Abstract
Leishmaniasis and American trypanosomiasis are parasitic diseases that cause significant clinical, social and economic impact on the population of tropical and subtropical countries. Their current treatment is limited and presents multiple drawbacks, including high toxicity, high cost, lengthy treatment plans, as well as the emergence of resistant species. Therefore, there is a need to find new lead compounds with high potency against parasites and low toxicity in patients. In the present work, the bioguided fractionation of an endemic plant from the Canary Islands, Withania aristata, led to the identification of withanolide-type metabolites (1–3) with leishmanicidal and trypanocidal activities. Compounds 1 and 3 showed a significant dose-dependent inhibition effect on the proliferation of L. amazonensis promastigotes and T. cruzi epimastigotes, higher than the reference drugs, miltefosine and benznidazole, respectively. Moreover, compounds 1–3 were more potent (IC50 0.055–0.663 µM) than the reference drug against the intracellular amastigote stage of L. amazonensis, with a high selectivity index on murine macrophage cells (SI 58.66–216.73). Studies on the mechanism of death showed that the compounds induced programmed cell death or that which was apoptosis-like. The present findings underline the potential of withanolides as novel therapeutic antikinetoplastid agents.
1. Introduction
Leishmaniasis and American trypanosomiasis (Chagas disease), protozoal diseases, are neglected tropical diseases causing considerable morbidity, affecting millions of people every year worldwide [1]. Leishmaniasis is an infectious disease caused by protozoal parasites of the genus Leishmania and is transmitted by the bite of a sand fly belonging to the genera Lutzomyia and Phlebotomus. Leishmaniasis presents three different forms: cutaneous, mucocutaneous and visceral [2]. Furthermore, American trypanosomiasis affects nearly 8 million people in endemic countries and the available treatments only include benznidazole or nifurtimox, which are effective only in the acute phase of the infection [3]. Despite advances in antiparasitic chemotherapy and supportive care, these diseases have remained a public health challenge, mostly due to problems associated with chemotherapeutic regimen. In fact, the treatment of these infections has been ineffective by variable sensitivity between species, toxicity, route of administration, requirement for long courses of administration, resistance, adverse effects and cost [4]. In this sense, natural products from plants are a promising source of novel lead compounds, characterized by unique chemical architectures, pharmacophores and inherent drug-like properties [5].
The genus Withania (Solanaceaea) is distributed in the Macaronesian region, the east of Mediterranean area, and south Asia [6], and some species are well known in folk medicine. The therapeutic potential of Withania species has been ascribed to the presence of withanolides, which are structurally diverse C28-steroidal compounds with an ergostane skeleton [7]. In particular, Withania aristata is an endemic plant from the Canary Islands, popularly known as orobal. This plant has been used in traditional medicine as a scarring agent, antispasmodic, as well as for rheumatism, eye diseases and otitis, insomnia, constipation and urinary pathologies [8]. Previous phytochemical studies on W. aristata described the isolation of withanolide-type metabolites with cytotoxic [9,10] and diuretic properties [11], including the well-known antiproliferative agent withaferin A [12].
Several typical markers of mammalian apoptosis have been found in Leishmania, suggesting the existence of an apoptosis-like death in this genus. These markers include: cell shrinkage, nuclear chromatin condensation, DNA fragmentation, membrane blebbing, mitochondrial transmembrane potential loss and phosphatidylserine exposure [13]. Moreover, Leishmania cell death appears very peculiar, since different stimuli can induce a form of cell death with the same phenotypic features as in mammalian apoptosis that could be named Leishmania apoptosis [14] or apoptosis-like.
The present work reports the identification of three known withanolides from the leaves of W. aristata through a bioassay-guided fractionation carried out against Leishmania spp. and Trypanosoma cruzi. In order to investigate the mechanism of action for their antiparasitic effects, a group of experiments was performed to study the induction of the apoptosis-like mechanism.
2. Results and Discussion
2.1. Bioassay-Guided Fractionation
The hexanes and acetone extracts of the leaves of Withania aristata were evaluated against promastigote forms of L. amazonensis and L. donovani, and on the epimastigote stage of T. cruzi. Cytotoxicity on murine macrophages was also assessed searching for selectivity (Table 1). In addition, miltefosine and benznidazole were evaluated for comparative purposes as reference drugs against leishmaniasis and Chagas disease respectively. Miltefosine shows IC50s of 2.64 µg/mL and 1.35 µg/mL against L. amazonensis and L. donovani, respectively, and CC50 of 29.42 µg/mL, whereas benznidazole showed an IC50 of 1.81 µg/mL against T. cruzi, with a CC50 of 104.1 µg/mL. The acetone extract showed activity against the three tested species, with IC50s between 2.87 and 20.25 µg/mL. The most sensitive species was L. amazonensis and the less sensitive one was L. donovani. The selectivity index (SI), ranging from 2.1 to 14.9, was a promising fact to continue with the bioassay-guided fractionation. Therefore, the acetone extract was submitted to vacuum liquid chromatography on silica gel affording three fractions, F1–F3 (Scheme 1). The most active fractions, F2 (IC50 values ranging from 1.02 to 12.73 µg/mL) and F3 (IC50 values ranging from 3.63 to 22.28 µg/mL), exhibited potent activity on L. amazonesis and T. cruzi, similar to the reference drugs. Furthermore, both fractions showed a good selectivity index (SI around 11.0) on murine macrophages (Table 1), highlighting these two fractions as the most promising ones.

