Antiviral Effect of Lithium Chloride and Diammonium Glycyrrhizinate on Porcine Deltacoronavirus In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells Culture and Reagents
2.2. Virus Stocks and Titration
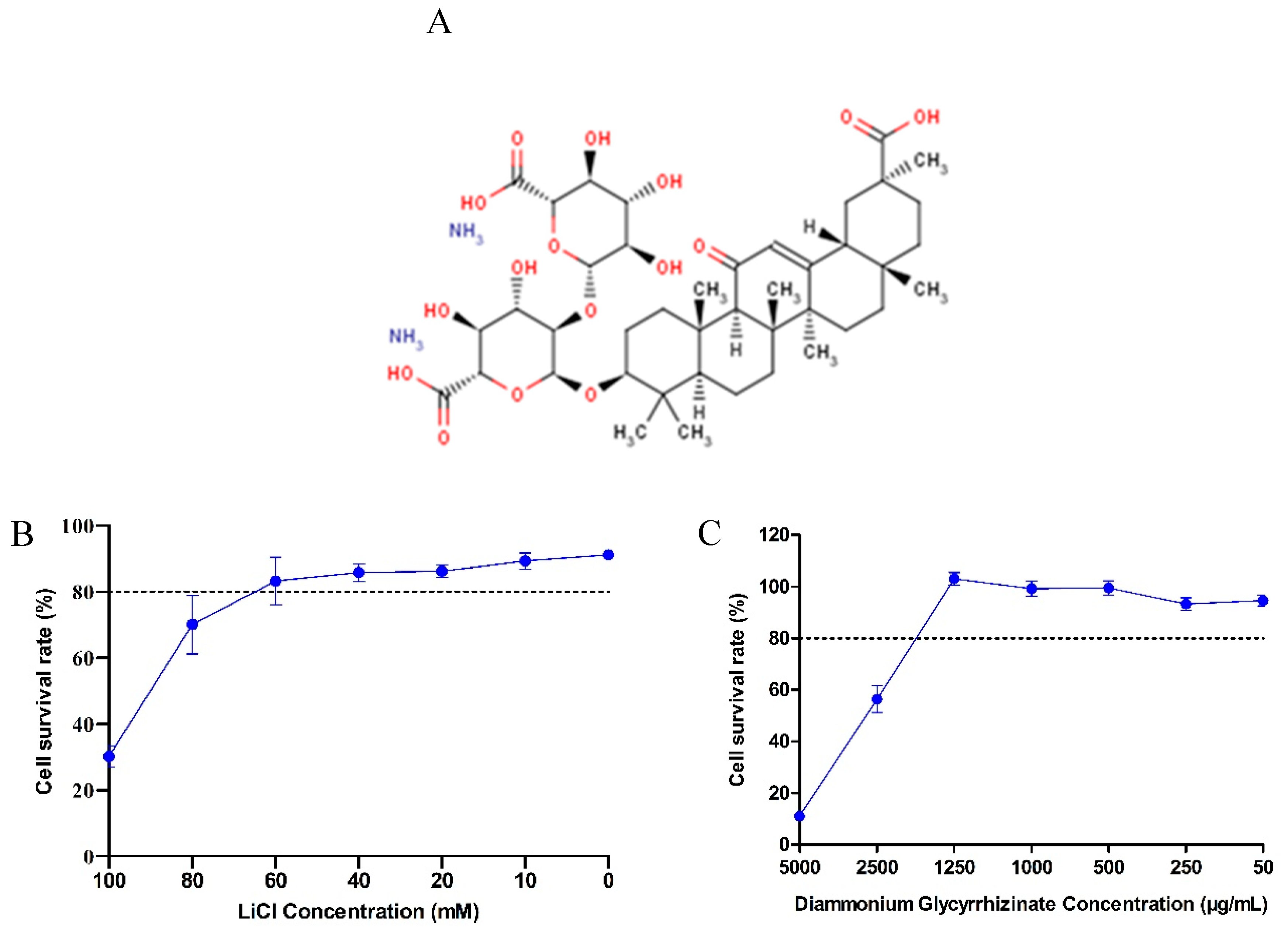
2.3. Cytotoxicity Assay
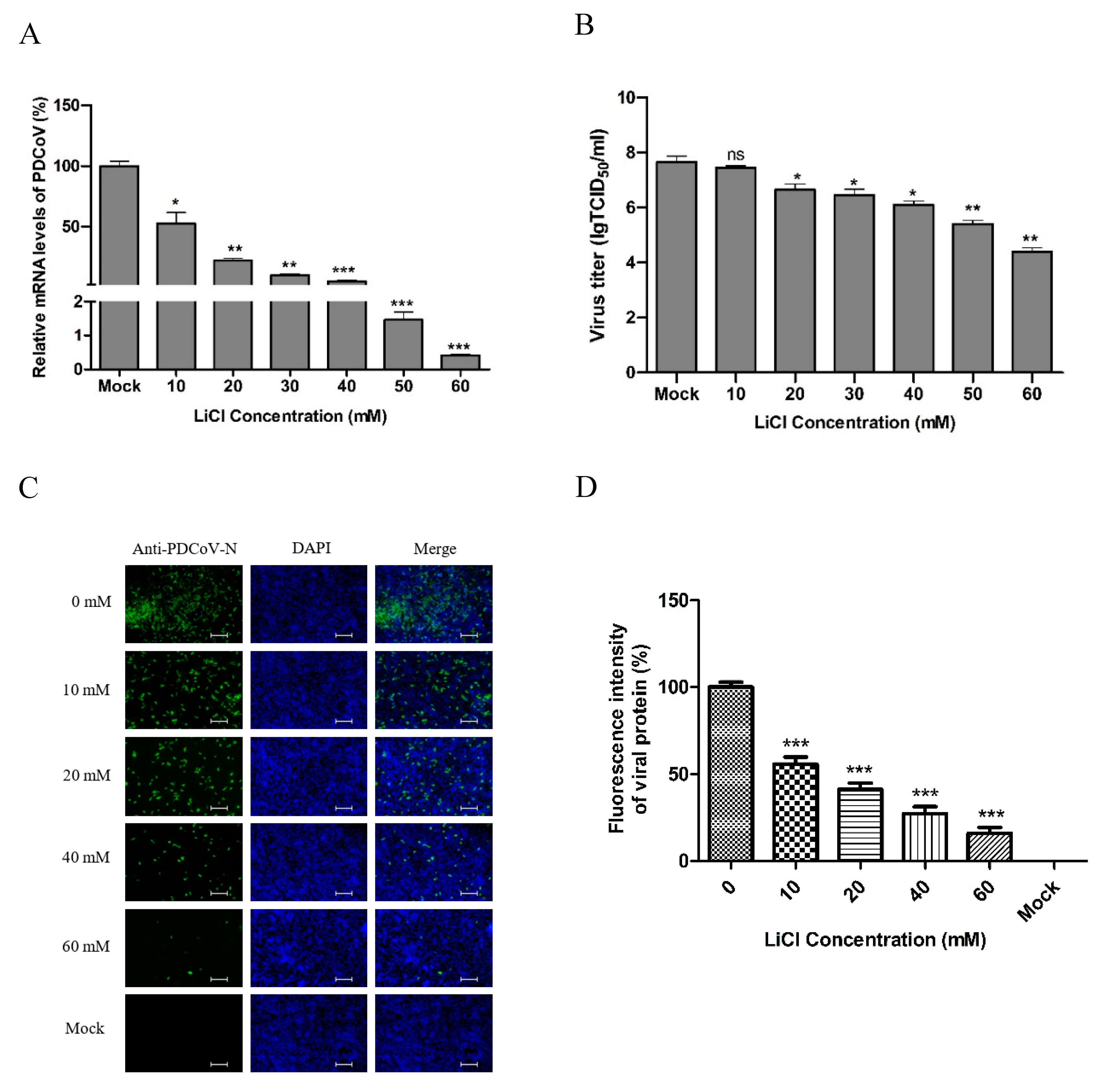
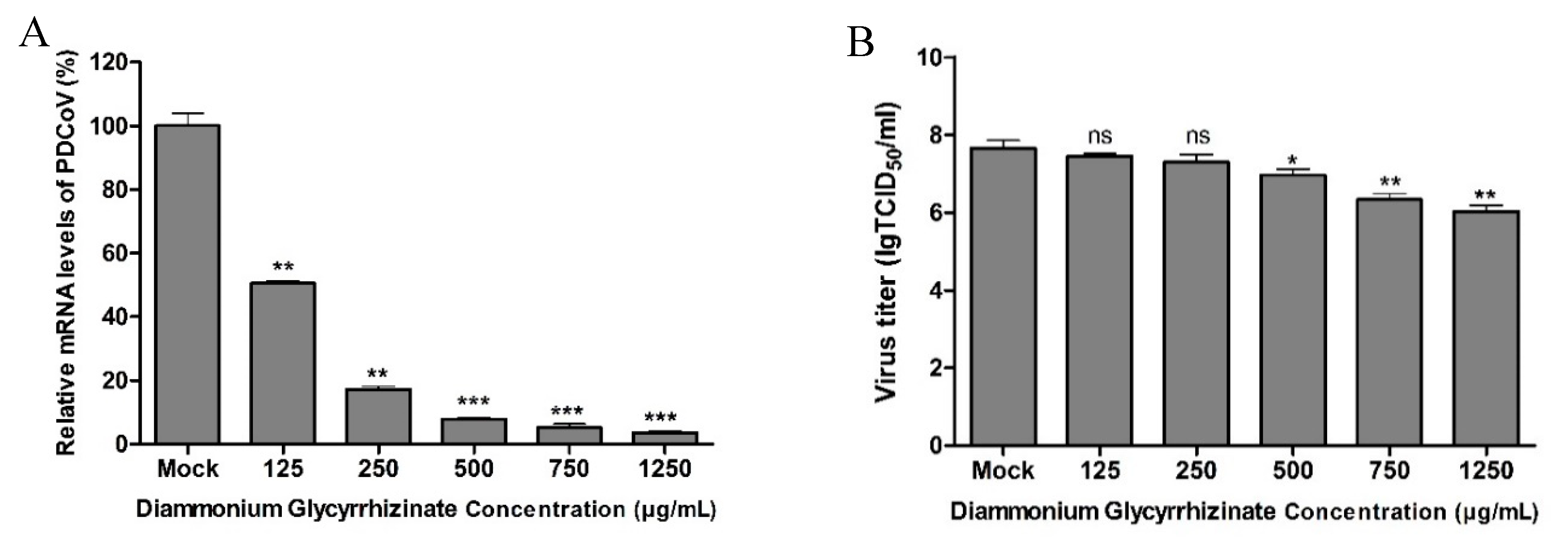
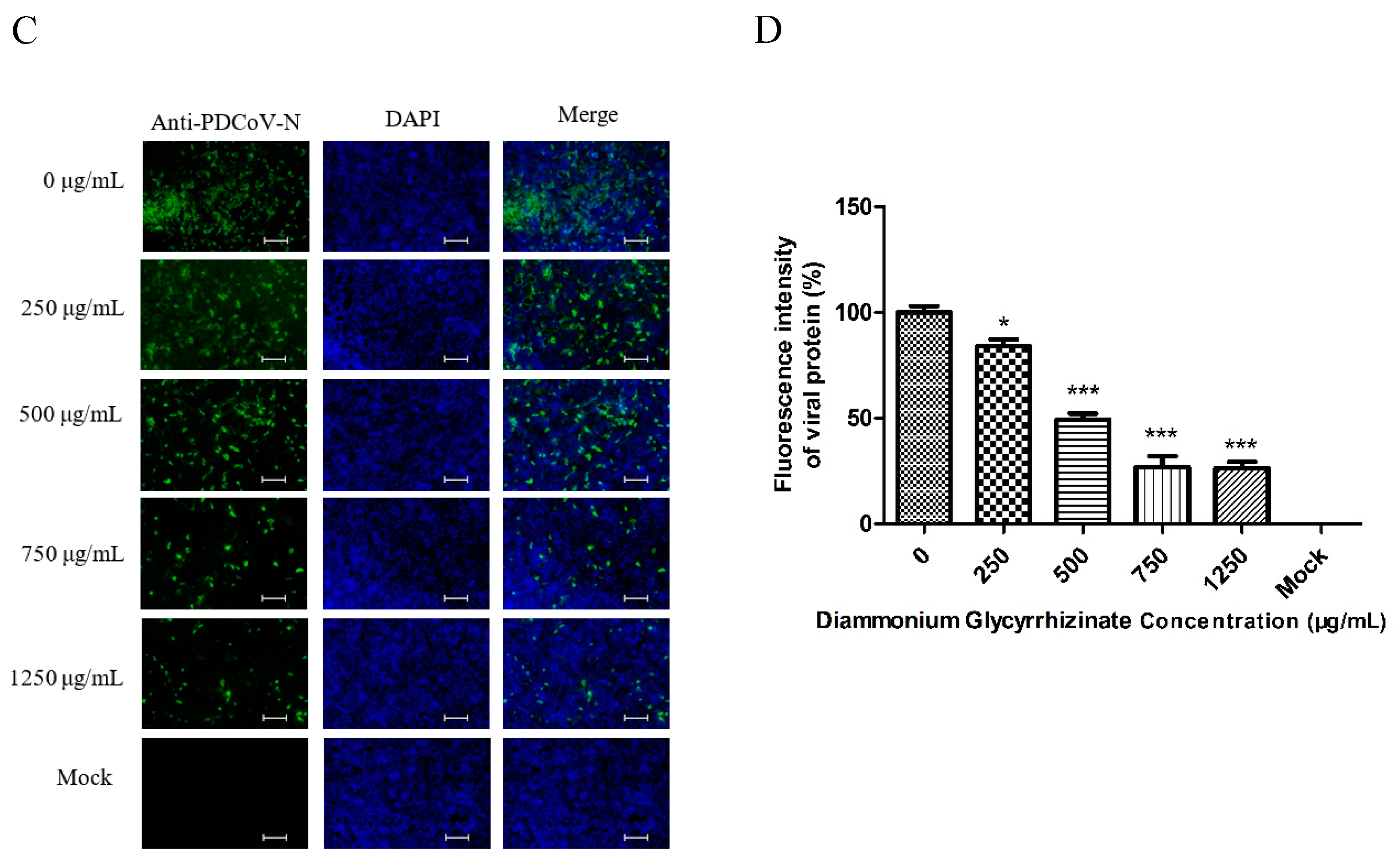
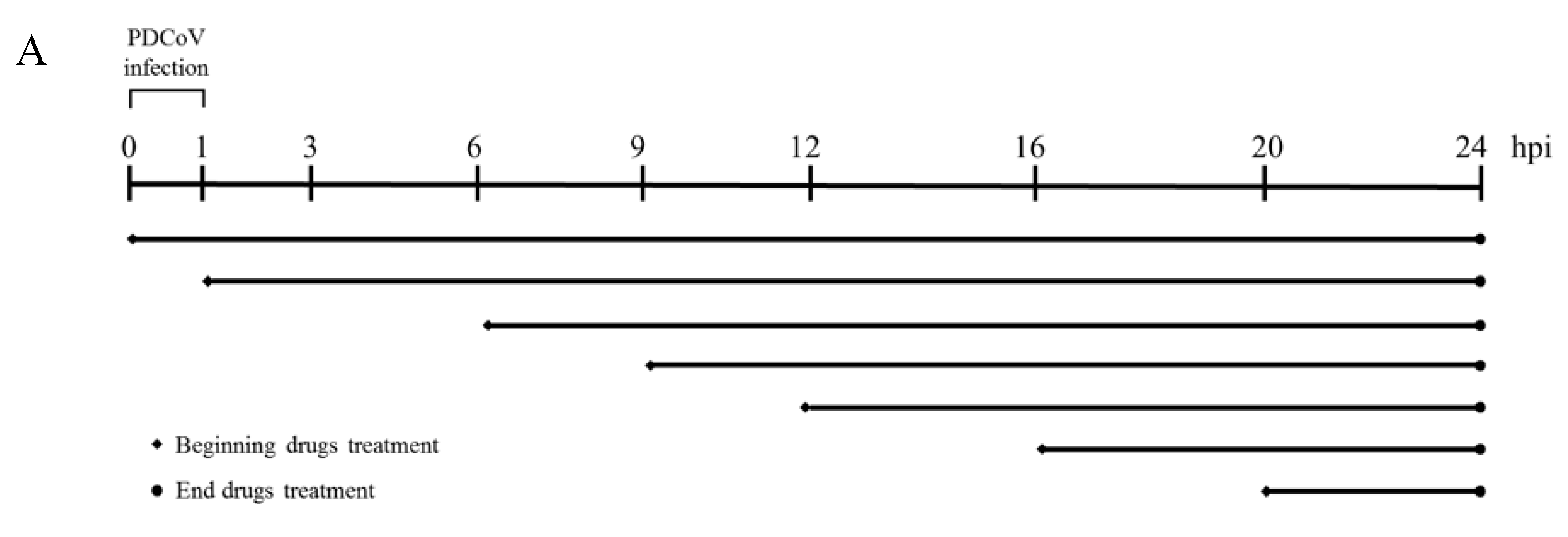
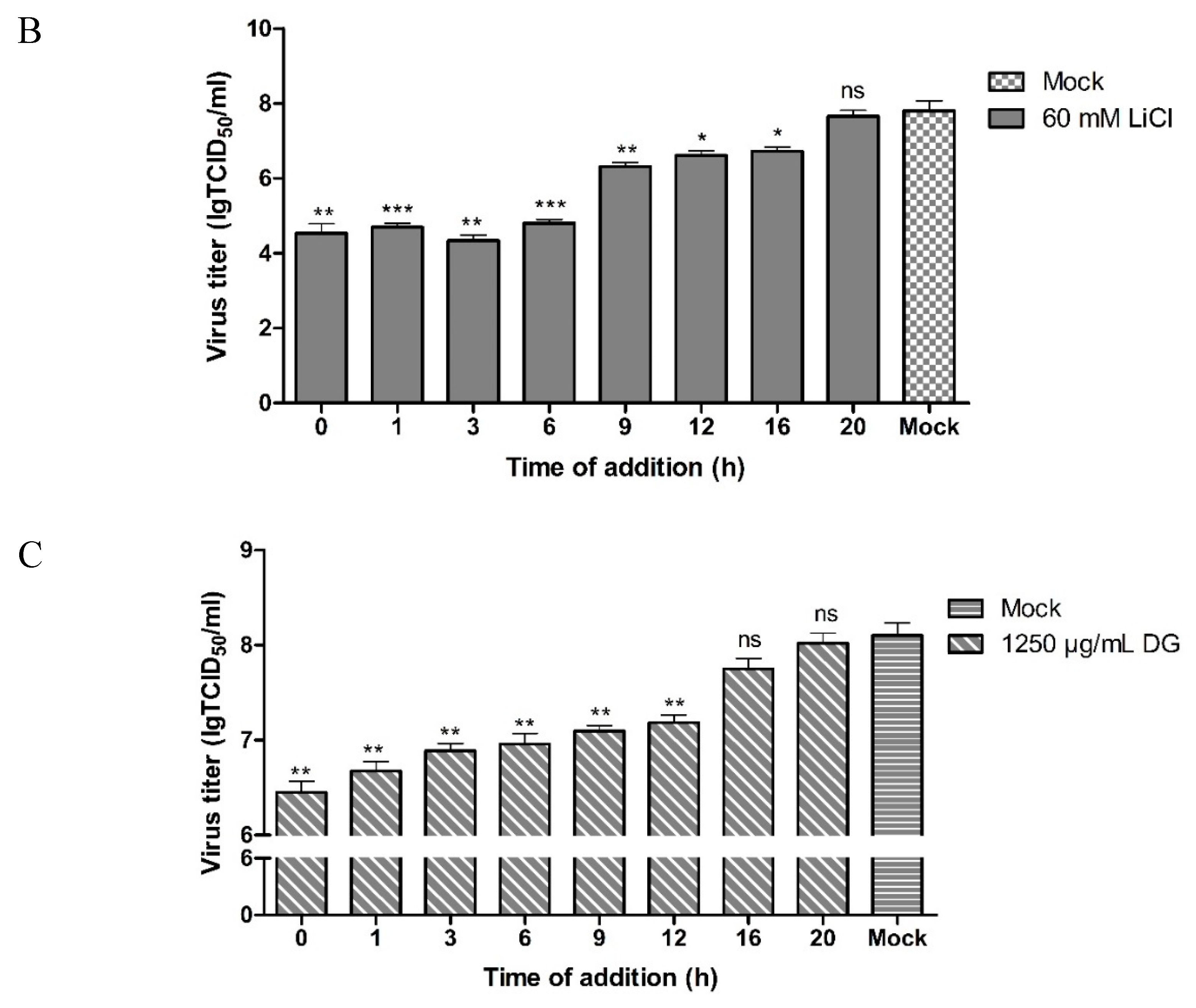
2.4. Antiviral Assay
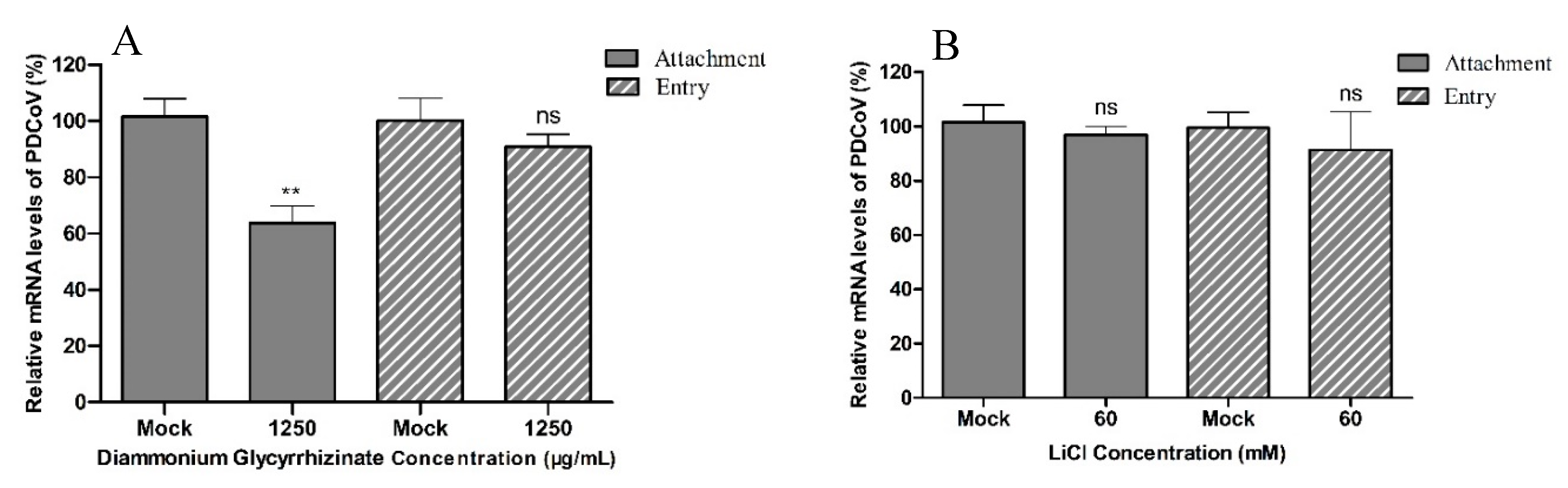
2.5. Analysis of the Effect of LiCl and DG on Viral Attachment
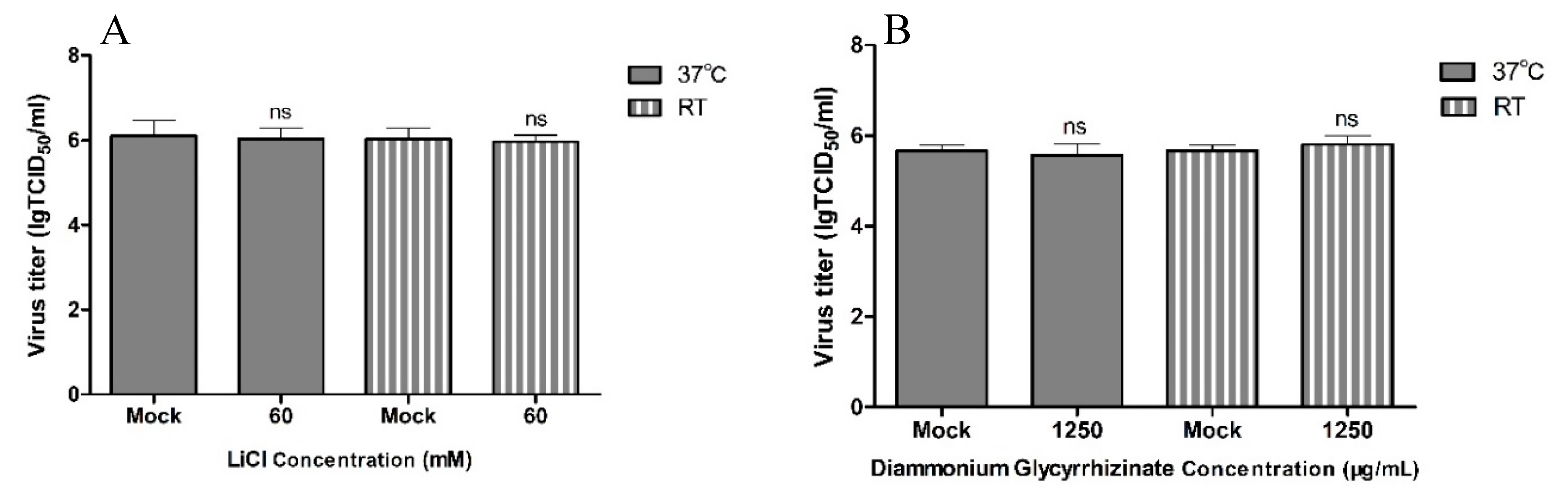
2.6. Analysis of the Viricidal Effect
2.7. Analysis of the Effect of LiCl and DG on Viral Entry
2.8. Real-Time qPCR
2.9. Indirect Immunofluorescence Assay (IFA)
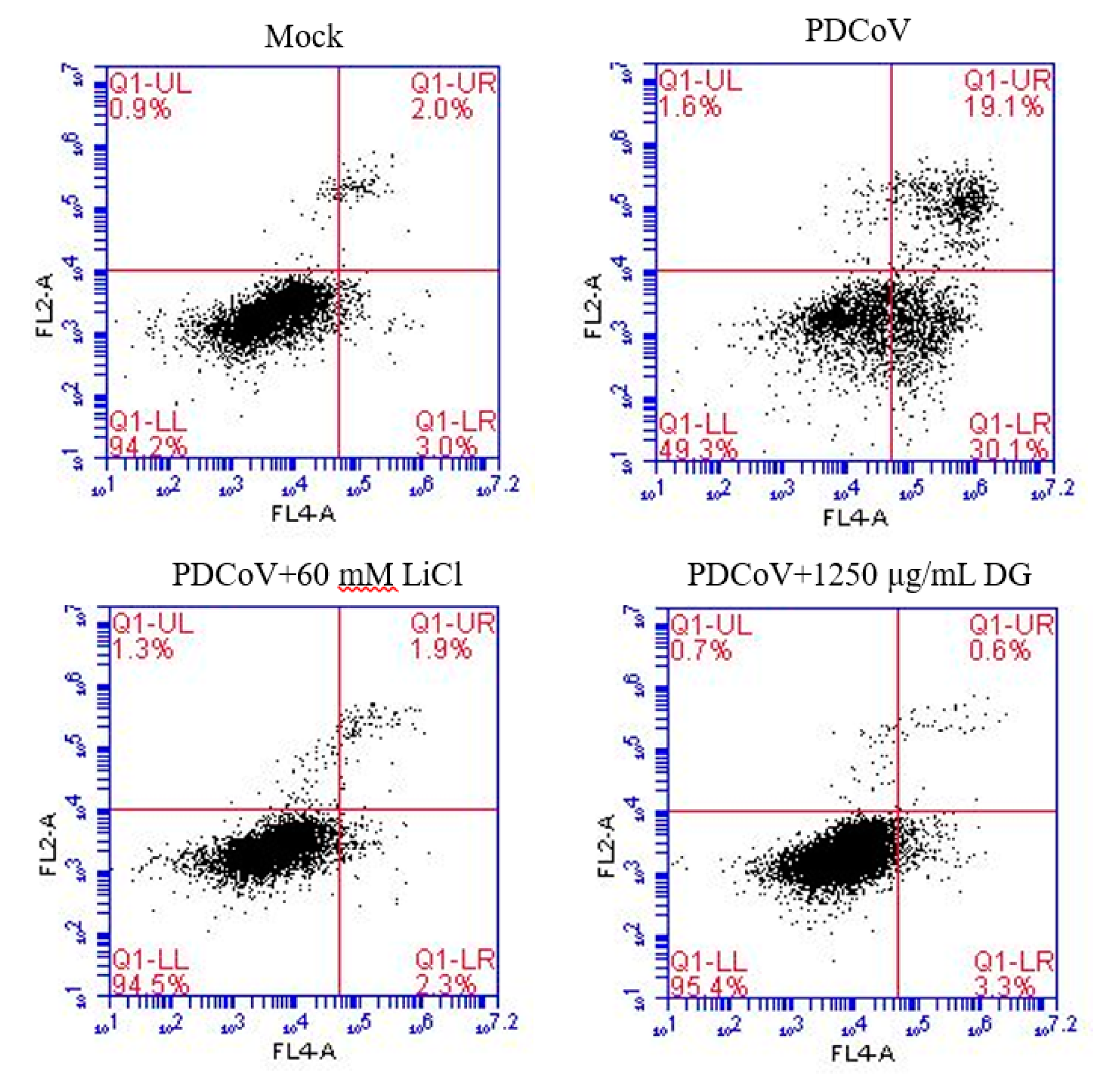
2.10. Analysis of Cell Apoptosis
2.11. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Marthaler, D.; Lindsey, R.; Yin, J.; James, C.; Kurt, R.; Albert, R. Rapid Detection, Complete Genome Sequencing, and Phylogenetic Analysis of Porcine Deltacoronavirus. Emerg. Infect. Dis. 2014, 20, 1347. