Abstract
Leishmaniasis remains a major world health problem, and in particular, Algeria ranks second for the incidence of cutaneous leishmaniasis. Pulicaria inuloides is a well-known Arabian Peninsula medicinal plant. In the present study, the chloroform, ethyl acetate and n-butanol extracts from the roots of Pulicaria inuloides were analyzed for antioxidant activity and its correlation with the total phenolic and flavonoid contents. The highest antioxidant activity using a DPPH assay was showed by the ethyl acetate extract (IC50 4.08 µg/mL), which also had the highest total phenolic content (307.12 µgAGE). Furthermore, P. inuloides root extracts were evaluated against Leishmania amazonensis and Leishmania donovani. The results highlighted the chloroform extract as the most active one against both tested Leishmania strains. A bioguided fractionation of the chloroform extract led to the isolation of (8R:8S)-(75:25 er)-10-isobutyryloxy-8,9-epoxy-thymol isobutyrate as the main bioactive component, showing a potent leishmanicidal activity on L. amazonensis promatigote and amastigote stages (IC50 5.03 and 2.87 µM, respectively) and a good selectivity index on murine macrophages (CC50 19.37 µM). This study provides the first report of the antioxidant and leishmanicidal activities of P. inuloides root extracts and the results point to this species as a source of potential bioactive agents.
1. Introduction
Leishmaniasis, a neglected tropical disease caused by protozoan parasites belonging to the genus Leishmania, remains a major world health problem [1]. In particular, Algeria is endemic for cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL) [2]. CL, also called “Biskra boil” in the local language, is a serious public health problem because the country has the second largest focus in the world after Afghanistan. It is reported to be endemic in 328 third sub-national administrative levels with 10 million populations at risk, rising 13106 numbers of cases reported in 2017 [3]. Regarding VL, the cases have been detected mainly from the central and eastern parts of the Tell region, and new foci have appeared and the number of cases has resurged, with 34 new cases reported in 2017 [4]. The Leishmaniasis National Control Program (LNCP) in Algeria was established in 2006, with a vector control program based on pyrethroids as insecticides, but without reservoir host control program [5]. The treatment of the population is provided free of charge in the public sector, with the following antileishmanial medicines included in the national List of Essential Medicines: amphotericin B deoxycholate, liposomal amphotericin B and meglumine antimoniate [6,7]. In spite of the high prevalence, current chemotherapy for leishmaniasis is compromised by high cost, toxicity associated with long-term treatments, route of administration, drug resistance, and different strain sensitivity to the available drugs [8]. These drawbacks lead to an urgent need to develop new treatments with acceptable efficacy and safety profile. In this regard, plants have demonstrated to be an important source of leishmanicidal drugs owing to their accessibility, structural diversity, low cost and possible rapid biodegradation [9].
The genus Pulicaria belonging to the Asteraceae family (tribe Inuleae) consists of about 100 species distributed in Europe, North Africa and Asia, particularly around the Mediterranean region [10,11]. Some Pulicaria species are used as traditional medicines for treatment of a variety of illnesses due to their anticancer, antioxidant, antimicrobial, antifungal and antimalarial properties, among others [11,12,13]. Pulicaria inuloides (Poir.) DC is a well-known Yemeni medicinal plant. The leaves are claimed to have antibacterial and antioxidant properties, and are also used to flavor foods and to make an herbal tea [14]. The chemical composition [14,15,16] and medicinal uses as anticancer, antibacterial and antioxidant [14,17] of the P. inuloides essential oil obtained by hydro-destillation of the leaves and flowers have been reported. Moreover, the antioxidant properties and total phenol and flavonoid contents of different extracts from the leaves of P. inuloides were analyzed by Al-Hajj and co-workers [15]. In addition, the isolation of ent-kaurane diterpenoids and flavonoids from the aerial part of P. inuloides was reported, along with their antimicrobial, cytotoxic and antioxidant evaluation [18].
Our previous works reported the in vitro antiprotozoal evaluation of extracts from the aerial part of P. inuloides, and the characterization of quercetagetin-3,5,7,3’-tetramethyl ether as the main component of the active CHCl3 extract [19]. In the present work, and as a continuation of the P. inuloides medicinal plant research, the antioxidant activity, total phenolic and flavonoid contents, and leishmanicidal properties of P. inuloides roots are reported. The bioassay-guided fractionation of the active CHCl3 extract led to the isolation and characterization of 10-isobutyryloxy-8,9-epoxythymol isobutyrate as the main leishmanicidal component, highlighting this plant as a promising source of antiparasitic agents.
2. Results and Discussion
2.1. DPPH Radical Scavenging Activity
Oxidative stress has been associated with the development and progression of several diseases, including cancer, inflammation, atherosclerosis, cardiovascular diseases or diabetes. Thus, administration of exogenous antioxidants is a promising way of combating the undesirable effects of reactive oxygen species (ROS) induced oxidative damage. In this regard, plants have demonstrated to be an important source of antioxidants such as polyphenols or flavonoids [20]. Moreover, taking into consideration that some Pulicaria species, and in particular P. inuloides, are claimed to have antioxidant properties [11], the antioxidant activity of P. inuloides roots was analyzed.
