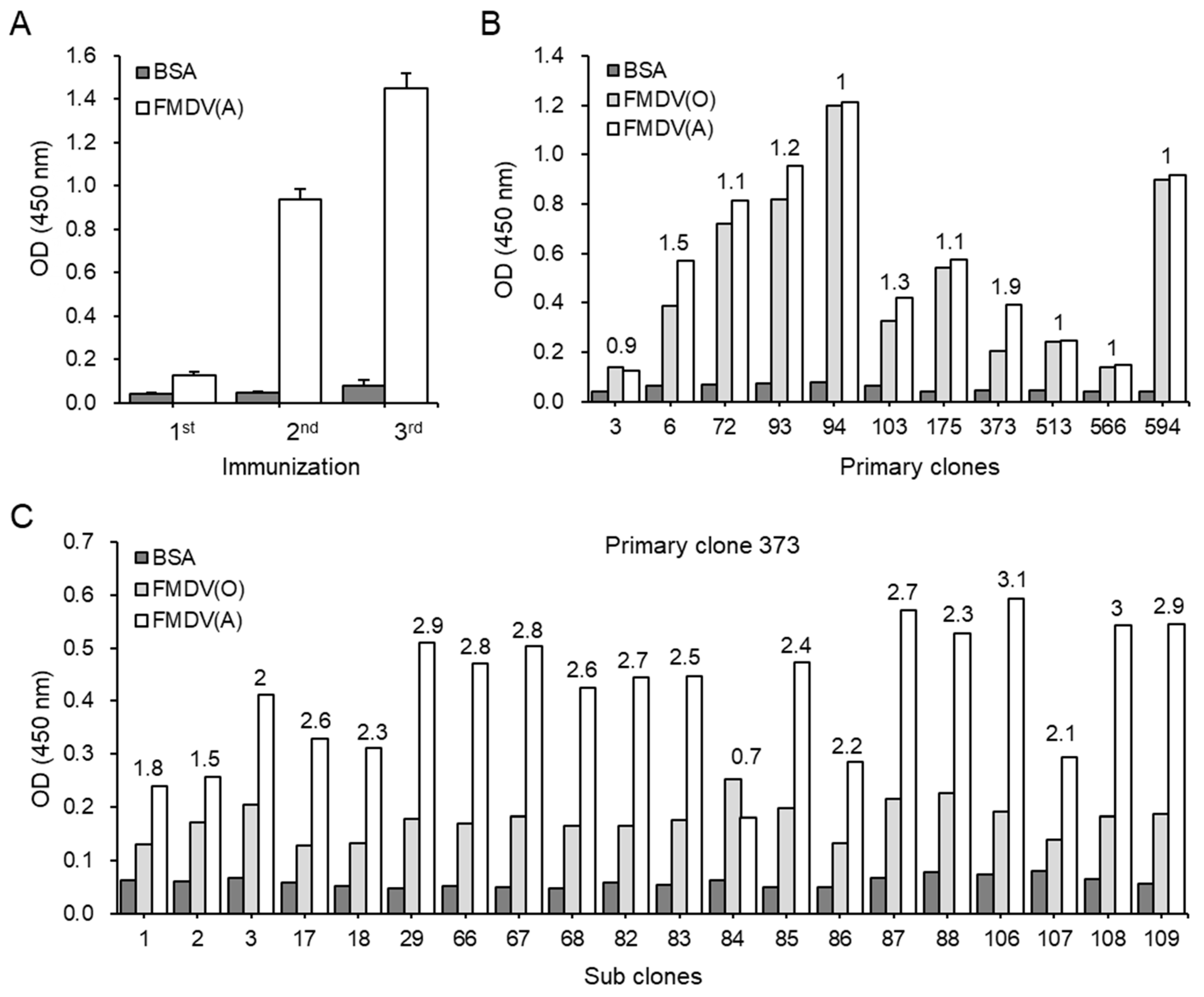
2.1. Production of Anti-FMDV Type A mAbs
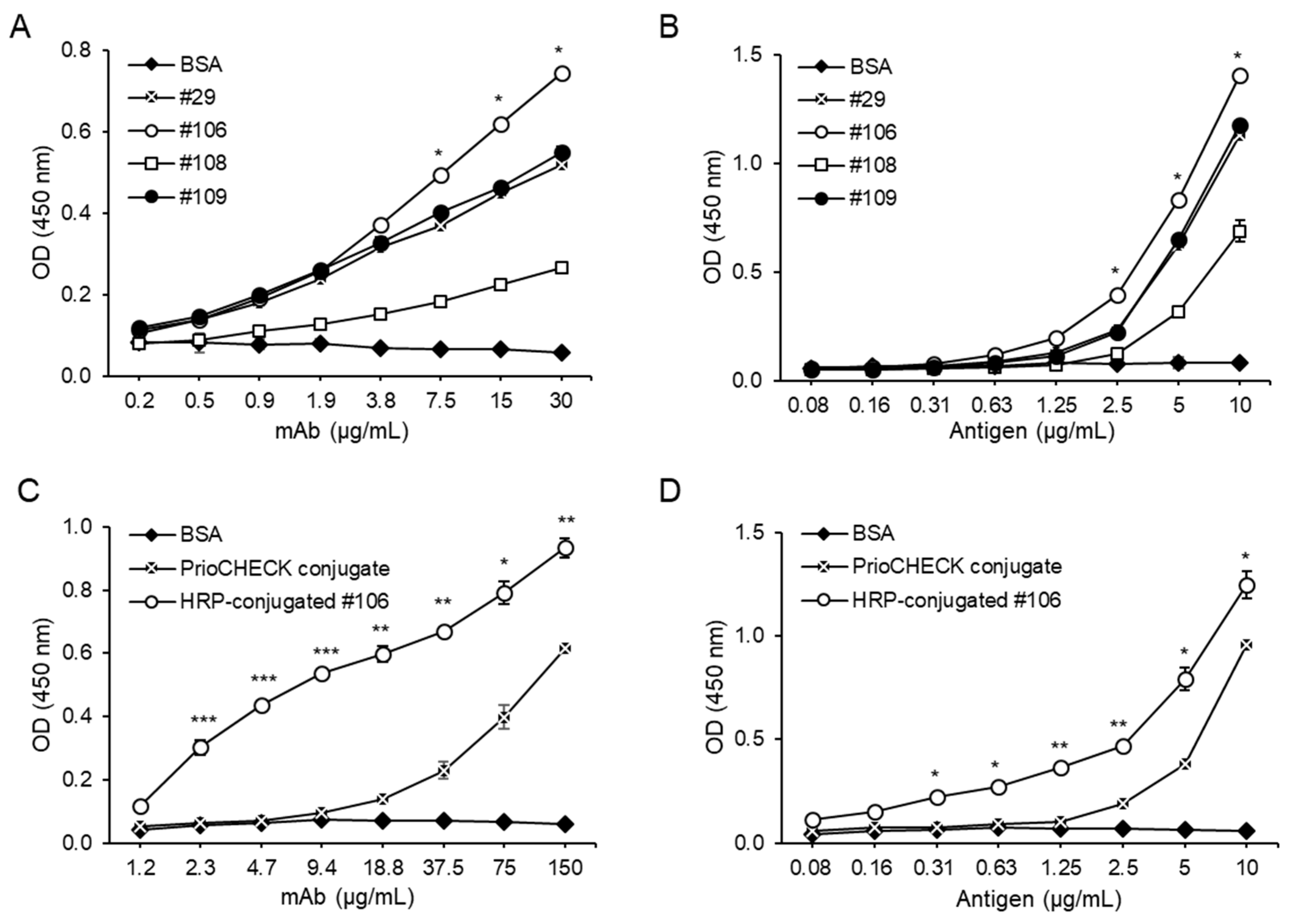
2.2. Comparison of Binding Reactivity and Characterization of the mAbs Against FMDV Type A
2.3. Specificity of the mAb against FMDV Serotypes
2.4. Evaluation of the mAb in SPCE for Detection of SP Abs from FMDV Type A-Vaccinated Cattle
3. Discussion
4. Materials and Methods
4.1. Mouse Immunization and Hybridoma Preparation
4.2. Isotype Identification
4.3. Conjugation of mAb with HRP
4.4. ELISA
4.5. Cattle Sample Collection
4.6. SPCE
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pereira, H. Foot-and-mouth disease. In Virus Disease of Food Animals; Gibbs, E.P.J., Ed.; ACA Press Inc: London, UK, 1981; Volume 56, pp. 333–363. [Google Scholar]
- Knight-Jones, T.; Rushton, J. The economic impacts of foot and mouth disease—What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Pendell, D.L.; Leatherman, J.; Schroeder, T.C.; Alward, G.S. The economic impacts of a foot-and-mouth disease outbreak: A regional analysis. Am. J. Agric. Econ. 2007, 39, 19–33. [Google Scholar] [CrossRef]
- Perry, B.D.; Rich, K.M. Poverty impacts of foot-and-mouth disease and the poverty reduction implications of its control. Vet. Rec. 2007, 160, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.M.; Belsham, G.J. Foot-and-mouth disease: Past, present and future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Di-Nardo, A.; Henstock, M. OIE/FAO Foot-and-Mouth Disease Reference Laboratory Network: Annual Report 2017. In Proceedings of the OIE/FAO FMD Laboratory Network Meeting, Pretoria, South Africa, 29–30 November 2017; pp. 6–17. [Google Scholar]