Table 1.
Activity a against promastigote stage of Leishmania spp., epimastigote stage of T. cruzi, and cytotoxicity against eucariotic cells of the extract, fractions and sub-fractions from Withania aristata leaves.
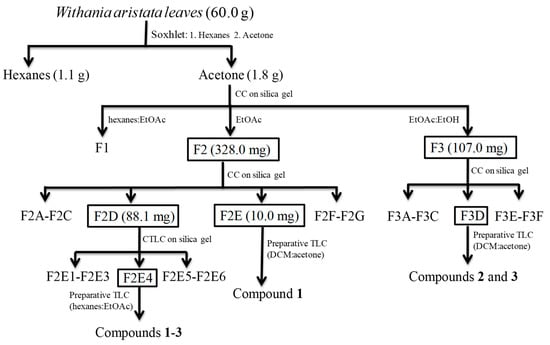
Scheme 1.
Flowchart of the kinetoplasticidal bioguided fractionation of Withania aristata leaves.
The most active fractions, F2 and F3, were further chromatographed on a silica gel column yielding seven (F2A–F2G) and six sub-fractions (F3A–F3F), respectively, which were assayed against the three parasite species. From Fraction 2, F2D exhibited a potent antikinetoplastid effect on L. amazonensis and T. cruzi, which was also higher than, and similar to, miltefosine (IC50 0.36 versus 2.64 µg/mL) and benznidazole (IC50 1.73 versus 1.81 µg/mL), respectively. Regarding fraction F3, sub-fraction F3D was the most active one on the three parasites species, showing IC50s of 1.01 and 3.67 µg/mL for L. amazonensis and L. donovani, respectively, and an IC50 of 3.59 µg/mL for T. cruzi. In addition, the active fractions showed a moderated cytotoxic profile, with CC50 values (>7.65 µg/mL) high enough to continue with the phytochemical analysis.
Therefore, sub-fraction F2D was submitted to purification steps, affording the known withanolides 1–3 (Scheme 1, Figure 1). Moreover, sub-fraction F2E showed to be the most potent one on L. amazonensis (IC50 value 0.19 µg/mL), which after preparative TLC yielded compound 1. Furthermore, sub-fraction F3D was submitted to preparative TLC, affording withanolides 2 and 3 (Scheme 1). Their chemical structures were identified as withaferin A (1) [10], 4β,17α,27-trihydroxy-1-oxo-witha-2,5,24-trienolide (2) [9] and witharistatin (3) [10] by spectrometric and spectroscopic data, including 1D and 2D NMR experiments, and comparison with data reported in the literature.
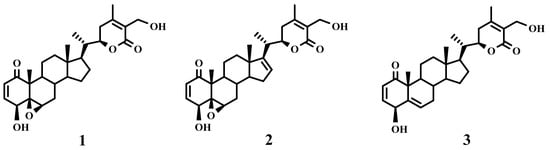
Figure 1.
Chemical structure of withanolides 1–3 isolated from Withania aristata.
Compounds 1–3 were evaluated against the three parasite species (Table 2). The results, over the promastigote stage, showed that compounds 1 and 3 were 7.8- and 2.3-fold more potent than the reference drug [15] against L. amazonensis (IC50 0.83 and 2.82 µM, respectively). Moreover, compounds 1 and 3 were 6.8- and 2.9-fold more potent than benznidazole against T. cruzi epimastigotes (IC50 1.02 and 2.41 µM, respectively). Regarding the selectivity index (SI) on macrophages, as shown in Table 2, compound 1 has a potent effect on L. amazonensis (SI 14.36 versus 11.14 for miltefosine).

Table 2.
Activity of withanolides 1–3 against the promastigote stage of Leishmania, epimastigote stage of T. cruzi and cytotoxicity against eucariotic cells.
Moreover, activity of the withanolides (1–3) against the intracelular amastigote stage of L. amazonensis (Table 3) was higher than that of miltefosine (IC50 3.12 µM), showing IC50 values ranging from 0.055 to 0.663 µM. In fact, withanolides 1 and 3 were 56- and 14.9-fold more potent than the reference drug. Besides, the three compounds showed a higher selectivity index than miltefosine (Table 3), showing compound 1 to have an SI of 216.73 versus 23.13 for miltefosine.

Table 3.
Activity of withanolides 1–3 against amastigote of L. amazonensis.