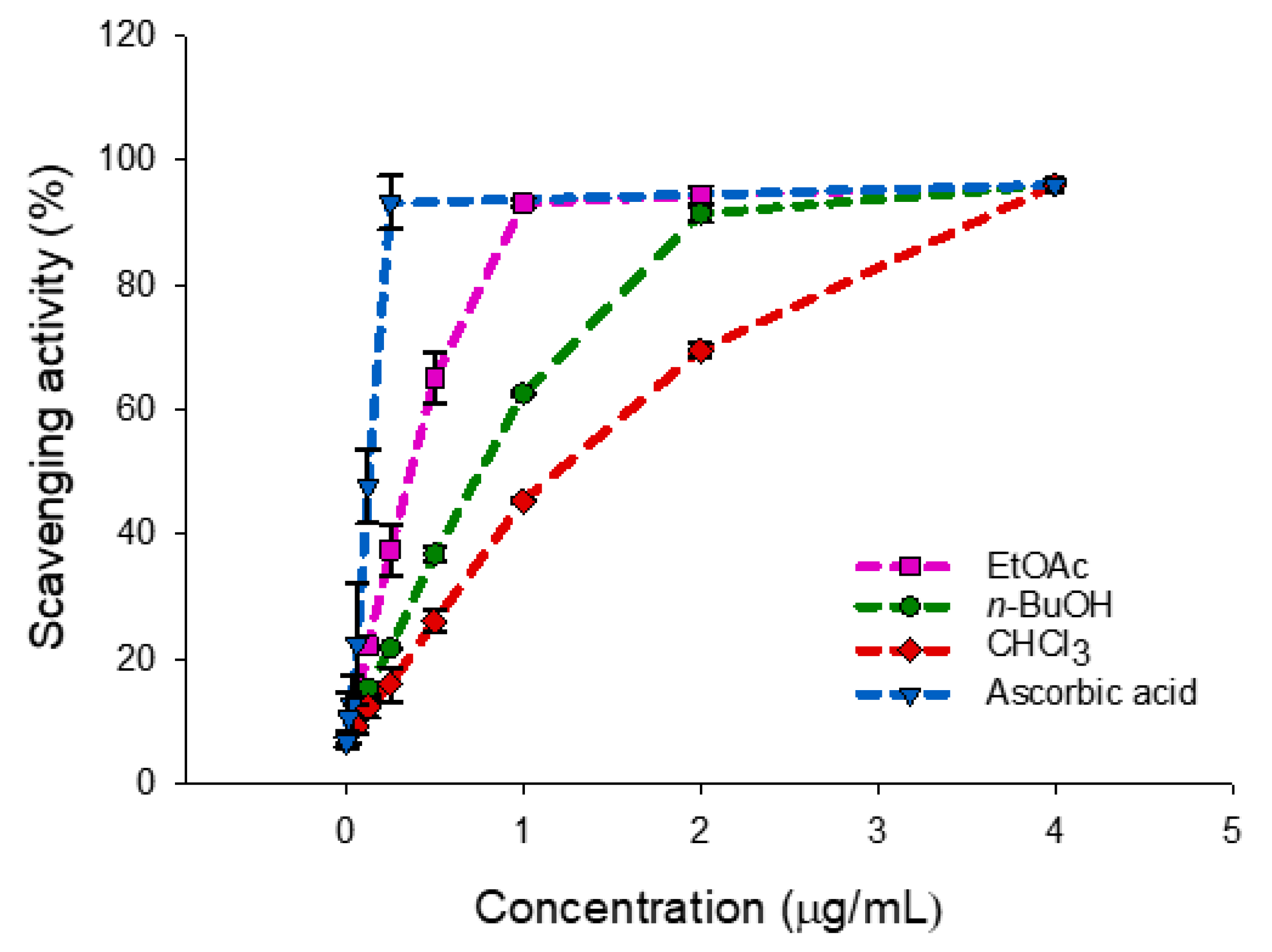
The results of the antioxidant activity (Table 1, Figure 1) by the DPPH radical scavenging assay of three extracts from the roots of P. inuloides indicated strong ability of the ethyl acetate extract to act as DPPH scavenger, with an IC50 value of 4.08 µg/mL, followed by the n-butanol and chloroform extracts (8.76 and 12.85 µg/mL, respectively). The antioxidant activity, in particular this showed by the ethyl acetate extract is significantly high when compared to the standard ascorbic acid (1.30 µg/mL). Therefore, to investigate the correlation between the antioxidant activity and presence of antioxidants compounds into the different extracts, the total phenolic and flavonoid contents were determined.

Table 1.
Antioxidant activity and phenolic and flavonoid contents of P. inuloides root extracts.
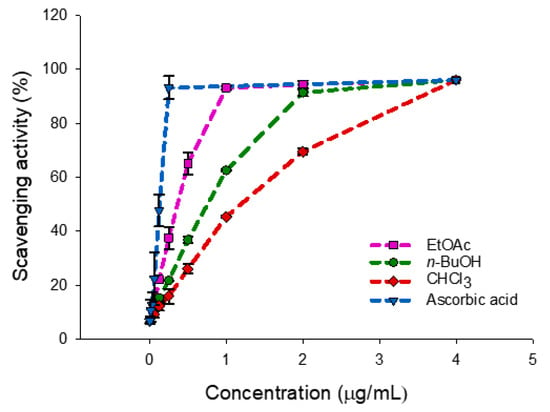
Figure 1.
DPPH radical-scavenging activity of Pulicaria inuloides roots (ethyl acetate, n-butanol and chloroform extracts) versus ascorbic acid. Data are expressed as mean ± standard deviation (n = 2).
2.2. Total Phenolic and Flavonoids Contents of Extracts
The amount of total phenols in the extracts was measured by the Foline-Ciocalteau method [21] in terms of gallic acid equivalent (GAE) from the calibration curve of gallic acid standard solution, using the equation: y = 0.004x + 0.017 with R² = 0.988, where y = absorbance at 765 nm and x = concentration of total phenolic content (Supplementary Materials, Figure S1). The ethyl acetate and n-butanol extracts showed the highest values (307.12 and 229.76 µgGAE/mg, respectively), whereas the chloroform extract showed a low total phenol content (32.28 µgGAE/mg) as seen in Table 1. These results indicated a direct correlation between the phenols content and the radical-scavenging capacity of the extracts.
Previous studies have reported the total phenolic content of P. inuloides essential oil (144 mgGAE/g) [14] and those of the methanolic, ethanolic and diethyl ether extracts of P. inuloides leaves (91.2, 89.9 and 64.9 mgGAE/g, respectively) [15]. Our results indicated that both, the ethyl acetate and n-butanol extracts from the roots of the plant have a higher total phenolic content than those for the essential oil and leaves extracts and by instant a higher antioxidant capacity. The total flavonoid content was calculated from a calibration curve (Supplementary Materials, Figure S1), and the results were expressed as µg quercetin equivalent per mg of extract (µgQE/mg). The total flavonoid content of the extracts was measured using the QE equation: y = 0.027x + 0.014 with R² = 0.999, where y = absorbance at 420 nm, and x = concentration of total flavonoid content. The results indicated low flavonoids content in the ethyl acetate and n-butanol extracts (5.08 and 6.31 µgQE/mg, respectively), while this type of compounds could not be detected in the chloroform extract (Table 1). A correlation between the total phenolic content and the antioxidant capacity of the extracts was observed. Previous reported studies revealed that phenolic compounds are major antioxidant constituents in the leaves of P. inuloides extracts [15], and the good correlation observed in our study support the hypothesis that phenolic compounds in P. inuloides roots contribute significantly to their antioxidant properties.