- Park, M.E.; You, S.H.; Lee, S.Y.; Lee, K.N.; Ko, M.K.; Choi, J.H.; Kim, B.; Lee, J.S.; Park, J.H. Immune responses in pigs and cattle vaccinated with half-volume foot-and-mouth disease vaccine. J. Vet. Sci. 2017, 18, 323–331. [Google Scholar] [CrossRef]
- Choi, J.H.; Jeong, K.; Kim, S.M.; Ko, M.K.; You, S.H.; Lyoo, Y.S.; Kim, B.; Ku, J.M.; Park, J.H. Synergistic effect of ribavirin and vaccine for protection during early infection stage of foot-and-mouth disease. J. Vet. Sci. 2018, 19, 788–797. [Google Scholar] [CrossRef]
- Horsington, J.; Nfon, C.; Bittner, H.; Durr, P.A.; Singanallur, N.; Alexandersen, S.; Vosloo, W. The protective capacity of high payload FMDV A22 IRQ vaccine in sheep against direct-contact challenge with a heterologous, contemporary FMDV A strain from South East Asia. PLoS ONE 2018, 13, e0195302. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2008. [Google Scholar]
- Clavijo, A.; Wright, P.; Kitching, P. Developments in diagnostic techniques for differentiating infection from vaccination in foot-and-mouth disease. Vet. J. 2004, 167, 9–22. [Google Scholar] [CrossRef]
- Haas, B. Application of the FMD liquid-phase blocking sandwich ELISA. Problems encountered in import/export serology and possible solutions. In Proceedings of the Session of the Research Group of the European Commission for the Control of Foot and Mouth Disease, Vienna, Austria, 19–22 September 1994; pp. 19–29. [Google Scholar]
- Mackay, D.K.; Bulut, A.N.; Rendle, T.; Davidson, F.; Ferris, N.P. A solid-phase competition ELISA for measuring antibody to foot-and-mouth disease virus. J. Virol. Methods 2001, 97, 33–48. [Google Scholar] [CrossRef]