2.2. Mechanisms of Cell Death
The programmed cell death or apoptosis-like death, is crucial for parasite development and pathogenesis, and the ability of a drug to modulate the life or death of a parasite is recognized for its immense therapeutic potential [16]. In this work, we carried out several experimental approaches to research the apoptotic potential of the withanolides 1–3 on L. amazonesis and T. cruzi.
2.2.1. Withanolides induced Mitochondrial Damage in L. amazonensis
The effect of the tested compounds on the mitochondrial membrane potential was determined by JC-1 fluorescence measure. Moreover, the tested compounds induced a noticeable decrease in the depolarization of L. amazonensis mitochondrial membrane (ΔΨm), since the JC-1 dye remained in the cytoplasm in its monomeric form (Figure 2), thereby showing no clear effect on T. cruzi. The mitochondrial damage was confirmed by quantifying the ATP level after 24 h. Compound 1 at IC90 produced a strong decrease in the total ATP level for L. amazonensis (Figure 3). Furthermore, this effect was not seen in T. cruzi, with an ATP level similar to the untreated cells and with a slight decrease when incubated with compound 1.
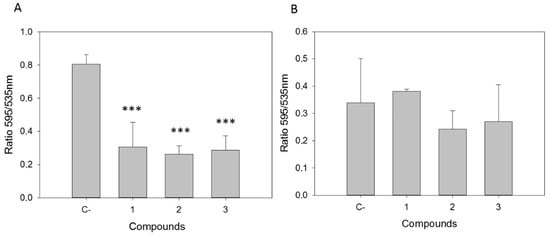
Figure 2.
Changes in the mitochondrial membrane potential (ΔΨm) after 24 h of incubation with the IC90 of withanolides 1–3 in (A) L. amazonensis and (B) T. cruzi. C-: Control. Error bars represent the standard deviation (SD). Each data point indicates the mean of the results of three measurements (***) p < 0.001.
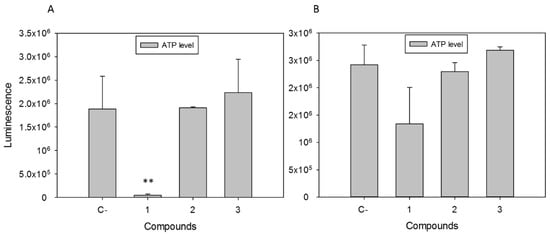
Figure 3.
ATP levels in relative luminescence units of (A) L. amazonensis and (B) T. cruzi after 24 h of incubation with the IC90 of compounds 1–3. C-: Control. Error bars represent the standard deviation (SD). Each data point indicates the mean of the results of three measurements (**) p < 0.01.
Due to T. cruzi epimastigotes being seemingly unaffected at the mitochondrial level by the assayed compounds, analysis of the cell death mechanism was continued only in the L. amazonensis species.
2.2.2. Withanolides Caused Chromatin Condensation in Treated Cells
In view of the obtained results, we decided to analyze the chromatin condensation event, a hallmark of apoptosis-like death, in which small and compact chromatin nuclei appear. To this end, parasites of L. amazonensis were incubated for 24 h with the IC90 of the withanolides, and subsequently, stained with Hoechst and propidium iodide. Condensation of chromatin in the parasites was clearly observed by fluorescence microscopy, since the treated cells showed a higher amount of bright blue in the nucleus (Figure 4). In addition, propidium iodide staining in red was observed in parasites of L. amazonensis treated with compound 1, indicating an advanced process of death, as confirmed by the transmitted light image, in which parasites appeared to be visibly damaged.
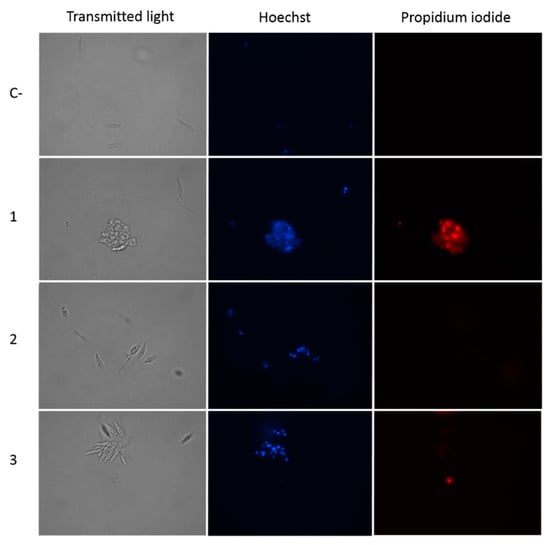
Figure 4.
Hoechst-propidium iodide staining: L. amazonensis incubated with IC90 of withanolides 1–3 and the chromatin condensation was analyzed after 24 h. Images (100×) show chromatin condensation (blue) in treated cells. Red fluorescence corresponds to the propidium iodide stain. C-: Control. Images were obtained using an EVOS FL Cell Imaging System.