2.3. Bioassay-Guided Fractionation
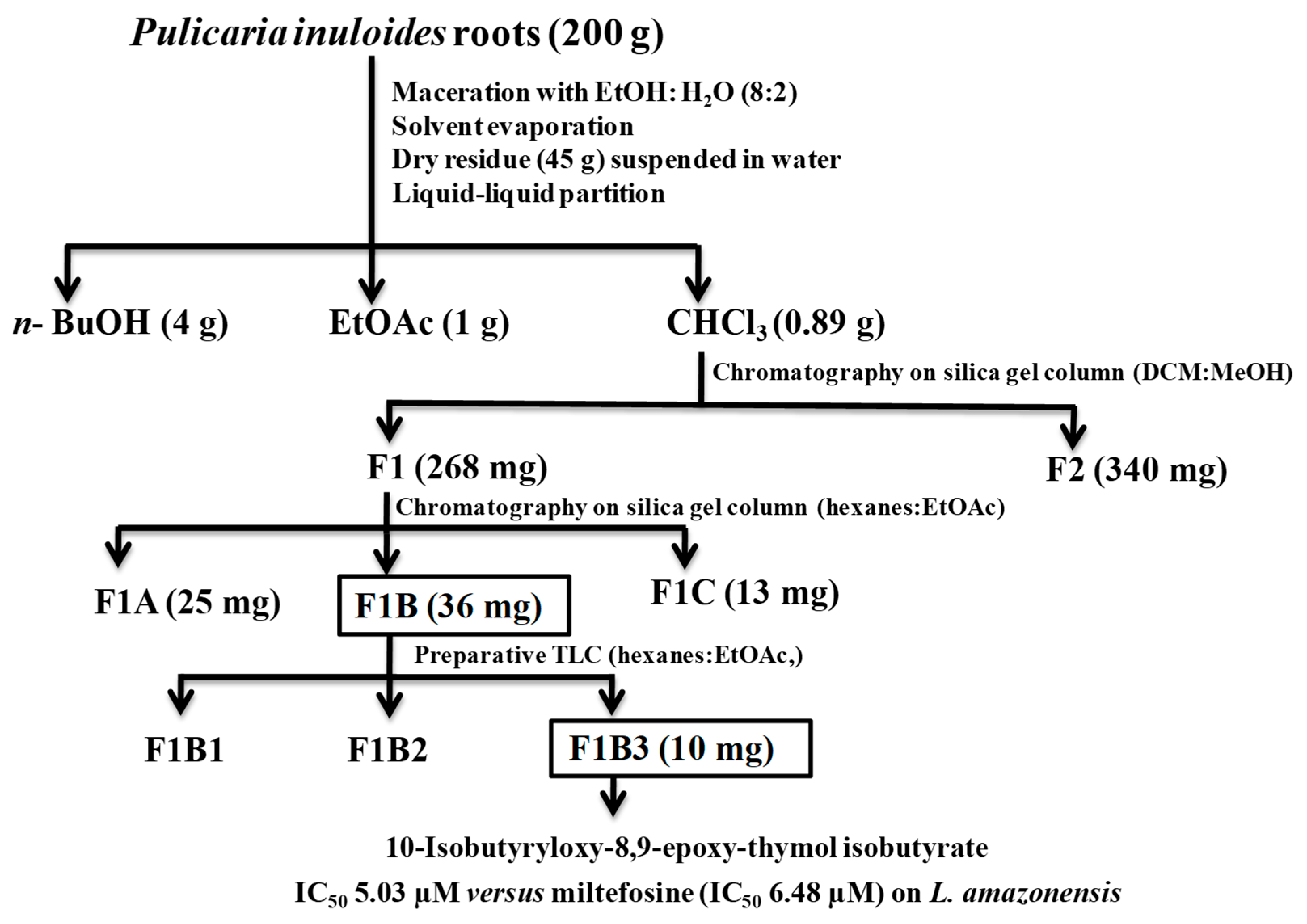
The roots of P. inuloides (200 g) were extracted with 80% ethanol, and the dry residue further suspended in water and extracted, successively, using three organic solvents to yield CHCl3, EtOAc and n-BuOH extracts. The highest extraction yield was obtained with n-BuOH (2%), followed by extraction with EtOAc (0.50%) and CHCl3 (0.44%). The three organic extracts were submitted to an in vitro leishmanicidal screening (Table 2, Scheme 1), revealing that the chloroform extract was the most active against L. amazonensis and L. donovani promastigote form. Therefore, the chloroform extract (0.89 g) was subjected to silica gel column chromatography, eluted with dichloromethane-methanol of increasing polarity to afford two main fractions: F1 (268.0 mg) and F2 (340.0 mg). The active fraction F1 was further chromatographed, using column chromatography on silica gel eluted with hexanes-ethyl acetate of increasing polarity to obtain three fractions: F1A (25.0 mg), F1B (131.0 mg), and F1C (13.0 mg).

Table 2.
Leishmanicidal screening a of extracts/fractions b from Pulicaria inuloides roots.
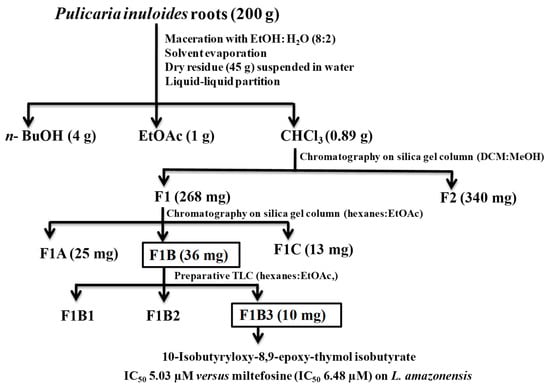
Scheme 1.
Flowchart of leishmanicidal bioguided fractionation of P. inuloides roots.
