Tuberculosis Epidemiology and Badger (Meles meles) Spatial Ecology in a Hot-Spot Area in Atlantic Spain
Abstract
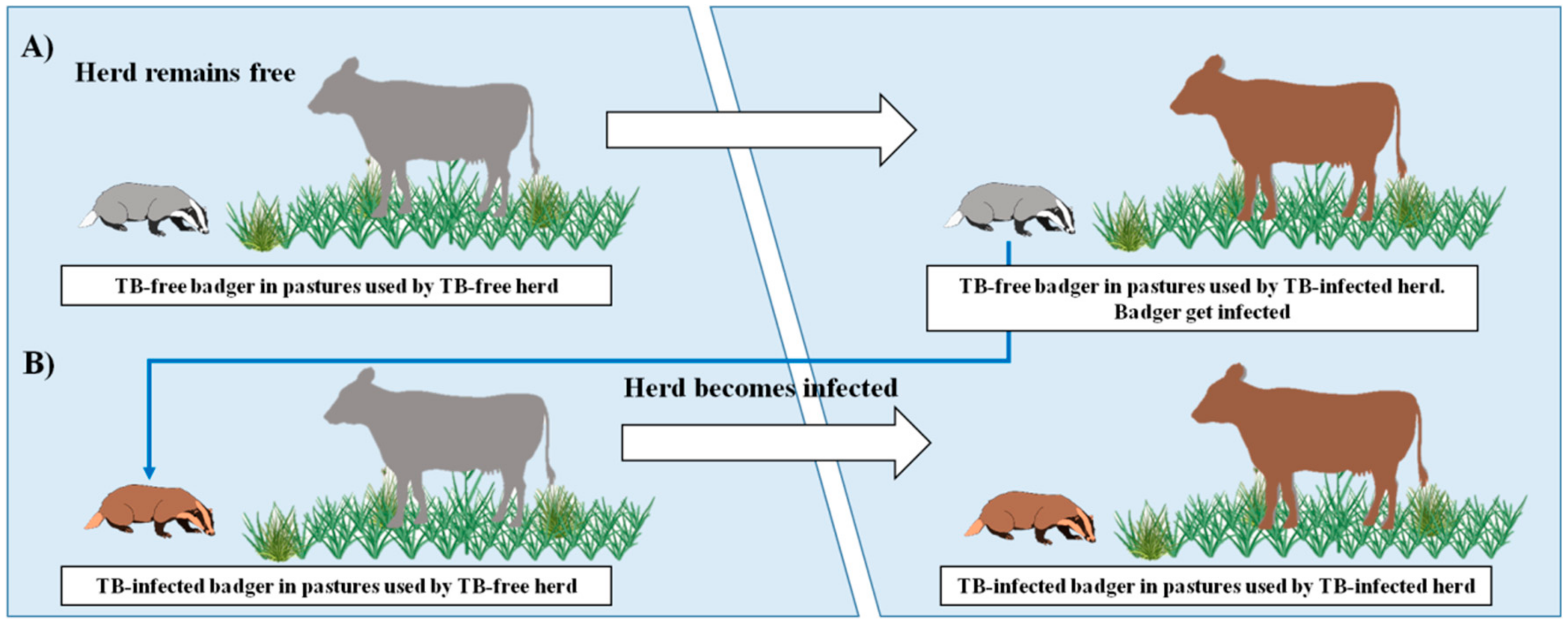
1. Introduction
2. Results
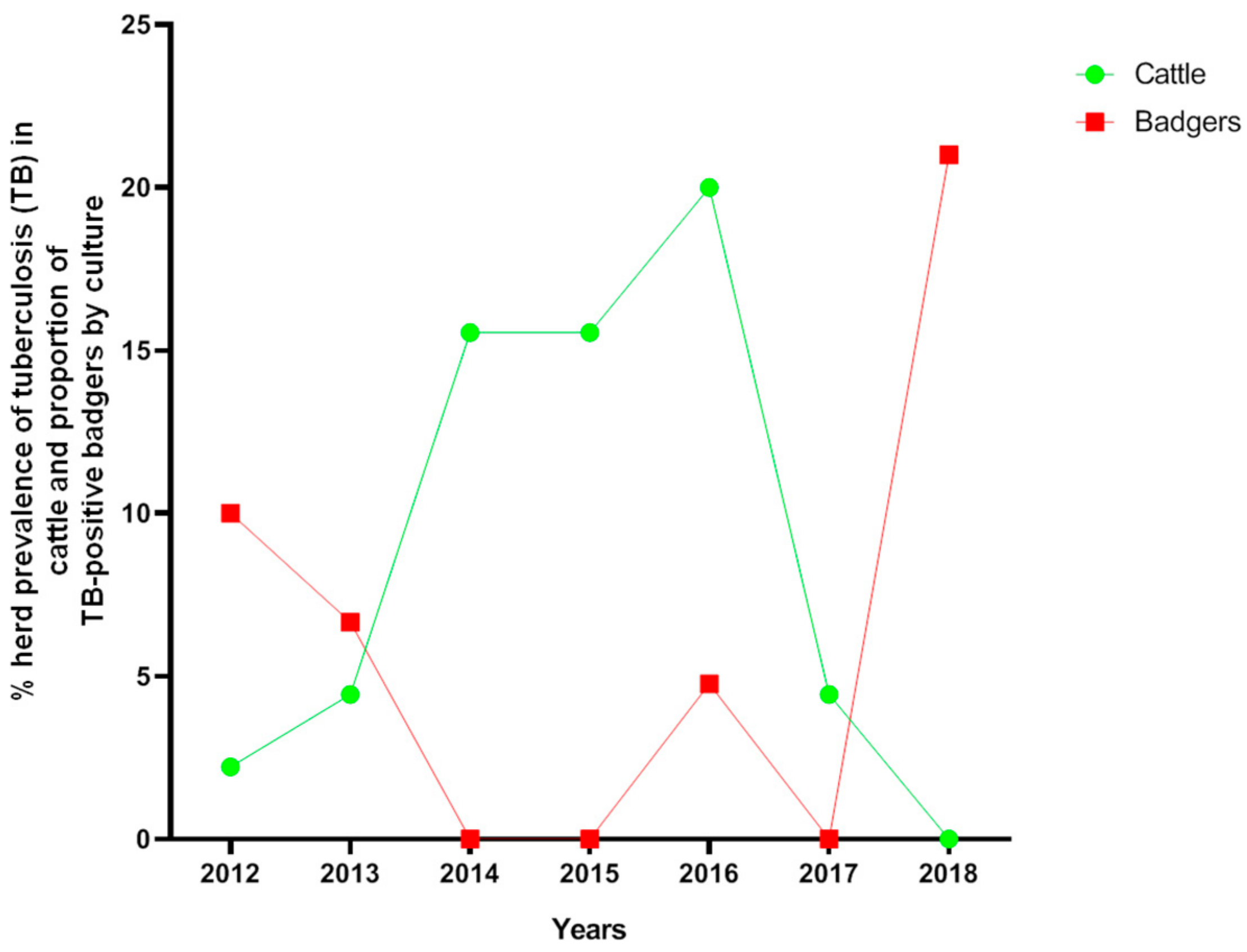
2.1. Medium-Term Description (2012–2018) of TB in Both Cattle and Badgers in the Hot-Spot Area
2.1.1. Cattle
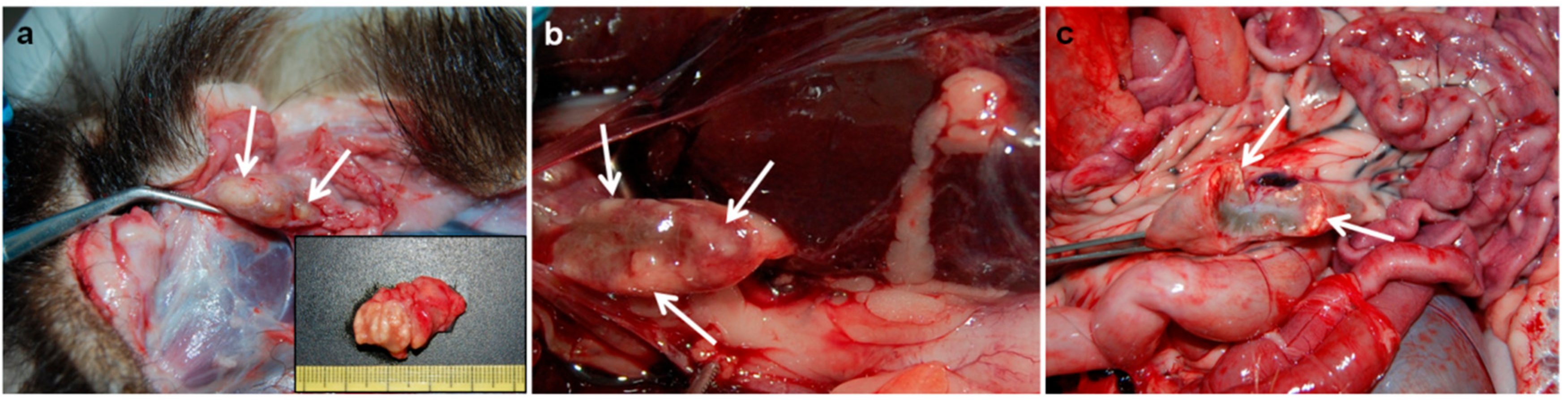
2.1.2. Badgers
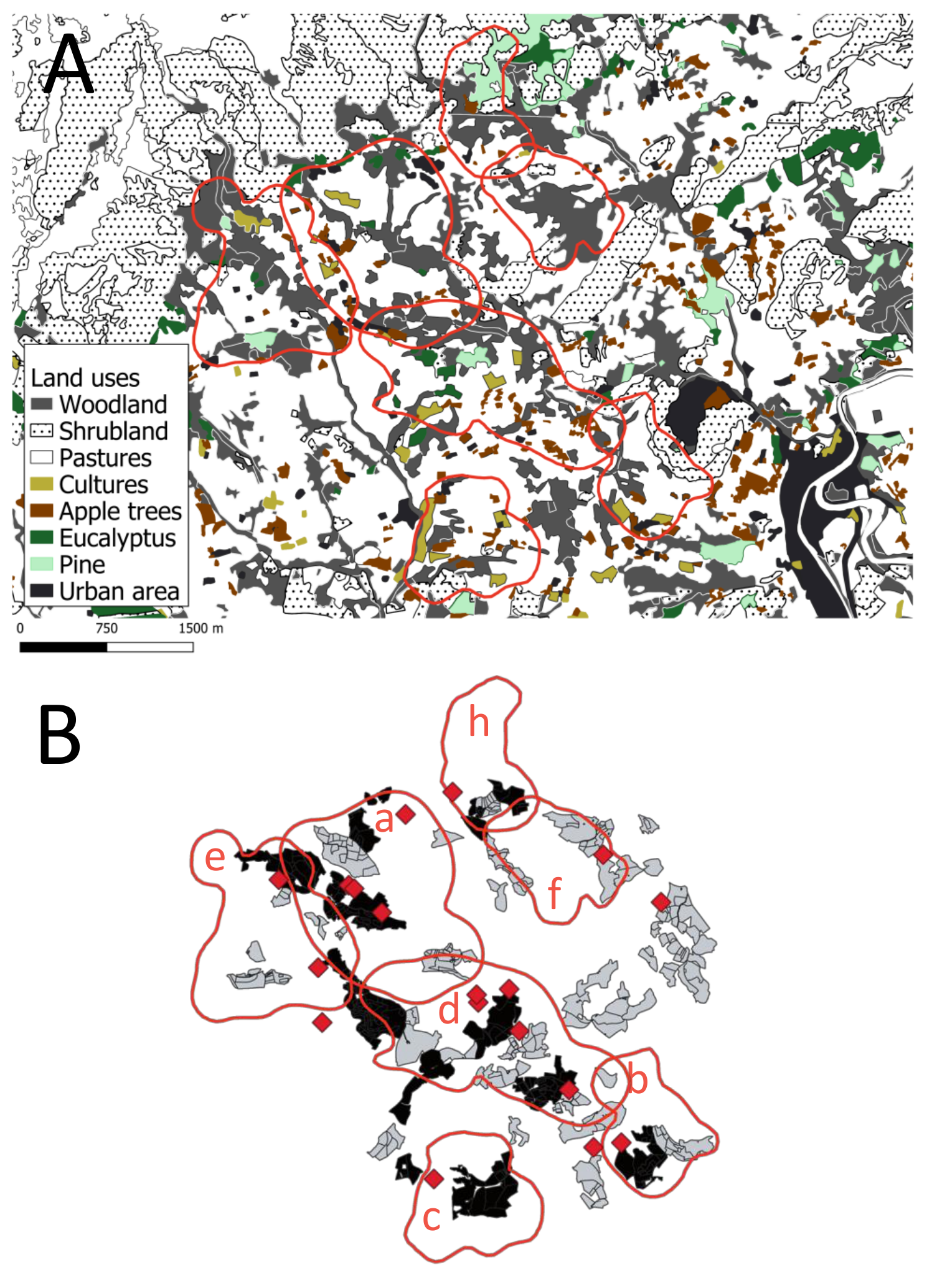
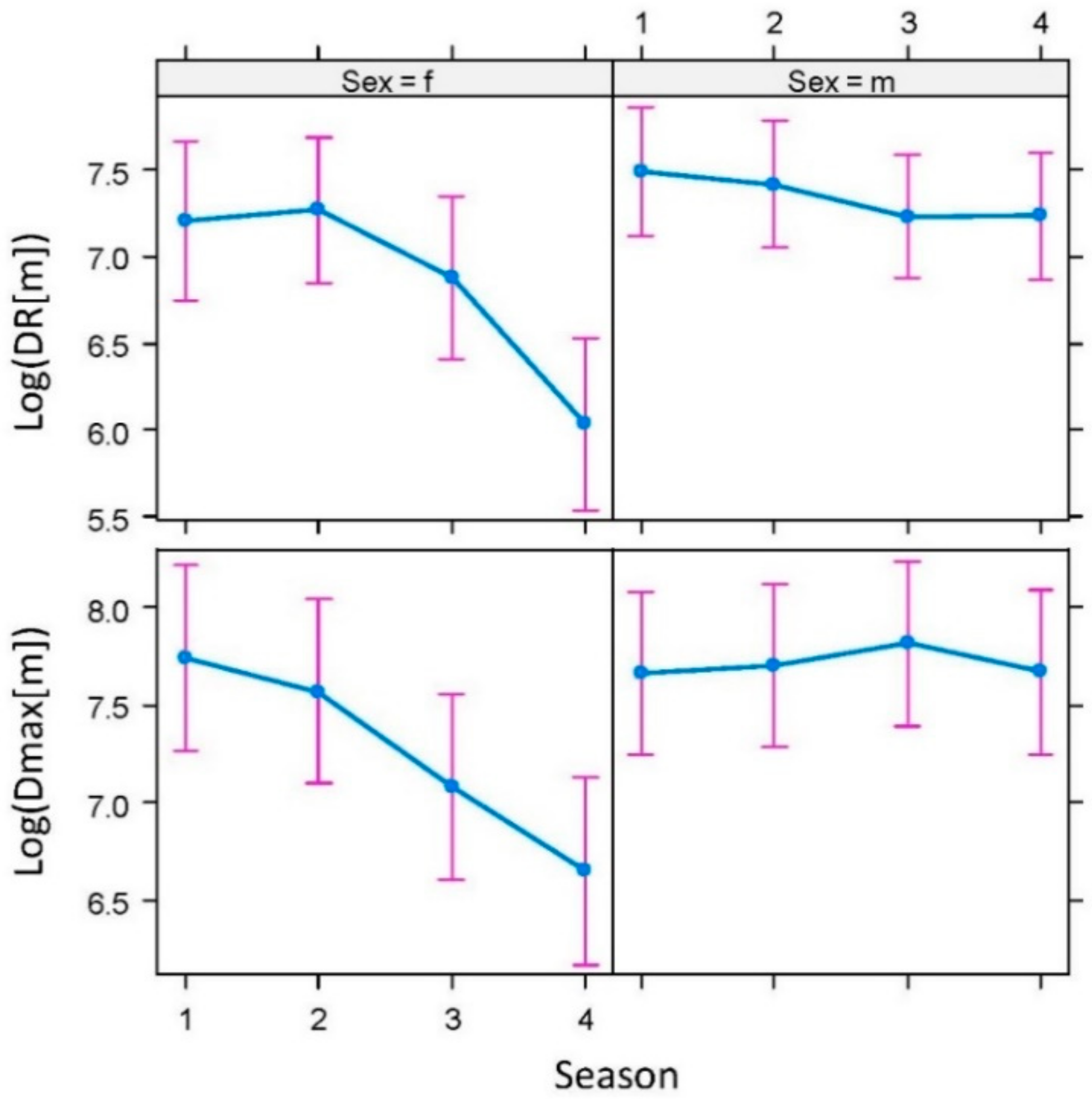
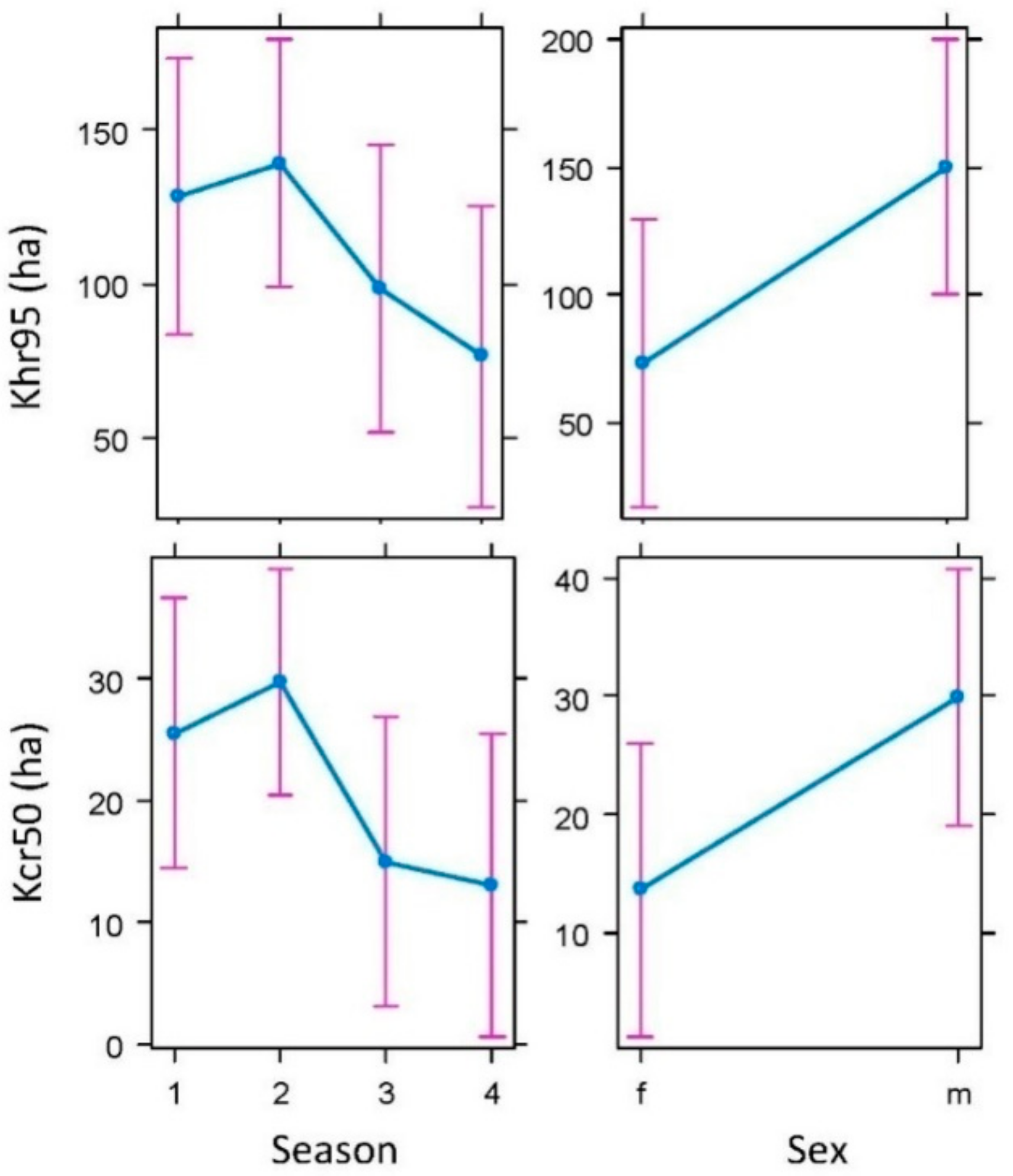
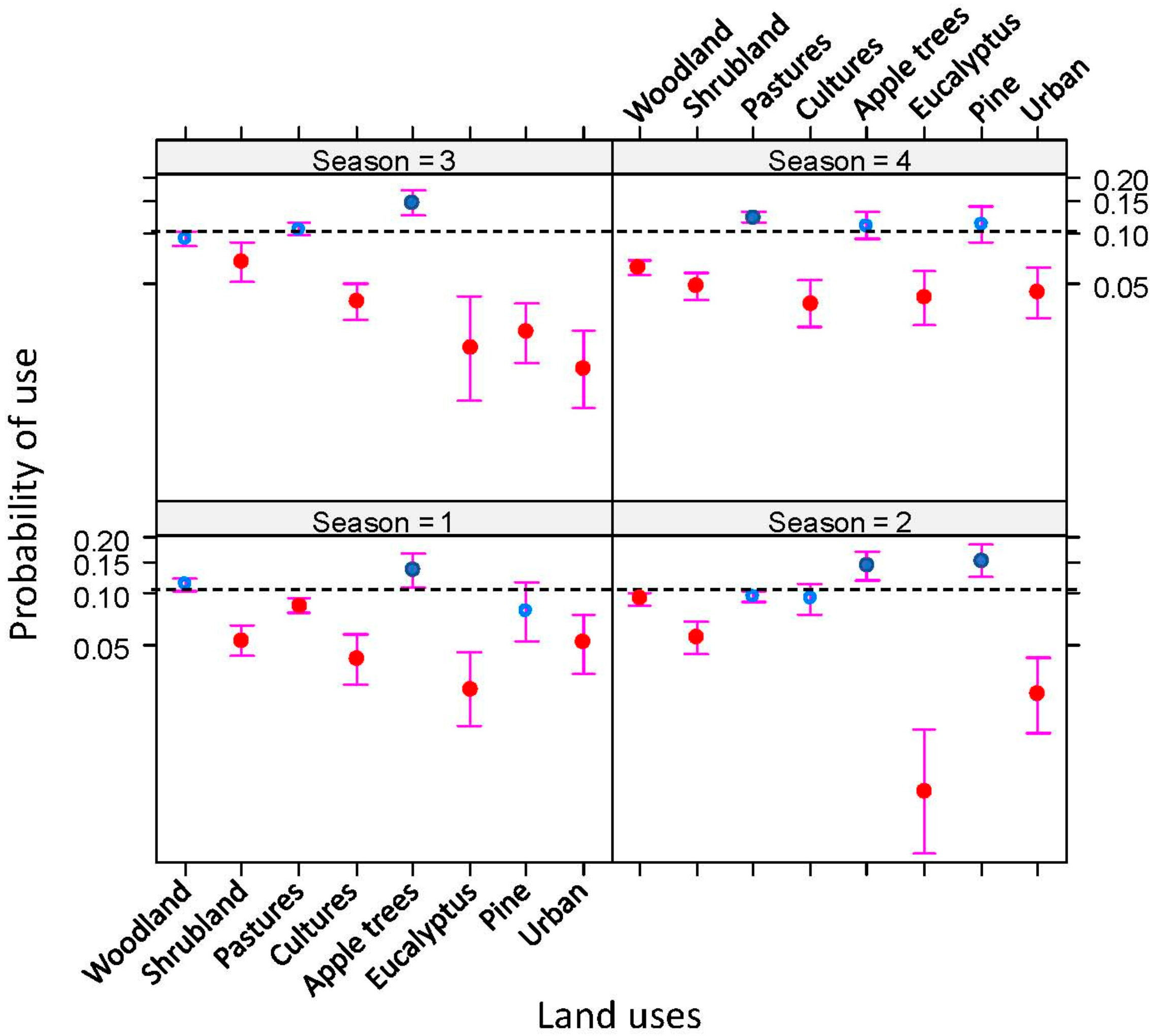
2.2. Spatial Ecology of Badgers
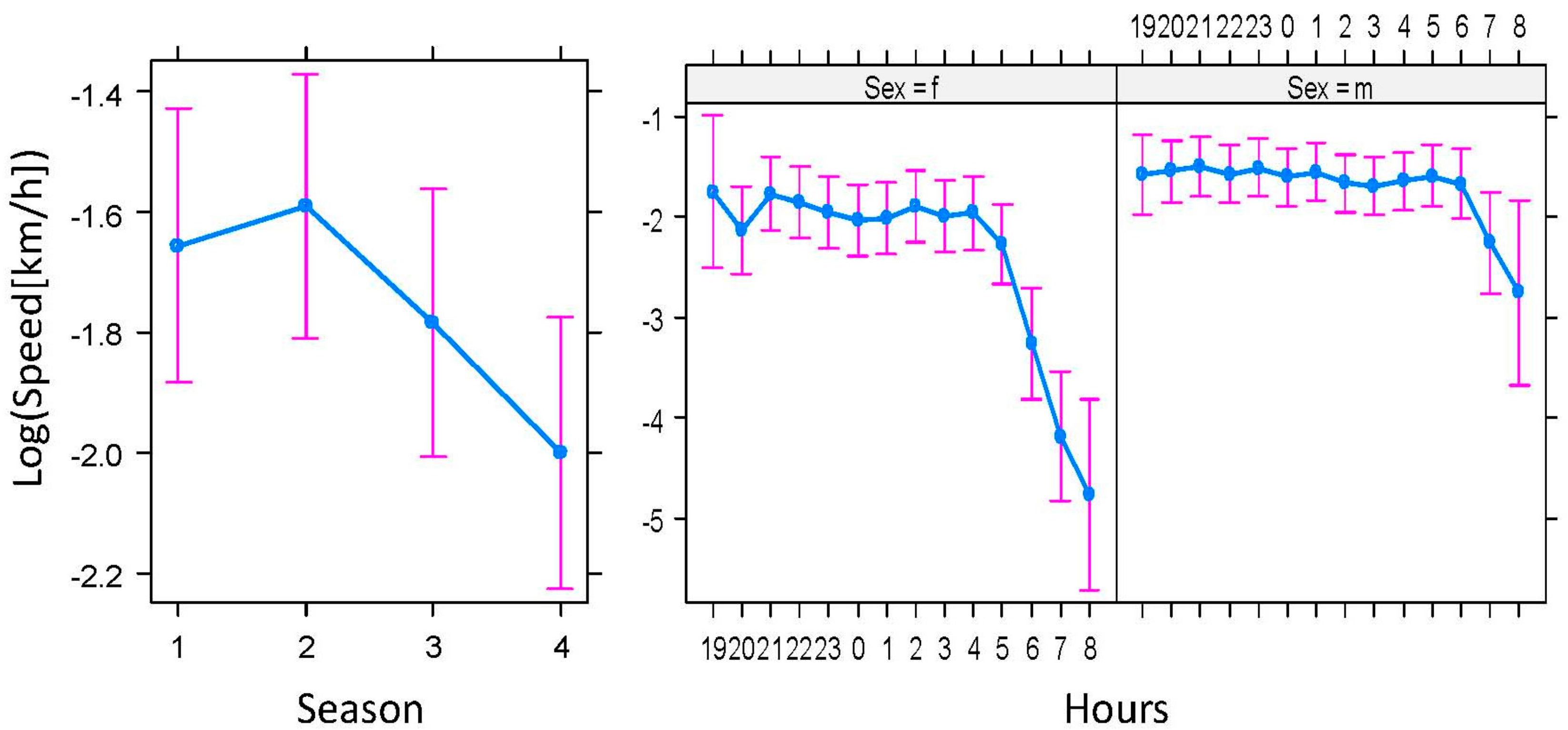
2.2.1. Activity, Movement, and Home Range
2.2.2. Badger Habitat Selection
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Study Area
4.3. Medium-Term (2012–2018) Description of TB in Cattle and Badgers in Parres
4.3.1. Cattle
4.3.2. Badgers
4.4. Badger Monitoring
4.5. Data Analysis
4.5.1. Basic Parameters of Badger Spatial Ecology: Activity, Movement, and Home Range
4.5.2. Badger Habitat Selection
4.5.3. TB Prevalence in Cattle Herds and Badgers
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riviere, J.; Carabin, K.; Le Strat, Y.; Hendrikx, P.; Dufur, B. Bovine tuberculosis surveillance in cattle and free-ranging wildlife in EU Member States in 2013: A survey-based review. Vet. Microbiol. 2014, 173, 323–331. [Google Scholar] [CrossRef] [PubMed]
- García-Saenz, A.; Saez, M.; Napp, S.; Casal, J.; Saez, J.L.; Acevedo, P.; Guta, S.; Allepuz, A. Spatio-temporal variability of bovine tuberculosis eradication in Spain (2006–2011). Spat. Spatiotemporal Epidemiol. 2014, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- MAPA. Available online: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/sanidad-animal/enfermedades/tuberculosis/Tuberculosis_bovina.aspx (accessed on 2 October 2019).
- Guta, S.; Casal, J.; Napp, S.; Saez, J.L.; García-Saenz, A.; Pérez de Val, B.; Romero, B.; Álvarez, J.; Allepuz, A. Epidemiological investigation of bovine tuberculosis herd breakdowns in Spain 2009/2011. PLoS ONE 2014, 9, e104383. [Google Scholar] [CrossRef] [PubMed]
- Gortázar, C.; Delahay, R.J.; McDonald, R.A.; Boadella, M.; Wilson, G.J.; Gavier-Widen, D.; Acevedo, P. The status of tuberculosis in European wild mammals. Mammal Rev. 2012, 42, 193–206. [Google Scholar] [CrossRef]