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Q.; Harmon, K.M.; Yoon, K.-J.; Schwartz, K.J.; Hoogland, M.J.; Gauger, P.C.; Main, R.G.; Zhang, J. Full-Length Genome Sequence of Porcine Deltacoronavirus Strain USA/Ia/2014/8734. Genome Announc. 2014, 2, e00278-14. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavir. J. Virol. 2012, 86, 3995. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.L.; Duchãªne, S.; Holmes, E.C. Comparative Analysis Estimates the Relative Frequencies of Co-Divergence and Cross-Species Transmission within Viral Families. PLoS Pathog. 2017, 13, e1006215. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.; Lai, S.T.; Poon, L.L.; Guan, Y.; Yam, L.Y.; Lim, W.; Nicholls, J.; Yee, W.K.; Yan, W.W.; Cheung, M.T. Coronavirus as a Possible Cause of Severe Acute Respiratory Syndrome. Lancet 2003, 361, 1319–1325. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.F.; Liu, J.; Lai, A.C.K.; Zhou, J.Y.; Liu, W.J.; Bi, Y.H.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.L.; Ralph, S.B. Recombination, Reservoirs, and the Modular Spike: Mechanisms of Coronavirus Cross-Species Transmission. J. Virol. 2010, 84, 3134. [Google Scholar] [CrossRef] [PubMed]