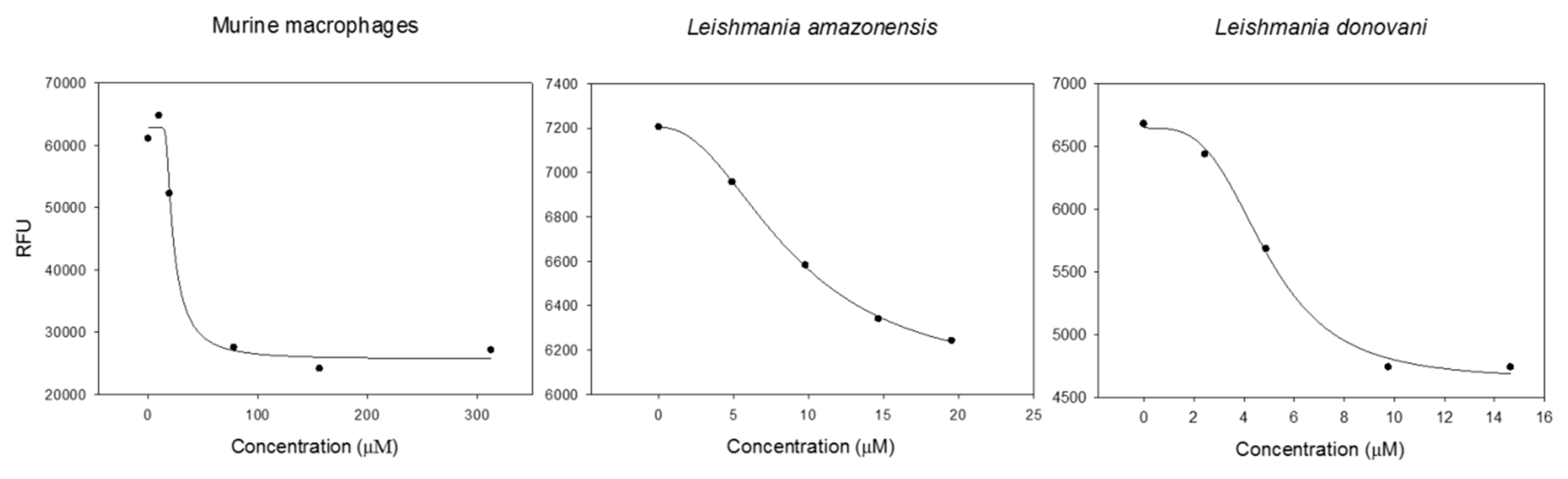
The most active fraction F1B against both Leishmania strains (Table 3) was subjected to preparative thin layer chromatography (PTLC) to afford three sub-fractions (F1B1-F1B3) (Scheme 1). Sub-fractions F1B1 and F1B2 were inactive at 200 µg/mL on L. amazonenis and L. donovani. On the other hand, sub-fraction F1B3 showed a potent leishmanicidal effect with IC50 values of 5.03 ± 0.29 µM and 4.65 ± 0.10 µM on both Leishmania strains, respectively, even somewhat higher on L. amazonensis that Miltefosine used as the reference drug (IC50 values of 6.48 µM and 3.32 µM on L. amazonensis and L. donovani, respectively, Table 4 and Figure 2). Moreover, evaluation of this active sub-fraction on murine macrophages (CC50 19.37 µM ± 0.23 µg/mL) in the search for selectivity indicated a moderate selective index (SI), with values of 3.9 and 4.2 for these Leishmania strains, respectively [22].

Table 3.
Antiparasitic screening a of the chloroform extract sub-fractions of P. inuloides roots.

Table 4.
Leishmanicidal and cytotoxic activity a of sub-fractions from P. inuloides roots.
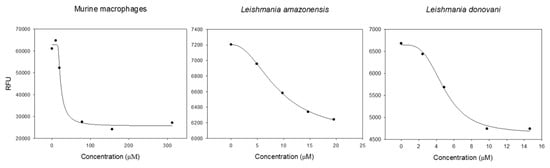
Figure 2.
EC50 curves of cytotoxicity and leismanicidal activity of sub-fraction F1B3.
Activity assays against L. amazonensis intracellular amastigotes (Table 4) revealed that F1B3 was also more potent (IC50 2.87 µM) that the reference drug, miltefosine (IC50 3.12 µM), which increases the interest of this active sub-fraction as potential leishmanicidal agent.
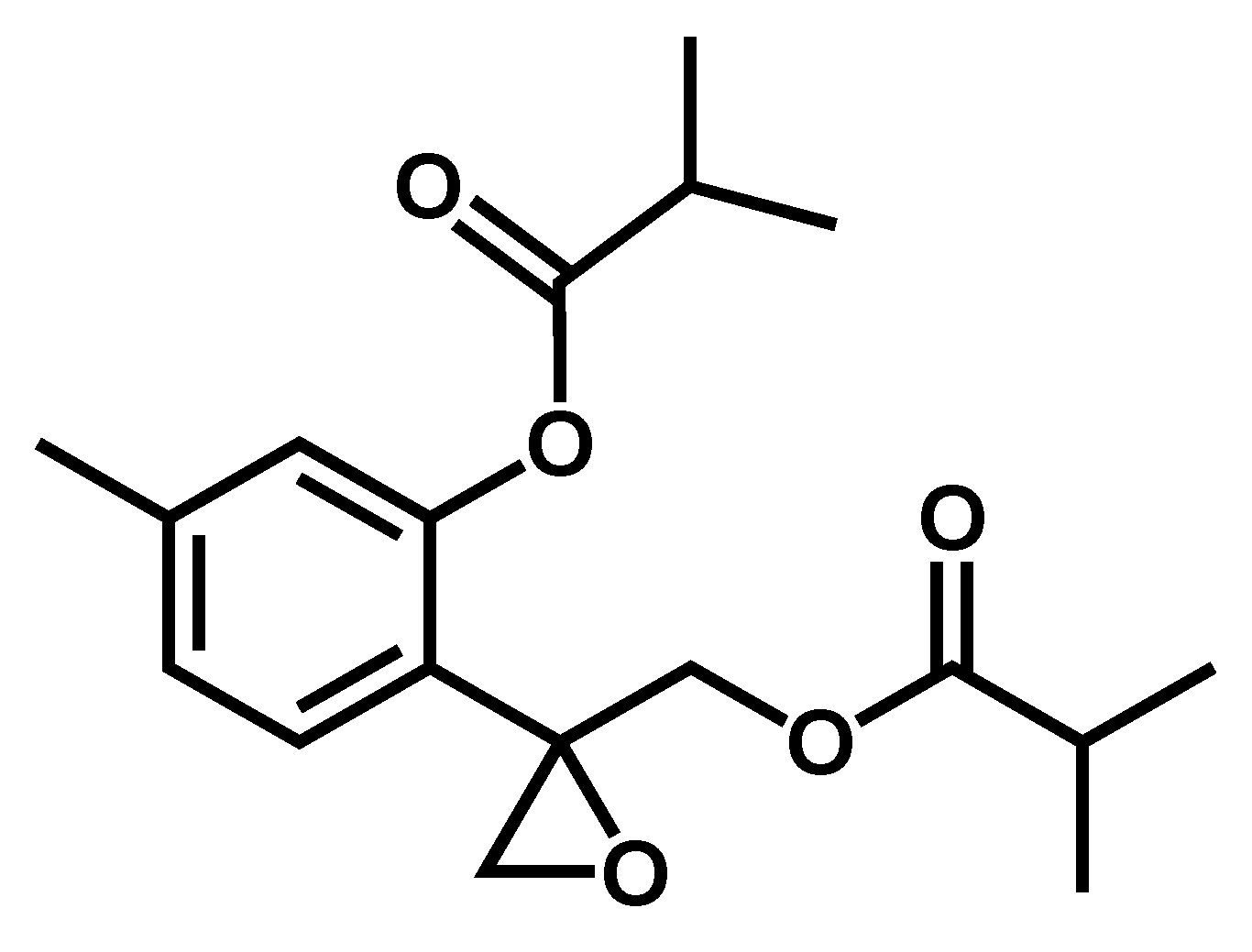
TLC analysis of fraction F1B3 suggested it could be a pure compound (Figure 3). Therefore, spectrometric and spectroscopic studies, including 1D- and 2D-NMR spectra (Supplementary Materials, Figures S2–S6), led to the characterization of F1B3 as 10-isobutyryloxy-8,9-epoxy-thymol isobutyrate (1) [23] as an enantiomeric mixture with around a 75:25 scalemic proportion as determined by the integration of the signals in its 1HNMR spectrum. Previously, the absolute configuration and scalemic proportion determination of epoxy-thymol derivatives using (S)-BINOL as a chiral solvating agent combined with vibrational circular dichroism (VCD) studies has been reported [24]. By applying this methodology the authors determined the enantiomeric ratio of this expoxy-thymol derivative as (8S:8R)-(75:25 er) with specific rotation value [α]436 +18.9. Compound 1 shows a specific rotation of [α]20D -18.5 value, suggesting its structure corresponds to (8R:8S)-(75:25 er)-10-isobutyryloxy-8,9-epoxy-thymol isobutyrate (1).
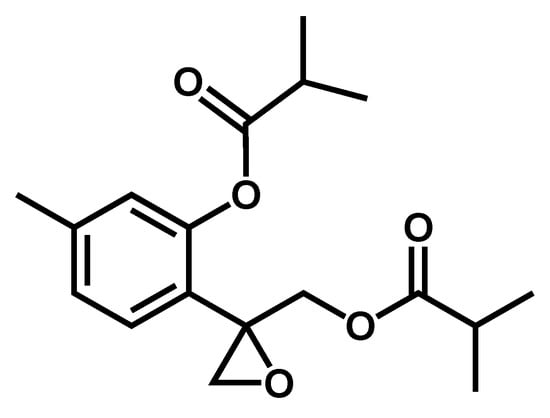
Figure 3.
Structure of the leismanicidal thymol derivative 1, isolated from the roots of P. inuloides by bioguided fractionation.