- Balseiro, A.; Rodríguez, O.; González-Quirós, P.; Merediz, I.; Sevilla, I.A.; Davé, D.; Dalley, D.J.; Lesellier, S.; Chambers, M.A.; Bezos, J. Infection of Eurasian badgers (Meles meles) with Mycobacterium bovis and Mycobacterium avium complex in Spain. Vet. J. 2011, 190, 21–25. [Google Scholar]
- Balseiro, A.; González-Quirós, P.; Rodríguez, O.; Copano, M.F.; Merediz, I.; De-Juan, L.; Chambers, M.A.; Delahay, R.J.; Marreros, N.; Royo, L.J.; et al. Spatial relationships between Eurasian badgers (Meles meles) and cattle infected with Mycobacterium bovis in Northern Spain. Vet. J. 2013, 197, 739–745. [Google Scholar]
- Cheeseman, C.L.; Wilesmith, J.W.; Stuart, F.A. Tuberculosis: The disease and its epidemiology in the badger; a review. Epidemiol. Infect. 1989, 103, 113–125. [Google Scholar] [CrossRef]
- Bourne, F.J.; Donelly, C.A.; Cox, D.R.; Gettinby, G.; Mclerney, J.P.; Morrison, W.I.; Woodroffe, R. Re: TB policy and the ISG’s findings. Vet. Rec. 2007, 161, 633–635. [Google Scholar] [CrossRef]
- Murphy, D.; Gormley, E.; Costello, E.; O´Meara, D.; Corner, L.A. The prevalence and distribution of Mycobacterium bovis infection in European badgers (Meles meles) as determined by enhanced post mortem examination and bacteriological culture. Res. Vet. Sci. 2010, 88, 1–5. [Google Scholar] [CrossRef]
- Clifton-Hadley, R.S.; Wilesmith, J.W.; Stuart, F.A. Mycobacterium bovis in the European badger (Meles meles): Epidemiological findings in tuberculous badgers from a naturally infected population. Epidemiol. Infect. 1993, 111, 9–19. [Google Scholar] [CrossRef]
- Gavier-Widen, D.; Chambers, M.A.; Palmer, N.; Newell, D.G.; Hewinson, R.G. Pathology of natural Mycobacterium bovis infection in Euopean badgers (Meles meles) and its relationship with bacterial excretion. Vet. Rec. 2001, 148, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.A.; Woodroffe, R.; Cox, D.R.; Bourne, J.; Gettinby, G.; Le Fevre, A.M.; McInerney, J.P.; Morrison, W.I. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature 2003, 426, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.A.; Woodroffe, R.; Cox, D.R.; Bourne, F.J.; Cheeseman, C.L.; Clifton-Hadley, R.S.; Wei, G.; Gettinby, G.; Gilks, P.; Jenkins, H.; et al. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 2006, 439, 843–846. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.; Haydon, D.T.; Kao, R.R. An ecological and comparative perspective on the control of bovine tuberculosis in Great Britain and Republic of Ireland. Prev. Vet. Med. 2012, 104, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Barasona, J.A.; Acevedo, P.; Ruíz-Fons, J.F.; Boadella, M.; Diez-Delgado, I.; Beltrán-Beck, B.; González-Barrio, D.; Queirós, J.; Montoro, V.; et al. Temporal trend of tuberculosis in wild ungulates from Mediterranean Spain. Transbound. Emerg. Dis. 2013, 1, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.W.; Kenny, K.; Fogarty, U.; O’Keeffe, J.J.; More, S.J.; McGrath, G.; Teeling, M.; Martin, S.W.; Dohoo, I.R. Spatial and temporal analyses of metrics of tuberculosis infection in badgers (Meles meles) from the Republic of Ireland: Trends in apparent prevalence. Prev. Vet. Med. 2015, 122, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Delahay, R.; Wilson, G.; Harris, D.; Macdonald, D.W. Badger Meles meles. In Mammals of the British Isles: Handbook, 4th ed.; Harris, S., Yalden, D., Eds.; The Mammal Society: Southampton, UK, 2008; Volume 1, pp. 425–436. [Google Scholar]
- Woodroffe, R.; Donnelly, C.A.; Ham, C.; Jackson, S.Y.B.; Moyes, K.; Chapman, K.; Stratton, N.G.; Cartwright, S.J. Badgers prefer cattle pasture but avoid cattle: Implications for bovine tuberculosis control. Ecol. Lett. 2016, 19, 1201–1208. [Google Scholar] [CrossRef]
- Payne, A.; Philipon, S.; Hars, J.; Dufour, B.; Gilot-Fromont, E. Wildlife Interactions on baited places and waterholes in a French area infected by bovine tuberculosis. Front. Vet. Sci. 2017, 3, 122. [Google Scholar] [CrossRef]
- Vicente, J.; Delahay, R.J.; Walker, N.J.; Cheeseman, C.L. Social organization and movement influence the incidence of bovine tuberculosis in an undisturbed high-density badger Meles meles population. J. Anim. Ecol. 2007, 76, 348–360. [Google Scholar] [CrossRef]
- Riordan, P.; Delahay, R.J.; Cheeseman, C.; Johnson, P.J.; Macdonald, D.W. Culling-induced changes in badger (Meles meles) behavior, social organization and the epidemiology of bovine tuberculosis. PLoS ONE 2011, 6, e28904. [Google Scholar] [CrossRef]
- Vial, F.; Donnelly, C.A. Localized reactive badger culling increases risk of bovine tuberculosis in nearby cattle herds. Biol. Lett. 2012, 8, 50–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byrne, A.W.; Quinn, J.L.; O´Keeffe, J.J.; Green, S.; Sleeman, D.P.; Martin, S.W.; Davenport, J. Large-scale movements in European badgers: Has the tail of the movement kernel been underestimated? J. Anim. Ecol. 2014, 83, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Gaughran, A.; Kelly, D.J.; MacWhite, T.; Mullen, E.; Maher, P.; Good, M.; Marples, N.M. Super-ranging. A new ranging strategy in European badgers. PLoS ONE 2018, 13, e0191818. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.A.; Nouvellet, P. The contribution of badgers to confirmed tuberculosis in cattle in high-incidence areas in England. PLoS Curr. 2013, 10, 5. [Google Scholar] [CrossRef]
- Delahay, R.J.; Smith, G.C.; Barlow, A.M.; Walker, N.; Harris, A.; Clifton-Hadley, R.S.; Cheeseman, C.L. Bovine tuberculosis infection in wild mammals in the South-West region of England: A survey of prevalence and a semi-quantitative assessment of the relative risks to cattle. Vet. J. 2007, 173, 287–301. [Google Scholar] [CrossRef]
- Mycobacterium bovis Spoligotype Database Website. Available online: www.Mbovis.org (accessed on 2 October 2019).
- Revilla, E.; Palomares, F. Does local feeding specialization exist in Eurasian badgers? Can. J. Zool. 2002, 80, 83–93. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Jedrzejewska, B.; Zalewski, A. Annual and circadian activity patterns of badgers Meles meles in Bialowieza Primeval Forest (eastern Poland) compared with other Palaearctic populations. J. Biogeogr. 2003, 30, 463–472. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Zalewski, A.; Bogumiła, J. Daily movement and territory use by badgers Meles meles in Białowieża Primeval Forest, Poland. Wild. Biol. 2006, 12, 385–391. [Google Scholar] [CrossRef][Green Version]
- Acevedo, P.; González-Quirós, P.; Etherington, T.R.; Prieto, J.M.; Gortázar, C.; Balseiro, A. Generalizing and transferring spatial models: A case study to predict Eurasian badger abundance in Atlantic Spain. Ecol. Model. 2014, 275, 1–8. [Google Scholar] [CrossRef]
- Fine, A.E.; Bolin, C.A.; Gardiner, J.C.; Kaneene, J.B. A study of the persistence of Mycobacterium bovis in the environment under natural weather conditions in Michigan, USA. Vet. Med. Int. 2011, 2011, 765430. [Google Scholar] [CrossRef]
- Zabala, J.; Garin, I.; Zuberogoitia, I.; Aihartza, J. Habitat selection and diet of badgers (Meles meles) in Biscay (northern Iberian Peninsula). Ital. J. Zool. 2002, 69, 233–238. [Google Scholar] [CrossRef]
- Kruuk, H. The Social Badger. Ecology and Behaviour of a Group Living Carnivore, 1st ed.; Oxford University Press: Oxford, UK, 1989; p. 155. [Google Scholar]