- Janetanakit, T.; Lumyai, M.; Bunpapong, N.; Boonyapisitsopa, S.; Chaiyawong, S.; Nonthabenjawan, N.; Kesdaengsakonwut, S.; Amonsin, A. Porcine Deltacoronavirus, Thailand, 2015. Emerg. Infect. Dis. 2016, 22, 757–759. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Chen, J.; Kong, X.; Shao, Y.; Han, Z.; Feng, L.; Cai, X.; Gu, S.; Liu, M. Isolation of Avian Infectious Bronchitis Coronavirus from Domestic Peafowl (Pavo Cristatus) and Teal (Anas). J. Gen.Virol. 2005, 86, 719–725. [Google Scholar] [CrossRef]
- Wang, L.; Beverly, B.; Yan, Z. Porcine Coronavirus Hku15 Detected in 9 US States, 2014. Emerg. Infect. Dis. 2014, 20, 1594–1595. [Google Scholar] [CrossRef]
- Li, W.; Hulswit, R.J.; Kenney, S.P.; Widjaja, I.; Jung, K.; Alhamo, M.A.; Bosch, B.J. Broad Receptor Engagement of an Emerging Global Coronavirus May Potentiate Its Diverse Cross-Species Transmissibility. Proc. Natl. Acad. Sci. USA 2018, 115, 201802879. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Choe, H. Angiotensin-Converting Enzyme 2 Is a Functional Receptor for the Sars Coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Müller, M.A.; Dijkman, R.; Thiel, V. Dipeptidyl Peptidase 4 Is a Functional Receptor for the Emerging Human Coronavirus-Emc. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, Y.; Zhang, W.; Qi, J.; Gao, G.F. Enabling the ’Host Jump’: Structural Determinants of Receptor-Binding Specificity in Influenza a Viruses. Nat. Rev. Microbiol. 2014, 12, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Sun, Y.; Rao, Z. Current Progress in Antiviral Strategies. Trends Pharm. Sci. 2014, 35, 86–102. [Google Scholar]
- Kaufmann, S.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-Directed Therapies for Bacterial and Viral Infections. Nat. Rev. Drug Discov. 2018, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.P.; Sasse, F.; BrãNstrup, M.; Diez, J.; Meyerhans, A. Antiviral Drug Discovery: Broad-Spectrum Drugs from Nature. Nat. Prod. Rep. 2014, 32, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.H.; Andrea, K.; Simon, V.A.; Peter, P.; Shaw, M.L. Broad-Spectrum Antiviral That Interferes with De Novo Pyrimidine Biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5777–5782. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Cao, L.; Gao, H.Y.; Wu, Y.; Wang, Y.X.; Fang, F.; Lan, T.L.; Lou, Z.Y.; Rao, Y. Discovery, Optimization, and Target Identification of Novel Potent Broad-Spectrum Antiviral Inhibitors. J. Med. Chem. 2019, 62, 4056–4073. [Google Scholar] [CrossRef]
- Adam, M.; Nichol, S.T.; Spiropoulou, C.F. Hantavirus Pulmonary Syndrome. Virus Res. 2011, 162, 138–147. [Google Scholar]
- Lin, Z.F.; Li, Y.H.; Gong, G.F.; Xia, Y.; Wang, C.B.; Chen, Y.; Hua, L.; Zhong, J.Y.; Tang, Y.; Liu, X.M.; et al. Restriction of H1n1 Influenza Virus Infection by Selenium Nanoparticles Loaded with Ribavirin Via Resisting Caspase-3 Apoptotic Pathway. Int. J. Nanomed. 2018, 13, 5787–5797. [Google Scholar] [CrossRef] [PubMed]
- Pires de Mello, C.; Drusano, G.; Rodriquez, J.; Kaushik, A.; Brown, A. Antiviral Effects of Clinically-Relevant Interferon-Α and Ribavirin Regimens against Dengue Virus in the Hollow Fiber Infection Model (Hfim). Viruses 2018, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, K.M.; Drusano, G.L.; D’Argenio, D.Z.; Brown, A.N. Chikungunya