Compound 1 has been isolated from 40 different plants that represent 60% of the total reported vegetal species containing epoxythymol derivatives. Only about 10% of the known functionalized thymol derivatives have been evaluated as antibacterial, anti-inflammatory, antioxidant, antiprotozoal, cytotoxic, piscicidal, or allelophatic agents [25]. Regarding epoxythymol derivatives, evaluation on Entamoeba histolytica and Giardia lamblia of thymol isobutyrate derivatives revealed a moderate antiprotozoal activity on both protozoa [26]. However, studies on their leishmanicidal or antioxidant activities have not been reported.
To our knowledge, this is the first report of thymol derivative 1 in Pulicaria genus. Previously, the leishmanicidal bioguided fractionation of the active chloroform extract from the aerial part of P. inuloides led to the isolation of quercetagetin-3,5,7,3’-tetramethyl ether as the main bioactive component, exhibiting a moderate leishmanicidal activity with an IC50 value of 0.483 mM on L. amazonensis [19]. Furthermore, the present study reveals that a thymol derivative, the major component from the roots of this species, is responsible for the leishmanicidal activity. This further supports P. inuloides as a source of promising leishmanicidal agents.
3. Materials and Methods
3.1. General Experimental Procedure
Optical rotation was measured on a Perkin Elmer 241 automatic polarimeter in CHCl3 at 20 °C. 1H (600 MHz), 13C (150 MHz) Nuclear Magnetic Resonance (NMR) spectra were recorded on a Bruker Avance 600 spectrometer, the chemical shifts are given in δ (ppm) with residual CDCl3 (δH 7.26, δC 77.0) as internal reference and coupling constants in Hz; the experiments were carried out with the pulse sequences given by Bruker. EIMS and HREIMS were collected with a Micromass Autospec spectrometer. Silica gel 60 (particle size 15–40 and 63–200 μm, Macherey-Nagel) was used for column chromatography, while silica gel 60 F254 (Macherey-Nagel) were used for analytical or preparative TLC. The spots were visualized by UV light and heating silica gel plates sprayed with H2O-H2SO4-AcOH (1:4:20). All solvents used were purchased from Sigma Aldrich.
3.2. Plant Material and Extraction
Pulicaria inuloides (Poir.) DC. was collected at flowering stage in 2013 in Bechar Dam Djorf Ettorba (80 km south west from Bechar) by Professor Samir Benayache. A voucher specimen (PU/105/VAR/05-15) was identified by Ben Abd El-Hakem Mohamed, Head of the Protection Plants Service in Algeria and was deposited in the Herbarium of the VARENBIOMOL Research Unit, Université des Frères Mentouri, Constantine, Algeria.