2.2.3. Withanolides Induce Cytoplasmic Membrane Permeability in Treated Cells.
The cytoplasmic membrane permeability assays on promastigotes of L. amazonensis with withanolides 1–3 (Figure 5) showed green fluorescence inside the cells, indicating that the plasmatic membrane permeability was slightly damaged, since the cells preserved their integrity and shape.
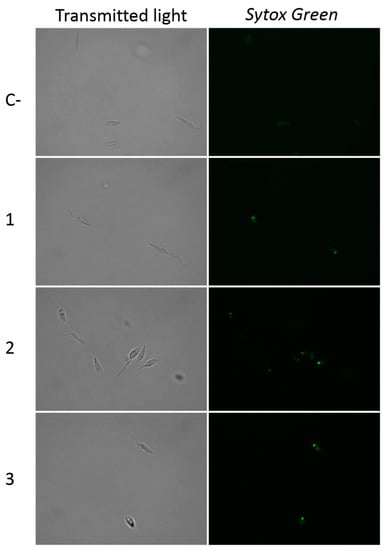
Figure 5.
Plasma membrane permeability alteration (Sytox® Green) caused by withanolides 1–3. C-: Control. Images (100×) are representative of the cell population observed in the performed experiments. Images were obtained using an EVOS FL Cell Imaging System.
2.2.4. Withanolides Induce Oxidative Stress in L. amazonensis
As could be observed in Figure 6, after 24 h of incubation of L. amazonensis promastigotes with the IC90 of the withanolides 1–3, the treated cells showed a higher amount of red staining in the cytoplasm. This corresponds with the reactive oxygen species (ROS) accumulation inside the cell, a well-established event on cells before undergoing a programmed cell death. ROS induced by chemotherapeutic agents is closely associated with mitochondrial function, cytochrome c release, and the apoptosis-like mechanism.
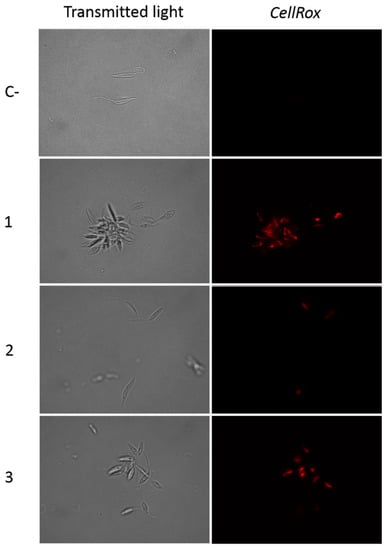
Figure 6.
CellROX Deep Red staining. Results after 24 h of incubation of L. amazonensis promastigotes with the IC90 of withanolides 1–3. C-: Control. Images were captured using an EVOS FL Cell Imaging system (Thermo Fisher Scientific, Waltham, MA, USA) (100×).
Some species belonging to the Withania genera have been shown to present antileishmanial activity. One of the most studied is Withania somnifera, a well-known plant in the Ayurvedic medicine system, which extracts exhibited activity against L. donovani parasites on in vivo models as well as against L. major promastigotes and intracellular amastigotes [17,18]. Withania coagulans aerial parts demonstrated activity against L. major [19]. Furthermore, to the best of our knowledge, only a study on the evaluation of Withania somnifera against T. cruzi has been reported [20].
Withanolides have attracted considerable attention due to their potential in drug research and development [7]. In particular, withaferin A, a well-known anticancer drug candidate, exerts its anticancer effect via induction of apoptosis in several human cancer cells [21]. Recent studies have revealed that withaferin A-analogues are potent apoptotic inducers in different tumor cell lines, evidenced by DNA fragmentation, chromatin condensation and phosphatidylserine exposure, indicating an apoptosis mechanism of action in cancer cells [22,23]. Moreover, the leishmanicidal activity of withaferin A has been reported against L. donovani, inducing an apoptosis-like cell death mechanism by inhibiting protein-kinase C, leading to the depolarization of mitochondrial membrane potential and releasing of cytochrome c into the cytosol, which induces formation of ROS inside cells, causing oxidative DNA lesions [24]. Our data agree with these previous studies, because depolarization of mitochondrial membrane potential was detected, as well as accumulation of ROS, DNA condensation and a lack of ATP, that proves the mechanism of apoptosis-like in L. amazonensis induced by withaferin A (1), as well as by compounds 2 and 3. These compounds could induce apoptosis-like through the mitochondrial pathway initiated by ROS production. Therefore, our proposal is that withanolides-type compounds could induce the formation of ROS, leading this excessive ROS to trigger apoptosis-like death by altering the mitochondrial membrane potential and damaging the respiratory chain, as has been proven in a human leukemia cell line [25].