Virus: In Vitro Response to Combination Therapy with Ribavirin and Interferon Alfa 2a. J. Infect. Dis. 2016, 214, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Skinner, G.R.B.; Hartley, C.; Buchan, A.; Harper, L.; Gallimore, P. The Effect of Lithium Chloride on the Replication Ofherpes Simplex Virus. Med. Microbiol. Immunol. 1980, 168, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Fandan, M.; Jiechao, Y.; Guangxing, L.; Xunliang, L.; Chao, W.; Georg, H. Action Mechanisms of Lithium Chloride on Cell Infection by Transmissible Gastroenteritis Coronavirus. PLoS ONE 2011, 6, e18669. [Google Scholar] [CrossRef] [PubMed]
- Ping, H.H.; Wen, L.B.; Li, J.R.; Wang, Y.; Ni, B.; Wang, R.; Wang, X.; Sun, M.X.; Fan, H.J.; Mao, X. Licl Inhibits Prrsv Infection by Enhancing Wnt/Β-Catenin Pathway and Suppressing Inflammatory Responses. Antiviral Res. 2015, 117, 99–109. [Google Scholar]
- Xiuwen, S.; Yin, J.; Ren, X. Antiviral Effect of Diammonium Glycyrrhizinate and Lithium Chloride on Cell Infection by Pseudorabies Herpesvirus. Antiviral Res. 2010, 85, 346–353. [Google Scholar]