- Molina-Vacas, G.; Bonet-Arboli, V.; Rafart-Plaza, E.; Rodríguez-Teijeiro, J.D. Spatial ecology of European badgers (Meles meles L.) in Mediterranean habitats of the north-eastern Iberian Peninsula. I: Home range size, spatial distribution and social organization. Vie et Milieu 2007, 59, 237–246. [Google Scholar]
- Macdonald, D.W.; Newman, C.; Dean, J.; Buesching, C.D.; Johnson, P.J. The distribution of Eurasian badger, Meles meles, setts in a high-density area: Field observations contradict the sett dispersion hypothesis. Oikos 2004, 106, 295–307. [Google Scholar] [CrossRef]
- Rosalino, L.M.; Macdonald, D.W.; Santos-Reis, M. Resource dispersion and badger population density in Mediterranean woodlands: Is food, water or geology the limiting factor? Oikos 2005, 110, 441–452. [Google Scholar] [CrossRef]
- Baudry, J.; Bunce, R.G.H.; Burel, F. Hedgerows: An international perspective on their origin, function and management. J. Environ. Manag. 2000, 60, 7–22. [Google Scholar] [CrossRef]
- Braña, F.; Prieto, L.; González-Quirós, P. Habitat change and timing of the dusk flight in Eurasian Woodcock: A trade-off between feeding and predator avoidance? Ann. Zool. Fenn. 2010, 47, 206–214. [Google Scholar] [CrossRef]
- Quirós, P. Las poblaciones reproductora e invernante de becada (Scolapax rusticola) en la cordillera Cantábrica: Uso del espacio y tendencias poblacionales. Ph.D. Thesis, Universidad de Oviedo, Oviedo, Spain, 15 January 2016. [Google Scholar]
- Garnett, B.T.; Delahay, R.J.; Roper, T.J. Use of cattle farm resources by badgers (Meles meles) and risk of bovine tuberculosis (Mycobacterium bovis) transmission to cattle. Proc. R. Soc. Lond. B 2002, 269, 1487–1491. [Google Scholar] [CrossRef]
- Benham, P.F.J.; Broom, D.M. Responses of dairy cows to badger urine and faeces on pasture with reference to bovine tuberculosis transmission. Br. Vet. J. 1991, 147, 517–531. [Google Scholar] [CrossRef]
- Cheeseman, C.L.; Mallinson, P.J. Behaviour of badgers (Meles meles) infected with bovine tuberculosis. J. Zool. 1981, 194, 284–289. [Google Scholar] [CrossRef]
- Tolhurst, B.A.; Delahay, R.J.; Walker, N.J.; Ward, A.I.; Roper, T.J. Behaviour of badgers (Meles meles) in farm buildings: Opportunities for the transmission of Mycobacterium bovis to cattle? Appl. Anim. Behav. Sci. 2009, 117, 103–113. [Google Scholar] [CrossRef]