The powdered air-dried roots of P. inuloides (200 g) were extracted three times with 80% ethanol (3 × 1 L) at 26 °C. The extracts were filtered through cotton and solvent was evaporated under reduced pressure to dryness. The dry residue (45 g) was further suspended in water and extracted, successively, with chloroform (3 × 100 mL), ethyl acetate (3 × 100 mL) and n-butanol (5 × 100 mL) at room temperature. Each extract was collected, separately, and concentrated by a rotary vacuum evaporator to remove the solvent to yield the CHCl3 (0.89 g, 0.44% w/w on dry plant material), EtOAc (1 g, 0.50% w/w) and n-BuOH (4 g, 2.0% w/w) extracts.
3.3. DPPH Radical Scavenging Assay
The antioxidant activity of the extracts has been evaluated using the DPPH method (2,2-diphenyl-1-picrylhydrazyl) [27]. All extracts have been prepared in methanol at a concentration of 8 mg/mL. Then, 30 μL of sample solutions at different concentrations were added to 3 mL of DPPH solution (0.04 mg/mL). After 30 min of incubation in the dark at room temperature, the absorbance of the samples was measured at 517 nm with an UV-Vis spectrophotometer (Thermo Scientific Evolution 300). The capability to scavenge the DPPH radical was calculated using the following equation: % inhibition = [(Ac – As)/Ac] x 100; where Ac is the blank control absorbance and is the sample absorbance at 517 nm. Ascorbic acid was used as a positive control. All determinations were carried out in duplicate, and the results were expressed as IC50 values (concentration of sample required to scavenge 50% DPPH free radicals) calculated from a calibration curve using Microsoft Excel [28].
3.4. Determination of Total Phenolic Content
The total phenolic content of extracts was measured by the Folin-Ciocalteu method [21] with slight modifications. Briefly, the extracts were prepared at a concentration of 1.0 mg/mL in distilled water. There were 500 μL aliquot transferred into a test tube and 1.0 mL of Folin-Ciocalteu reagent (1 N) was added. The mixture was allowed to stand at room temperature for 4 min, and afterward, 5 mL of 20% sodium carbonate were added to the mixture and was kept at room temperature for 2 h prior to recording the absorbance at 765 nm with an UV-Vis spectrophotometer. All determinations were carried out in duplicate. The total phenolic content was calculated from a calibration curve, and the results are expressed as µg gallic acid equivalents per mg of extract (µgGAE/mg).
3.5. Determination of Total Flavonoid Content
The total flavonoid content in extracts was determined by the aluminium trichloride method [29], using quercetin as the reference compound. Briefly, a volume of 2 mL of ethanolic solution of extract (1 mg/mL) was mixed with 2 mL of 2 % AlCl3. After incubation at room temperature for 1 h, the absorbance was measured at 420 nm. All determinations were carried out in duplicate. The total flavonoid content was calculated from a calibration curve, and the results were expressed as µg quercetin equivalent per mg of extract (µgQE/mg).
3.6. Parasite Strains
The leishmanicidal activity was evaluated against promastigotes of L. amazonensis (MHOM/BR/77/LTB0016) and L. donovani (MHOM/IN/90/GE1F8R). Promastigotes were cultured in Schneider’s medium (Sigma-Aldrich, Madrid, Spain) supplemented with 10% fetal bovine serum at 26 °C and were grown to the log phase as per previous methods. For some of the assays, the parasites were also cultured in RPMI 1640 medium (Gibco), with or without phenol red [30].
3.7. In Vitro Effect on Promastigote Forms of Leishmania spp
The activity of crude extracts and fractions were determined by the modified alamarBlue® assay (Invitrogen/Life Technologies, Madrid, Spain) as previously described [31]. This simple and rapid test is based on oxido/reduction reaction. Briefly, the oxidized, blue, non-fluorescent Alamar Blue is reduced to a pink fluorescent dye in the medium by cell activity. This reaction could be measured either by colorimetric or fluorimetric [32]. Samples were dissolved in dimethyl sulfoxide (DMSO) and further dilutions were made in RPMI 1640 medium. The final DMSO concentration never exceeded 0.1% (v/v) with no effect on the parasites proliferation or morphology. Promastigotes of L. amazonensis and L. donovani were grown at 26 °C in RPMI 1640 modified medium (Gibco) and supplemented with 10% heat-inactivated fetal bovine serum. Logarithmic phase cultures were used for experimental purposes, and the in vitro susceptibility assay was performed in sterilized 96-wellmicrotiter plates (Corning™). To these wells, 106/well parasites were added, and the samples at the concentration to be tested. The final volume was 200 μL in each well. After an incubation of 72 h, analysis of the plates was carried out visually using an inverted microscope. Subsequently, in the case of the pure compound, the plates were analyzed on an EnSpire multimode plate reader (PerkinElmer, MA, USA) using a test wavelength of 570 nm and a reference wavelength of 630 nm. Miltefosine, kindly provided by Æterna Zentaris Inc., was used as reference drug. Percentage of inhibition, 50% inhibitory concentrations (IC50) for the active samples, was calculated by linear regression analysis with 95% confidence limit. All experiments were performed three times each in duplicate, and the mean values were calculated. A non-parametric regression, adjusting the data to a four-parameter logistic curve was used for analysis of the data. The obtained inhibition curves statistical analysis was undertaken using Sigma Plot 12.0 software program (Systat Software Inc.).
3.8. In Vitro Effect on Leishmania Amazonensis Amastigote Stage
Activity assay against intracellular amastigotes was performed according to Jain et al. (2012) [33]. Macrophages (J774A.1 cell line) were placed in a 96-well flat bottom plate at a density of 2 × 105/mL in RPMI supplemented with 10% SBF and incubated for an hour at 37 °C in 5 % CO2 environment. Additionally, 100 µL of stationary phase promastigotes (7 days old culture) were added in a 10:1 ratio, and plates were re-incubated at 37 °C overnight to allow a maximum infection. After incubation, free promastigotes were washed off with the same medium at least 3 times. 50 μL of culture medium (RPMI-1640 with 10% FBS) were added into each well. Subsequently, a serially dilution of test compounds was made in a 96-deep well plate with the same medium, and then 50 μL of this serially-diluted standard were added to each well. The plates were incubated at 37 °C, 5% CO2 for 24 h. After incubation, the medium from each well was removed, and 30 μL of Schneider (with 0.05% SDS) was added to each well. Plate was shacked for 30 sec. and 170 μL of Schneider medium were added to each well. AlamarBlue at 10% was added into each well of the 96-well plates and incubated at 26 °C for 72 h to allow transformation of rescued amastigotes to promastigotes. After incubation, the emitted fluorescence was measured in a Perkin Elmer EnSpire spectrofluorometer at 585 nm.