3. Material and Methods
3.1. General Procedures
Optical rotations were recorded at 25 °C on a Perkin Elmer 241 automatic polarimeter in CHCl3 and the [α]D values are given in 10−1 deg cm2/g. IR (film) spectra were recorded on a Bruker IFS 55 spectrophotometer. NMR spectra were performed on Bruker Avance 500 and 600 spectrometers at 300 ⁰K. The EIMS and HREIMS data were obtained on a Micromass Autospec spectrometer. HRESIMS (positive mode) were performed on an LCT Premier XE Micromass Electrospray spectrometer. Silica gel 60 (particle size 15–40 and 63–200 μm, Macherey-Nagel) was used for column chromatography (CC), while silica gel 60 F254 was used for analytical thin layer chromatography (TLC). Centrifugal planar chromatography was performed by a Chromatotron instrument (model 7924T, Harrison Research Inc., Palo Alto, CA, USA) on manually coated silica gel 60 GF254 (Merck, Kenilworth, NJ, USA) using 1, 2, or 4 mm plates. The developed TLC plates were visualized by UV light and then spraying with HOAc-H2SO4-H2O (80:16:4), followed by heating at 100 °C for 3 min. All the used solvents were purchased from Panreac. Reagents, deuterated solvents and benznidazole were provided by Sigma-Aldrich. For bioassays, Schneider’s medium (Sigma-Aldrich, St. Louis, MO, USA), Alamar Blue® reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA), EnSpire® Multimode Plate Reader (Perkin Elmer, Waltham, MA, USA), RPMI 1640 and LIT media (Gibco, Thermo Fisher, Madrid, Spain), and Leika DMIL inverted microscope (Leika, Wetzlar, Germany) were used. Miltefosine, used as a reference drug, was purchased from Æterna Zentaris, Charleston, SC, USA.
3.2. Plant Material
W. aristata leaves were harvested in Icod de los Vinos (Tenerife, Canary Islands, Spain). A voucher specimen (TFC 53219) is stored at the Herbarium of the Department of Botany, University of La Laguna, Tenerife. The identification was performed by MSc Cristina González Montelongo.
3.3. Extraction, Bioassay-Guided Fractionation and Isolation
The air-dried powdered W. aristata leaves (60.0 g) were extracted successively with hexanes and acetone in a Soxhlet apparatus. The organic extracts were concentrated under reduced pressure to give hexanes (1.1 g, 1.8%) and acetone (1.8 g, 3.0%) extracts. Both extracts were assayed against two species of Leishmania spp. promastigote form and epimastigote stage of T. cruzi for their anti-protozoal activity. After the preliminary screening, the active acetone extract was subjected to a bioassay-guided fractionation procedure. In this way, the extract was fractioned by liquid chromatography on silica gel, eluted with hexanes/EtOAc (2:8, 0.5 L), EtOAc (0.5 L) and EtOAc/EtOH (8:2, 0.5 L), affording three fractions (F1–F3). The most active F2 fraction (328.0 mg) was chromatographed on silica gel column eluting with mixtures of increasing polarity of hexanes/EtOAc (2:8 to 0:10) and EtOAc/EtOH (10:0 to 9:1) to yield 39 sub-fractions, which were combined on the basis of their TLC profile in seven sub-fractions (F2A to F2G). Sub-fraction F2D (88.1 mg) was chromatographed on silica gel by centrifugal thin-layer chromatography (CTLC) on a 1 mm plate, using mixtures of dichloromethane (DCM)/acetone from 9:1 to 7:3) as eluent to give six sub-fractions (F2D1 to F2D6). Sub-fraction F2D4 (19.4 mg) was further purified by preparative TLC with hexanes/EtOAc (1:10) to yield compounds 1 (11.2 mg), 2 (1.9 mg) and 3 (2.4 mg). Sub-fraction F2E (10.0 mg) was further purified by preparative TLC with DCM/acetone (8.5:1.5) to yield compound 1 (7.3 mg). Fraction F3 (107.0 mg) was chromatographed on a silica gel column eluting with mixtures of increasing polarity of hexanes/EtOAc (9:1 to 0:10) and EtOAc/EtOH (10:0 to 8:2) to yield 31 sub-fractions, which were combined on the basis of their TLC profile in six sub-fractions (F3A–F3F). Sub-fraction F3D (7.2 mg) was further purified by preparative TLC with DCM/acetone (8:2) to yield compounds 2 (4.1 mg) and 3 (2.9 mg). The structures of the compounds were identified as withaferin A (1) [10], 4β,17α,27-trihydroxy-1-oxo-witha-2,5,24-trienolide (2) [9] and witharistatin (3) [10] by NMR spectroscopy and mass spectrometry, and comparison with data reported in the literature.
3.4. Cultures
To perform the experiments, promastigotes of L. amazonensis (MHOM/BR/77/LTB0016) and L. donovani (MHOM/IN/90/GE1F8R) and epimastigote T. cruzi (Y strain) were employed. Leishmania species were cultured in Schneider’s medium (Sigma-Aldrich, Madrid, Spain) at 26 °C supplemented with 10% fetal bovine serum (VWR, Biowest, Nuaillé, France) as well as in RPMI 1640 medium (Gibco, Thermo Fisher, Madrid, Spain). Epimastigotes of T. cruzi were cultured in Liver Infusion Tryptose (LIT) medium supplemented with 10% fetal bovine serum at 26 °C. For the cytotoxicity assays, murine macrophage cell line J774A.1 (ATCC TIB-67) was maintained at 37 °C in a 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% fetal bovine serum.