- Payne, A.; Chappa, S.; Hars, J.; Dufour, B.; Gilot-Fromont, E. Wildlife visits to farm facilities assessed by camera traps in a bovine tuberculosis-infected area in France. Eur. J. Wild. Res. 2016, 62, 33–42. [Google Scholar] [CrossRef]
- Cresswell, W.J.; Harris, S. Foraging behaviour and home range utilization in a suburban badger (Meles meles) population. Mammal Rev. 1988, 18, 37–49. [Google Scholar] [CrossRef]
- Da Silva, J.; Woodroffe, R.; Macdonald, D.W. Habitat, food availability and group territoriality in the European badger, Meles meles. Oecologia 1993, 95, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Garnett, B.T.; Delahay, R.J.; Roper, T.J. Ranging behavior of European badger (Meles meles) in relation to bovine tuberculosis (Mycobacterium bovis) infection. Appl. Anim. Behav. Sci. 2005, 94, 331–340. [Google Scholar] [CrossRef]
- Weber, N.; Carter, S.P.; Dall, S.R.X.; Delahay, R.J.; McDonald, J.L.; Bearhop, S.; McDonald, R.A. Badger social networks correlate with tuberculosis infection. Curr. Biol. 2013, 23, R915–R916. [Google Scholar] [CrossRef]
- Ninyerola, M.; Pons, X.; Roure, J.M. Atlas Climático Digital de la Península Ibérica. Metodología y Aplicaciones en Bioclimatología y Geobotánica, 1st ed.; Universidad Autónoma de Barcelona: Bellatera, Spain, 2005. [Google Scholar]
- Coetsier, C.; Vannuffel, P.; Bloondel, N.; Denef, J.F.; Cocito, C.; Gala, J.L. Duplex PCR for differential identification of Mycobacterium bovis, M. avium, and M. avium subsp. paratuberculosis in formalin-fixed paraffin-embedded tissues from cattle. J. Clin. Microbiol. 2000, 38, 3048–3054. [Google Scholar]
- Rodríguez-Campos, S.; Aranaz, A.; de Juan, L.; Sáez-Llorente, J.L.; Romero, B.; Bezos, J.; Jiménez, A.; Mateos, A.; Domínguez, L. Limitations of spoligotyping and variable number tandem repeat typing for molecular tracing of Mycobacterium bovis in a high diversity setting. J. Clin. Microbiol. 2011, 49, 3361–3364. [Google Scholar] [CrossRef]
- Infantes-Lorenzo, J.A.; Dave, D.; Moreno, I.; Anderson, P.; Lesellier, S.; Gormley, E.; Domínguez, L.; Balseiro, A.; Gortázar, C.; Domínguez, M.; et al. New serological platform for detecting antibodies against Mycobacterium tuberculosis complex in European badgers. Vet. Med. Sci. 2019, 5, 61–69. [Google Scholar] [CrossRef]
- Greenwald, R.; Esfandiari, J.; Lesellier, S.; Houghton, R.; Pollock, J.; Aagaard, C.; Andersen, P.; Hewinson, R.G.; Chambers, M.; Lyashchenko, K. Improved serodetection of Mycobacterium bovis infection in badgers (Meles meles) using multiantigen test formats. Diagn. Microbiol. Infec. Dis. 2003, 46, 197–203. [Google Scholar] [CrossRef]