- Paiba, G.A.; Anderson, J.; Paton, D.J.; Soldan, A.W.; Alexandersen, S.; Corteyn, M.; Wilsden, G.; Hamblin, P.; MacKay, D.K.; Donaldson, A.I. Validation of a foot-and-mouth disease antibody screening solid-phase competition ELISA (SPCE). J. Virol. Methods 2004, 115, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Sevik, M.; Ozturk, F.F.J.T.J.O.V.; Sciences, A. Comparative evaluation of liquid-phase blocking ELISA and solid-phase competition ELISA methods for the detection of antibodies to the structural proteins of foot-and-mouth disease types O and A viruses. Turk. J. Vet. Anim. Sci. 2013, 37, 523–528. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Ko, Y.; Kim, S.; Lee, H.; Park, J.; Yeh, J.; Kim, M.; Lee, Y.; Sohn, H. Diagnosis and Control Measures of the 2010 Outbreak of Foot-and-Mouth Disease A Type in the Republic of Korea. Transbound. Emerg. Dis. 2013, 60, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Park, W.; King, D.P.; Kim, H. Phylogenomics and molecular evolution of foot-and-mouth disease virus. Mol. Cells 2011, 31, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, G.B. Horseradish peroxidase labeling of IgG antibody. In The Protein Protocols Handbook; Human Press: Totowa, NJ, USA, 2009; pp. 681–683. ISBN 978-1-58829-880-5. [Google Scholar]
- Pinto, J. Foot-and-Mouth Disease Situation Food and Agriculture Organization of the United Nations Monthly Report; FAO: Roma, Italy, 2017; pp. 21–25. [Google Scholar]
- FAO calls for urgent action to tackle FMD outbreak in Egypt. Vet. Rec. 2012, 170, 350.