3.5. Leishmanicidal and Trypanocidal Assays Cytotoxic Effect Evaluation
3.5.1. Leishmanicidal Activity Assay
Promastigote in logarithmic phase were used for plate preparation, and the in vitro antiprotozoal assay was performed in 96-well plates. The different fractions or compounds were serially diluted on the wells and 106 parasites/mL were finally added. The activity of the fraction was calculated by the alamarBlue® method [26]. In addition, the active pure molecules were tested against the amastigote stage of L. amazonensis.
The amastigote activity was performed according to Jain et al. [27]. After allowing the transformation of rescued amastigotes to promastigotes, the activity was evaluated by AlamarBlue method. After incubation, the fluorescence was measured in a Perkin Elmer EnSpire spectrofluorometer and fluorescence was measured at emission peak 585 nm.
3.5.2. Trypanocidal Capacity Assay
For the evaluation against the epimastigote stage of T. cruzi, assays were performed following the same procedure mentioned in Section 3.5.1., modifying the density to 2 × 105 parasite/well and using LIT medium.
3.5.3. Cytotoxicity Assay
The cytotoxicity was evaluated using a macrophage cell line J774A.1 in RPMI medium. Macrophages were plated and at 105 macrophages/well and, subsequently, serial dilution of samples were added to the plates. After 24 h of incubation cell viability was determined by the alamarBlue® [28].
3.6. Mechanisms of Cell Death
3.6.1. Mitochondrial Membrane Potential evaluation
To perform the experiment, JC-1 Mitochondrial Membrane, Potential Assay Kit, Cayman Chemical, was employed according the instructions of the kit. Red and green fluorescence was measured with an Enspire microplate reader (PerkinElmer, Waltham, MA, USA).
3.6.2. Measurement of ATP
To determine the ATP level of the parasites, the Cell Titer-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, USA) was used following the manufacturer’s instructions after incubation of the parasites with the calculated IC90 of the tested withanolides for 24 h.
3.6.3. Chromatin Condensation Determination
The detection of condensed chromatin, was performed using the Vybrant™ Apoptosis Assay Kit #5, Hoechst 33342/Propidium Iodide (Invitrogen, Carlsbad, CA, USA). Parasites were incubated with the IC90 of the pure compounds for 24 h, then collected and centrifuged. The cell pellet was resuspended in RPMI, added to a black plate and incubated with the Hoechst 33342 and the propidium iodide. Then the plate was protected from light for 20 min at 4 °C. The EVOS FL Cell Imaging System (Invitrogen, ThermoFisher, Carlsbad, CA, USA) was used to observe the cells, using the DAPI (Hoechst) and RFP (PI) Light Cubes.
3.6.4. Plasma Membrane Permeability
In order to detect membrane permeability alterations SYTOX® Green dye was used. Briefly, 106 parasites/mL previously incubated with the IC90 of the compounds for 24 h, were incubated with SYTOX® Green (Molecular Probes, Eugene, OR, USA) at 1 μM. The fluorescence was monitored in an EVOS FL Cell Imaging System AMF4300, Life Technologies, USA. This dye is impermeable to intact cells, but when their membrane permeability is changed, SYTOX Green has the capacity to get inside the cell and attach to DNA, increasing its fluorescence >500 times.
3.6.5. Oxidative Stress
CellRox Deep Red Oxidative Stress Reagent (Thermo Fisher Scientific, Waltham, MA, USA) is a probe designed to measure reactive oxygen species (ROS) in live cells, exhibiting strong fluorogenic signal under oxidative state. Following the manufacturer’s protocol, parasites were incubated with the compounds at the IC90 concentration for 24 h, then washed, and incubated with 5 μM of CellRox Reagent for 30 min at 26 °C. Then, parasites were centrifuged and resuspended in buffer. H2O2 at 600 μM for 30 min was used as positive control [29]. A fluorescence microscope (EVOS FL) was used to observe the cells. The signal for Deep Red is localized in the cytoplasm.
3.7. Statistical Analysis
Data are presented as mean ± SE. Non-linear regression analysis was used for the IC50 (inhibitory concentration 50) and CC50 (cytotoxic concentration 50) calculations. All determinations were performed in triplicate and the data shown are representative results from at least three independent experiments. Statistical differences between means were tested using one-way analysis of variance (ANOVA; three or more samples), using the SigmaPlot 12.0 software. A significance level of p < 0.05 was used.