- Cutrera, A.P.; Antinuchi, C.D.; Mora, M.S.; Vassallo, A.I. Home-range and activity patterns of the South American subterranean rodent Ctenomys talarum. J. Mammal. 2006, 87, 1183–1191. [Google Scholar] [CrossRef]
- Rowcliffe, J.M.; Carbone, C.; Kays, R.; Kranstauber, B.; Jansen, P.A. Bias in estimating animal travel distance: The effect of sampling frequency. Methods Ecol. Evol. 2014, 3, 653–662. [Google Scholar] [CrossRef]
- Worton, B.J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Seaman, D.E.; Powell, R.A. An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 1996, 77, 2075–2085. [Google Scholar] [CrossRef]
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Seaman, D.E.; Millspaugh, J.J.; Kernohan, B.J.; Brundige, G.C.; Raedeke, K.J.; Gitzen, R.A. Effects of sample size on kernel home range estimates. J. Wildl. Manag. 1999, 63, 739–747. [Google Scholar] [CrossRef]
- Hemson, G.; Johnson, P.; South, A.; Kenward, R.; Ripley, R.; MacDonald, D. Are kernels the mustard? Data from global positioning system (GPS) collars suggests problems for kernel home range analyses with least squares cross validation. J. Anim. Ecol. 2005, 74, 455–463. [Google Scholar] [CrossRef]
- Börger, L.; Franconi, N.; De Michele, G.; Gantz, A.; Meschi, F.; Manica, A.; Lovari, S.; Coulson, T. Effects of sampling regime on the mean and variance of home range size estimates. J. Anim. Ecol. 2006, 75, 1393–1405. [Google Scholar] [CrossRef]
- Fox, J. Effect displays in R for generalised linear models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar] [CrossRef]
- Zuur, A.F.; Leno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Manly, B.F.J.; McDonald, L.L.; Thomas, D.L.; McDonald, T.L.; Erickson, W.P. Resource Selection by Animals: Statistical Design and Analysis for Field Studies, 1st ed.; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Gillies, C.S.; Hebblewhite, M.; Nielsen, S.E.; Krawchuk, M.A.; Aldridge, C.L.; Frair, J.L.; Saher, D.J.; Stevens, C.E.; Jerde, C.L. Application of random effects to the study of resource selection by animals. J. Anim. Ecol. 2006, 75, 887–898. [Google Scholar] [CrossRef]
- SITPA-IDEAS. Available online: http://sitpa.cartografia.asturias.es/sitpav30/pages/presentation/presentation.aspx (accessed on 2 October 2019).