3.9. Cytotoxicity Assay
Cytotoxicity was evaluated after 24 h incubation of macrophages J774A.1 (ATCC TIB-67) murine macrophage cell line with different concentrations of samples. The viability of the macrophages was determined with alamarBlue® assay as previously described [34]. Briefly, the macrophages at concentration of 105 cells/mL cultured in RPMI medium supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 were incubated with different concentrations of sample and AlamarBlue reagent is added at 10% of the final volume. After 24 h of incubation, the plates were analyzed on an EnSpire multimode plate reader (PerkinElmer, MA, USA) using a test wavelength of 570 nm and a reference wavelength of 630 nm. Dose response curves were plotted and the CC50 was obtained. The analyses were performed in triplicate.
3.10. Leishmanicidal Bioguided Fractionation
Fractionation of the active chloroform extract was performed against the promastigote stage of L. amazonensis and L. donovani (Scheme 1). The CHCl3 extract (0.89 g) was subjected to column chromatography on silica gel and eluted with dichloromethane-methanol of increasing polarity (CH2Cl2-MeOH; 100:0, 70:30, 30:70, 0:100). Four fractions of 50 mL were collected, and then combined in two fractions on the basis of their thin layer chromatography (TLC) profiles. Based on the results of leishmanicidal screening assay, the active fraction F1 (268 mg) was submitted to further silica gel column chromatography eluted with hexanes-ethyl acetate of increasing polarity (100:0 to 0:100). Three sub-fractions (F1A-F1C) were obtained based on their TLC profile, and tested for their activity against L. amazonensis and L. donovani. The active F1B fraction (36 mg) was purified on a preparative silica gel thin layer chromatography (PTLC), using mixtures of hexanes-ethyl acetate (50:50) to obtain sub-fractions F1B1-F1B3. Sub-fraction F1B3 yielded compound 1, as a pale yellow amorphous solid (10 mg), which was identified as 10-isobutyryloxy-8,9-epoxy-thymol isobutyrate by comparison of its spectroscopic and spectrometric data with those previously reported [23,24].
4. Conclusions
This study aimed to investigate the therapeutic potential of P. inuloides, an Algerian species used for traditional medicine. The results reported herein indicated that the ethyl acetate extract of the roots possesses a significant antioxidant profile, in accordance with a high total phenolic content. Moreover, the leishmanicidal bioguided fractionation against L. amazonensis and L. donovani of the active chloroform extract led to the isolation of a thymol derivative as the main bioactive component, showing higher potency that the reference drug against promastigote and amastigote forms of L. amazonensis. This study, and the previously reported one on the aerial part of this species, underlines this plant as being a potential source of therapeutic agents. Further studies will be conducted in intracellular amastigote form of the parasites to explore the potential of P. inuloides and its metabolites as alternative or complementary leishmanicidal remedies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/8/4/201/s1, Figure S1: Calibration graphs for total phenolic and flavonoid contents, Figures S2–S6: 1D and 2D NMR spectra of thymol dervative 1.
Author Contributions
Data curation, All authors; Formal analysis, I.S., H.F. and A.L-A.; Funding acquisition, I.L.B., J.E.P. and J.L.-M.; Investigation, all authors.; Methodology, I.A.J., J.E.P. and J.L.-M.; Supervision, I.L.B., I.A.J., J.E.P., S.B. and J.L.-M.; Writing—original draft, I.L.B., H.F. and J.L.-M.; Writing—review & editing, I.L.B., J.L.-M. and A.L.-A.
Funding
This study was supported by PI18/01380 and RICET (RD16/0027/0001 of the program of Redes Temáticas de Investigación Cooperativa, FIS), Spanish Ministry of Health, Spain, and RTI2018-094356-B-C21 Spanish MINECO co-funded by the European Regional Development Fund (FEDER) projects. HF was supported by a stage grant of the Ministry of Higher Education and Scientific Research of Algeria (Exceptional National Program 2016–2017). IS thanks the Cabildo de Tenerife (Agustín de Betancourt Program).
Conflicts of Interest
The authors declare no conflict of interest
References
- WHO. Leishmaniasis. 2019. Available online: https://www.who.int/es/news-room/fact-sheets/detail/leishmaniasis (accessed on 29 July 2019).
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; López-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Guidelines. Diagnosis and treatment of leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 2017, 96, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Khezzani, B.; Bouchemal, S. Demographic and spatio-temporal distribution of cutaneous leishmaniasis in the Souf oasis (Eastern South of Algeria): Results of 13 years. Acta Trop. 2017, 166, 74–80. [Google Scholar] [CrossRef] [PubMed]
- WHO. Leishmaniasis. Comprehensive Analytical Profile: Algeria. 2017. Available online: http://www.aho.afro.who.int/profiles_information/index.php/Algeria:Index (accessed on 29 July 2019).
- Kardjadj, M.; Ben-Mahdi, M.H. Epidemiology of dog-mediated zoonotic diseases in Algeria: A One Health control approach. New Microbe New Infect. 2019, 28, 17–20. [Google Scholar] [CrossRef] [PubMed]
- WHO. Algeria. Leishmaniasis Country Profiles. 2014. Available online: https://www.who.int/leishmaniasis/burden/Leishmaniasis_Algeria/en (accessed on 29 July 2019).