4. Conclusions
In summary, the bioassay-guided fractionation of W. aristata against Leishmania spp. and T. cruzi was employed to identify drug candidates for the treatment of leishmaniasis and/or Chagas disease. We have successfully identified three potent and selective withanolides with significant leishmanicidal profiles, and high selectivity indexes for L. amazonensis. In addition, withanolides induced several ultrastructural and morphological changes in promastigotes of L. amazonensis. Mitochondrial membrane potential changes, ROS production, phosphatidylserine externalization, cell shrinkage, a rounded shape of the parasites, nuclear condensation and no change in membrane permeability were observed in parasites that were treated with the mentioned molecules. The major finding in the present study was that these withanolides induced programmed cell death in L. amazonensis, sharing several phenotypic characteristics with other cases of programmed cell death in metazoans, and exhibiting them as promising candidates against leishmaniasis. These findings support future investigations for further biochemical studies to unveil the mechanism of action of these withanolides, which can lead to the optimization of treatment for leishmaniasis.
Author Contributions
Data curation, All authors; Formal analysis, I.S. and A.L.-A.; Funding acquisition, I.L.B., J.E.P. and J.L.-M.; Investigation, all authors; Methodology, I.A.J., J.E.P. and J.L.-M.; Supervision, I.L.B., I.A.J., J.E.P. and J.L.-M.; Writing—original draft, I.L.B. and A.L.-A.; Writing—review and editing, I.L.B., I.A.J. and A.L.-A.
Funding
This study was supported by PI18/01380 and RICET (RD16/0027/0001 of the program of Redes Temáticas de Investigación Cooperativa, FIS), Spanish Ministry of Health, Spain, and RTI2018-094356-B-C21 Spanish MINECO co-funded by the European Regional Development Fund (FEDER) projects. MRB was also funded by RICET. ALA and IS were funded by the Cabildo de Tenerife (Agustin de Betancourt Program). DSNH was funded by ACIISI, Gobierno de Canarias (FEDER).
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Neglected Tropical Diseases. 2019. Available online: http://www.who.int/neglected_diseases/diseases/en/ (accessed on 19 April 2019).
- Rao, S.P.S.; Barrett, M.P.; Dranoff, G.; Faraday, C.J.; Gimpelewicz, C.R.; Hailu, A.; Jones, C.L.; Kelly, J.M.; Lazdins-Helds, J.K.; Mäser, P.; et al. Drug discovery for kinetoplastid diseases: Future directions. ACS Infect. Dis. 2019, 5, 152–157. [Google Scholar] [CrossRef]
- Bustos, P.L.; Milduberger, N.; Volta, B.J.; Perrone, A.E.; Laucella, S.A.; Bua, J. Trypanosoma cruzi infection at the maternal-fetal interface: Implications of parasite load in the congenital transmission and challenges in the diagnosis of infected newborns. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.S.; Khare, S.; Kumar, A.B.; Supek, F.; Buchynskyy, A.; Mathison, C.J.N.; Chennamaneni, N.K.; Pendem, N.; Buckner, F.S.; Gelb, M.H.; et al. Recent developments in drug discovery for leishmaniasis and human african trypanosomiasis. Chem. Rev. 2014, 114, 11305–11347. [Google Scholar] [CrossRef] [PubMed]
- Cheuka, P.M.; Mayoka, G.; Mutai, P.; Chibale, K. The role of natural products in drug discovery and development against neglected tropical diseases. Molecules 2017, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Eich, E. Solanaceae and Convolvulaceae: Secondary Metabolites: Biosynthesis, Chemotaxonomy, Biological and Economic Significance; Springer: Berlin/Heidelberg, Germany, 2008; pp. 16–21. [Google Scholar]
- Chen, L.X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.J. Más de 100 Plantas Medicinales. Medicina Popular Canaria Monografías; Obra Social de La Caja de Canarias; Imprenta Pérez Galdós: Las Palmas de Gran Canaria, España, 2007. [Google Scholar]
- Llanos, G.G.; Araujo, L.M.; Jiménez, I.A.; Moujir, L.M.; Vázquez, J.T.; Bazzocchi, I.L. Withanolides from Withania aristata and their cytotoxic activity. Steroids 2010, 7, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Llanos, G.G.; Araujo, L.M.; Jiménez, I.A.; Moujir, L.M.; Bazzocchi, I.L. Withaferin A-related steroids from Withania aristata exhibit potent antiproliferative activity by inducing apoptosis in human tumor cells. Eur. J. Med. Chem. 2012, 54, 499–511. [Google Scholar] [CrossRef]
- Benjumea, D.; Martín-Herrera, D.; Abdala, S.; Gutiérrez-Luis, J.; Quiñones, W.; Cardona, D.; Torres, F.; Echeverri, F. Withanolides from Whitania aristata and their diuretic activity. J. Ethnopharmacol. 2009, 123, 351–355. [Google Scholar] [CrossRef]