- SIGPAC. Available online: http://sigpac.asturias.es/VisorSigPacHTML5/ (accessed on 2 October 2019).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using Ime4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
| Individual ID | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3.1 | 3.2 | 4.1 | 4.2 | 5 | 6 | 7 | 9 | ||
| Sex | M | F | M | F | M | F | M | F | M | M | 6 M & 4 F |
| Social Group | a | b | c | d | e | f | g | h | 8 | ||
| N Relocations | |||||||||||
| Spring | 144 | 359 | 409 | 1074 | 1986 | ||||||
| Summer | 312 | 58 | 209 | 727 | 164 | 616 | 254 | 2340 | |||
| Autumn | 377 | 1209 | 1082 | 2668 | |||||||
| Winter | 444 | 956 | 742 | 725 | 1911 | ||||||
| Total | 833 | 58 | 568 | 2380 | 409 | 164 | 956 | 1690 | 742 | 2061 | 8905 |
| N Nights | |||||||||||
| Spring | 19 | 26 | 42 | 82 | 169 | ||||||
| Summer | 63 | 9 | 17 | 55 | 16 | 44 | 20 | 224 | |||
| Autumn | 59 | 90 | 84 | 233 | |||||||
| Winter | 45 | 83 | 72 | 48 | 248 | ||||||
| Total | 141 | 9 | 43 | 190 | 42 | 16 | 83 | 126 | 72 | 152 | 874 |
| Home Range (ha) Kernel 95% | |||||||||||
| Spring | 212 | 93 | 203 | 91 | NA | ||||||
| Summer | 193 | 80 | 154 | 109 | 102 | 91 | 72 | NA | |||
| Autumn | 170 | 59 | 38 | NA | |||||||
| Winter | 22 | 168 | 3358 | 26 | NA | ||||||
| Total | 175 | 80 | 120 | 74 | 203 | 102 | 168 | 83 | 3558 | 42 | NA |
| Home Range (ha) Kernel 50% | |||||||||||
| Spring | 59 | 25 | 31 | 17 | NA | ||||||
| Summer | 51 | 18 | 45 | 20 | 16 | 26 | 17 | NA | |||
| Autumn | 37 | 9 | 6 | NA | |||||||
| Winter | 5 | 16 | 225 | 7 | NA | ||||||
| Total | 44 | 18 | 31 | 10 | 30 | 16 | 16 | 20 | 225 | 7 | NA |
| Daily Distance (m) | |||||||||||
| Spring | 1193 | 2305 | 2861 | 2328 | 2329 | ||||||
| Summer | 1198 | 803 | 2531 | 2277 | 1664 | 2359 | 1232 | 1831 | |||
| Autumn | 1256 | 1630 | 1382 | 1449 | |||||||
| Winter | 814 | 2618 | 2212 | 1271 | 1907 | ||||||
| Dispersal Distance (m) | |||||||||||
| Spring | 1969 | 1637 | 3781 | 1924 | NA | ||||||
| Summer | 1961 | 1322 | 1799 | 3339 | 1986 | 1620 | 1329 | NA | |||
| Autumn | 2216 | 2040 | 1528 | NA | |||||||
| Winter | 1324 | 2793 | 18286 | 1300 | NA | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo, P.; Prieto, M.; Quirós, P.; Merediz, I.; de Juan, L.; Infantes-Lorenzo, J.A.; Triguero-Ocaña, R.; Balseiro, A. Tuberculosis Epidemiology and Badger (Meles meles) Spatial Ecology in a Hot-Spot Area in Atlantic Spain. Pathogens 2019, 8, 292. https://doi.org/10.3390/pathogens8040292
Acevedo P, Prieto M, Quirós P, Merediz I, de Juan L, Infantes-Lorenzo JA, Triguero-Ocaña R, Balseiro A. Tuberculosis Epidemiology and Badger (Meles meles) Spatial Ecology in a Hot-Spot Area in Atlantic Spain. Pathogens. 2019; 8(4):292. https://doi.org/10.3390/pathogens8040292
Chicago/Turabian StyleAcevedo, Pelayo, Miguel Prieto, Pablo Quirós, Isabel Merediz, Lucía de Juan, José Antonio Infantes-Lorenzo, Roxana Triguero-Ocaña, and Ana Balseiro. 2019. "Tuberculosis Epidemiology and Badger (Meles meles) Spatial Ecology in a Hot-Spot Area in Atlantic Spain" Pathogens 8, no. 4: 292. https://doi.org/10.3390/pathogens8040292
APA StyleAcevedo, P., Prieto, M., Quirós, P., Merediz, I., de Juan, L., Infantes-Lorenzo, J. A., Triguero-Ocaña, R., & Balseiro, A. (2019). Tuberculosis Epidemiology and Badger (Meles meles) Spatial Ecology in a Hot-Spot Area in Atlantic Spain. Pathogens, 8(4), 292. https://doi.org/10.3390/pathogens8040292