- Ahmed, H.; Salem, S.; Habashi, A.; Arafa, A.; Aggour, M.; Salem, G.; Gaber, A.; Selem, O.; Abdelkader, S.; Knowles, N.J.T.; et al. Emergence of Foot-and-Mouth Disease Virus SAT 2 in Egypt During 2012. Transbound. Emerg. Dis. 2012, 59, 476–481. [Google Scholar] [CrossRef]
- Ducancel, F.; Muller, B.H. Molecular engineering of antibodies for therapeutic and diagnostic purposes. MAbs 2012, 4, 445–457. [Google Scholar] [CrossRef]
- Tiller, K.E.; Tessier, P.M. Advances in Antibody Design. Annu. Rev. Biomed. Eng. 2015, 17, 191–216. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Brenner, J.S.; Motley, W.; Parhiz, H.; Greineder, C.F.; Villa, C.H.; Marcos-Contreras, O.A.; Tsourkas, A.; Muzykantov, V.R. Molecular engineering of antibodies for site-specific covalent conjugation using CRISPR/Cas9. Sci. Rep. 2018, 8, 1760. [Google Scholar] [CrossRef]
- Abubakar, M.; Kanwal, S.; Saeed, A. Persistence, emergence and distribution of foot and mouth disease virus (FMDV); global and pakistan perspectives. Pak. J. Life Soc. Sci. 2012, 10, 84–90. [Google Scholar]
- Sobrino, F.; Saiz, M.; Jimenez-Clavero, M.A.; Nunez, J.I.; Rosas, M.F.; Baranowski, E.; Ley, V. Foot-and-mouth disease virus: A long known virus, but a current threat. Vet. Res. 2001, 32, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.L.; Jeong, J.Y.; Choi, H.Y.; Zhang, Y.; Liu, Y.; Lee, H.J.; Choi, J.C.; Lee, S.H.; Lee, B.J.; Lee, S.W.; et al. Evaluation and optimization of a conventional SPCE for FMD post-vaccination monitoring. BMC Vet. Res. 2018, 14, 371. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Li, J.; Shao, J.J.; Cong, G.Z.; Du, J.Z.; Gao, S.D.; Chang, H.Y. Develope monoclonal antibody against foot-and-mouth disease virus A type. Virol. Sin. 2011, 26, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Jo, Y.J.; Sung, J.H.; Hong, J.K.; Hwang, J.H.; Park, J.H.; Lee, K.N.; Park, S.G. Monoclonal and polyclonal antibodies specific for foot and mouth disease virus type A and type O VP1. Hybridoma 2012, 31, 358–363. [Google Scholar] [CrossRef]
- Yang, M.; Xu, W.; Bittner, H.; Horsington, J.; Vosloo, W.; Goolia, M.; Lusansky, D.; Nfon, C. Generation of mAbs to foot-and-mouth disease virus serotype A and application in a competitive ELISA for serodiagnosis. Virol. J. 2016, 13, 195. [Google Scholar] [CrossRef]
- Heo, C.K.; Woo, M.K.; Yu, D.Y.; Lee, J.Y.; Yoo, J.S.; Yoo, H.S.; Ko, J.H.; Kim, J.M.; Choi, J.Y.; Kim, I.G.; et al. Identification of autoantibody against fatty acid synthase in hepatocellular carcinoma mouse model and its application to diagnosis of HCC. Int. J. Oncol. 2010, 36, 1453–1459. [Google Scholar]
| MAb | Isotype | Light chain | EC50 against Inactivated FMDV Type A (μg/mL) |
|---|---|---|---|
| #29 | IgG2b | κ | 12 |
| #106 | IgG2b | κ | 5 |
| #108 | IgG2b | κ | 124 |
| #109 | IgG2b | κ | 10 |
| HRP-conjugated #106 | IgG2b | κ | 5 |
| PrioCHECK conjugate | IgG1 | κ | 69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.T.; Yang, J.; Byun, J.-W.; Pyo, H.M.; Park, M.-Y.; Ku, B.K.; Nah, J.; Ryoo, S.; Wee, S.-H.; Choi, K.-S.; et al. Development of Monoclonal Antibody Specific to Foot-and-Mouth Disease Virus Type A for Serodiagnosis. Pathogens 2019, 8, 301. https://doi.org/10.3390/pathogens8040301
Nguyen QT, Yang J, Byun J-W, Pyo HM, Park M-Y, Ku BK, Nah J, Ryoo S, Wee S-H, Choi K-S, et al. Development of Monoclonal Antibody Specific to Foot-and-Mouth Disease Virus Type A for Serodiagnosis. Pathogens. 2019; 8(4):301. https://doi.org/10.3390/pathogens8040301
Chicago/Turabian StyleNguyen, Quyen Thi, Jihyun Yang, Jae-Won Byun, Hyun Mi Pyo, Mi-Young Park, Bok Kyung Ku, Jinju Nah, Soyoon Ryoo, Sung-Hwan Wee, Kang-Seuk Choi, and et al. 2019. "Development of Monoclonal Antibody Specific to Foot-and-Mouth Disease Virus Type A for Serodiagnosis" Pathogens 8, no. 4: 301. https://doi.org/10.3390/pathogens8040301
APA StyleNguyen, Q. T., Yang, J., Byun, J.-W., Pyo, H. M., Park, M.-Y., Ku, B. K., Nah, J., Ryoo, S., Wee, S.-H., Choi, K.-S., & Poo, H. (2019). Development of Monoclonal Antibody Specific to Foot-and-Mouth Disease Virus Type A for Serodiagnosis. Pathogens, 8(4), 301. https://doi.org/10.3390/pathogens8040301