- Chirumamilla, C.S.; Perez-Novo, C.; Van, O.X.; Berghe, W.V. Molecular insights into cancer therapeutic effects of the dietary medicinal phytochemical withaferin A. Proc. Nutr. Soc. 2017, 76, 96–105. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, A.; Alzate, J.F.; MacLeod, E.T.; Kurt, L.C.G.; Fasel, N.; Hurd, H. Apoptotic markers in protozoan parasites. Parasites Vectors 2010, 3, 104. [Google Scholar] [CrossRef]
- Basmaciyan, L.; Azas, N.; Casanova, M. A potential acetyltransferase involved in Leishmania major metacaspase-dependent cell death. Parasites Vectors 2019, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- López-Arencibia, A.; García-Velázquez, D.; Martín-Navarro, C.M.; Sifaoui, I.; Reyes-Batlle, M.; Lorenzo-Morales, J.; Piñero, J. In Vitro Activities of Hexaazatrinaphthylenes against Leishmania spp. Antimicrob. Agents Chemother. 2015, 59, 2867–2874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vandana, D.R.; Tiwari, R.; Katyal, A.; Pandey, K.C. Metacaspases: Potential Drug Target Against Protozoan Parasites. Front Pharmacol. 2019, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Dayakar, A.; Veronica, J.; Sundar, S.; Maurya, R. An in vitro study of apoptotic like death in Leishmania donovani promastigotes by withanolides. Parasitol. Int. 2013, 62, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Veronica, J.; Sundar, S.; Maurya, R. Alcoholic Fractions F5 and F6 from Withania somnifera Leaves Show a Potent Antileishmanial and Immunomodulatory Activities to Control Experimental Visceral Leishmaniasis. Front. Med. (Lausanne) 2017, 4, 55. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Murata, M.; Nakane, T.; Shirota, O.; Sekita, S.; Fuchino, H.; Shinwari, Z.K. Leishmanicidal active withanolides from a Pakistani medicinal plant, Withania coagulans. Chem. Pharm. Bull. (Tokyo) 2012, 60, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.; Al-Sokari, S.; Mothana, R.; Hamed, M.; Wagih, M.; Cos, P.; Maes, L. In vitro antiprotozoal activity of five plant extracts from Albaha region. World J. Pharm. Res. 2016, 5, 338–346. [Google Scholar]
- Lee, I.; Choi, B.Y. Withaferin-A a natural anticancer agent with pleitropic mechanisms of action. Int. J. Mol. Sci. 2016, 17, 290–312. [Google Scholar] [CrossRef]
- Llanos, G.G.; Araujo, L.M.; Jiménez, I.A.; Moujir, L.M.; Rodríguez, J.; Jiménez, C.; Bazzocchi, I.L. Structure-based design, synthesis, and biological evaluation of withaferin A-analogues as potent apoptotic inducers. Eur. J. Med. Chem. 2017, 140, 52–64. [Google Scholar] [CrossRef]
- Perestelo, N.R.; Llanos, G.G.; Reyes, C.P.; Amesty, A.; Sooda, K.; Afshinjavid, S.; Jiménez, I.A.; Javid, F.; Bazzocchi, I.L. Expanding the Chemical Space of Withaferin A by Incorporating Silicon to Improve Its Clinical Potential on Human Ovarian Carcinoma Cells. J. Med. Chem. 2019, 62, 4571–4585. [Google Scholar] [CrossRef]
- Sen, N.; Banerjee, B.; Das, B.B.; Ganguly, A.; Sen, T.; Pramanik, S.; Mukhopadhyay, S.; Majumder, H.K. Apoptosis is induced in leishmanial cells by a novel protein kinase inhibitor withaferin A and is facilitated by apoptotic topoisomerase I-DNA complex. Cell Death Differ. 2007, 14, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, M.L.; Quintana-Aguiar, J.; De La Rosa, J.V.; López-Blanco, F.; Castrillo, A.; Gallardo, G.; Tabraue, C. Phenalenone-photodynamic therapy induces apoptosis on human tumor cells mediated by caspase-8 and p38-MAPK activation. Mol. Carcinog. 2018, 57, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Chammem, N.; Reyes-Batlle, M.; Mejri, M.; Lorenzo-Morales, J.; Abderabba, M.; Piñero, J.E. Activity of olive leaf extracts against the promastigote stage of Leishmania species and their correlation with the antioxidant activity. Exp. Parasitol. 2014, 141, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012, 30, 4054. [Google Scholar] [CrossRef] [PubMed]
- Fadel, H.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Hajaji, S.; Chiboub, O.; Jiménez, I.A.; Bazzocchi, I.L.; Lorenzo-Morales, J.; Benayache, S.; et al. Assessment of the antiprotozoal activity of Pulicaria inuloides extracts, an Algerian medicinal plant: Leishmanicidal bioguided fractionation. Parasitol. Res. 2018, 117, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Codonho, B.S.; Costa, S.S.; Peloso, E.F.; Joazeiro, P.P.; Gadelha, F.R.; Giorgio, S. HSP70 of Leishmania amazonensis alters resistance to different stresses and mitochondrial bioenergetics. Memórias do Instituto Oswaldo Cruz 2016, 111, 460–468. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).