- Adel, A.; Boughoufalah, A.; Saegerman, C.; Deken, R.D.; Bouchene, Z.; Soukehal, A.; Berkvens, D.; Boelaert, M. Epidemiology of visceral leishmaniasis in Algeria: An update. PLoS ONE 2014, 9, e99207. [Google Scholar] [CrossRef]
- Eddaikra, N.; Ait-Oudhia4, K.; Kherrachi1, I.; Oury, B.; Moulti-Mati, F.; Benikhlef, R.; Harrat, Z.; Sereno, D. Antimony susceptibility of Leishmania isolates collected over a 30-year period in Algeria. PLoS Negl. Trop. Dis. 2018, 12, e0006310. [Google Scholar] [CrossRef]
- Domingues, P.L.F.; Cruz, L.A.; Santos-Gomes, G.; Rodrigues, E.; Laurenti, M.D.; Ghilardi, L.J.H. Conventional versus natural alternative treatments for leishmaniasis: A review. Curr. Top. Med. Chem. 2018, 18, 1275–1286. [Google Scholar]
- Williams, C.A.; Harborne, J.B.; Greenham, J.R.; Grayer, R.J.; Kite, G.C.; Eagles, J. Variations in lipophilic and vacuolar flavonoids among European Pulicaria species. Phytochemistry 2003, 64, 275–283. [Google Scholar] [CrossRef]
- Liu, L.L.; Yang, J.L.; Shi, Y.P. Phytochemicals and biological activities of Pulicaria species. Chem. Biodivers. 2010, 7, 327–349. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Kriegisch, S.; Harms, M.; Wende, K.; Lindequist, U. Assessment of selected Yemeni medicinal plants for their in vitro antimicrobial, anticancer, and antioxidant activities. Pharm. Biol. 2011, 49, 200–210. [Google Scholar] [CrossRef]
- Awadh, A.N.A.; Julich, W.D.; Kusnick, C.; Lindequist, U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J. Ethnopharmacol. 2001, 74, 173–179. [Google Scholar]
- Al-Hajj, N.Q.M.; Wang, H.X.; Ma, C.; Lou, Z.; Bashari, M.; Thabit, R. Antimicrobial and antioxidant activities of the essential oils of some aromatic medicinal plants (Pulicaria inuloides-Asteraceae and Ocimum forskolei-Lamiaceae). Trop. J. Pharm. Res. 2014, 13, 1287–1293. [Google Scholar] [CrossRef]
- Al-Hajj, N.Q.M.; Wan, H.; Gasmalla, M.A.A.; Ma, C.; Thabit, R.; Rahman, M.R.T.; Tang, Y. Chemical composition and antioxidant activity of the essential oil of Pulicaria inuloides. J. Food Nutr. Res. 2014, 2, 221–227. [Google Scholar] [CrossRef]
- Al-Hajj, N.Q.M.; Ma, C.; Thabit, R.; Al-alfarga, A.; Gasmalla, M.A.A.; Musa, A.; Aboshora, W.; Wang, H. Chemical composition of essential oil and mineral contents of Pulicaria inuloides. J. Acad. Ind. Res. 2014, 2, 675–678. [Google Scholar]
- Al-Hajj, N.Q.M.; Algabr, M.N.; Omar, K.A.; Wang, H. Anticancer, antimicrobial and antioxidant activities of the essential oils of some aromatic medicinal plants (Pulicaria inuloides-Asteraceae). J. Food Nutr. Res. 2017, 5, 490–495. [Google Scholar] [CrossRef]
- Galala, A.A.; Sallam, A.; Abdel-Halim, O.B.; Gedara, S.R. New ent-kaurane diterpenoid dimer from Pulicaria inuloides. Nat. Prod. Res. 2016, 30, 2468–2475. [Google Scholar] [CrossRef] [PubMed]
- Fadel, H.; Sifaoui, I.; López, A.A.; Reyes, B.M.; Hajaji, S.; Chiboub, O.; Jiménez, I.A.; Bazzocchi, I.L.; Lorenzo, J.M.; Benayache, S.; et al. Assessment of the antiprotozoal activity of Pulicaria inuloides extracts, an Algerian medicinal plant: Leishmanicidal bioguided fractionation. Parasitol. Res. 2018, 117, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Likhitwitayawuid, K.; Angerhofer, C.K.; Chai, H.; Pezzuto, J.M.; Cordell, G.A.; Ruangrungsi, N. Cytotoxic and antimalarial alkaloids from the tubers of Stephania pierrei. J. Nat. Prod. 1993, 56, 1468–1478. [Google Scholar] [CrossRef]
- Mossa, J.S.; El-Feraly, F.S.; Muhammad, I.; Zaw, K.; Mbwambo, Z.H.; Pezzuto, J.M.; Fong, H.H.S. Sesquiterpene lactones and thymol esters from Vicoa pentanema. J. Nat. Prod. 1997, 60, 550–555. [Google Scholar] [CrossRef]
- Arreaga-González, H.M.; Pardo-Novoa, J.C.; del Río, R.E.; Rodríguez-García, G.; Torres-Valencia, J.M.; Manríquez-Torres, J.J.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Methodology for the absolute configuration determination of epoxythymols using the constituents of Ageratina glabrata. J. Nat. Prod. 2018, 81, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Talavera-Alemán, A.; Rodríguez-García, G.; López, Y.; García-Gutiérrez, H.A.; Torres-Valencia, J.M.; del Río, R.E.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Systematic evaluation of thymol derivatives possessing stereogenic or prostereogenic centers. Phytochem. Rev. 2016, 15, 251–277. [Google Scholar] [CrossRef]
- Bustos-Brito, C.; Vázquez-Heredia, V.J.; Calzada, F.; Yépez-Mulia, L.; Calderón, J.S.; Hernández-Ortega, S.; Esquivel, B.; García-Hernández, N.; Quijano, L. Antidiarrheal thymol derivatives from Ageratina glabrata. Structure and absolute configuration of 10-benzoyloxy-8,9-epoxy-6-hydroxythymol isobutyrate. Molecules 2016, 21, 1132. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. The use and abuse of the DPPH radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, H.; Kolak, U.; Meric, C. Antioxidant, anticholinesterase and antibacterial activities of Jurinea consanguinea DC. Rec. Nat. Prod. 2011, 5, 43–51. [Google Scholar]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Isla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- López-Arencibia, A.; Martín-Navarro, C.; Sifaoui, I.; Reyes-Batlle, M.; Wagner, C.; Lorenzo-Morales, J.; Maciver, S.K.; Piñero, J.E. Perifosine mechanisms of action in Leishmania species. Antimicrob. Agents Chemother. 2017, 61, e02127-16. [Google Scholar] [CrossRef]
- Cabrera-Serra, M.G.; Lorenzo-Morales, J.; Romero, M.; Valladares, B.; Piñero, J.E. In Vitro activity of perifosine: A novel alkylphospholipid against the promastigote stage of Leishmania species. Parasitol. Res. 2007, 100, 1155–1157. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pogman, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012, 70, e4054. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Martín-Navarro, C.M.; López-Arencibia, A.; Santana-Morales, M.A.; Afonso, L.R.N.; Maciver, S.K.; Valladares, B.; Martínez-Carretero, E. Therapeutic potential of a combination of two gene-specific small interfering. Antimicrob. Agents Chemother. 2010, 54, 5151–5155. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).