- Jie, L.H.; Gao, D.S.; Li, Y.T.; Wang, Y.S.; Liu, H.Y.; Zhao, J. Antiviral Effect of Lithium Chloride on Porcine Epidemic Diarrhea Virus in Vitro. Res. Vet. Sci. 2018, 118, 288–294. [Google Scholar]
- Wang, Z.W.; Sun, N.; Wu, C.H.; Jiang, J.B.; Bai, Y.S.; Li, H.Q. In Vitro Antiviral Activity and Underlying Molecular Mechanisms of Dipotassium Glycyrrhetate against Porcine Reproductive and Respiratory Syndrome Virus. Antiviral Ther. 2013, 18, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Takei, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Effect of Glycyrrhizin, an Active Component of Licorice Roots, on Hiv Replication in Cultures of Peripheral Blood Mononuclear Cells from Hiv-Seropositive Patients. Pathobiology 2003, 70, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hitoshi, S.; Goto, W.; Yamamura, J.I.; Kurokawa, M.; Kageyama, S.; Takahara, T.; Watanabe, A.; Shiraki, K. Therapeutic Basis of Glycyrrhizin on Chronic Hepatitis B. Antiviral Res. 1996, 30, 171–177. [Google Scholar]
- Marc, C.J.; Biziagos, E.; Jouan, A.; Deloince, R. Studies on Mechanism of Action of Glycyrrhizin against Hepatitis a Virus Replication in Vitro. Antiviral Res. 1994, 23, 63–76. [Google Scholar]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an Active Component of Liquorice Roots, and Replication of Sars-Associated Coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Li, J.; Yin, J.; Sui, X.; Li, G.; Ren, X. Comparative Analysis of the Effect of Glycyrrhizin Diammonium and Lithium Chloride on Infectious Bronchitis Virus Infection in Vitro. Avian Pathol. 2009, 38, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Ji, C.M.; Yang, Y.L.; Liang, Q.Z.; Zhao, P.; Xu, L.D.; Lei, X.M.; Luo, W.T.; Qin, P.; et al. Porcine Deltacoronavirus Engages the Transmissible Gastroenteritis Virus Functional Receptor Porcine Aminopeptidase N for Infectious Cellular Entry. J. Virol. 2018, 92, JVI003-18. [Google Scholar]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695. [Google Scholar] [CrossRef]
- Masahiko, I.; Sato, A.; Hirabayashi, K.; Tanabe, F.; Shigeta, S.; Baba, M.; de Clercq, E.; Nakashima, H.; Yamamoto, N. Mechanism of Inhibitory Effect of Glycyrrhizin on Replication of Human Immunodeficiency Virus (Hiv). Antiviral Res. 1988, 10, 289–298. [Google Scholar]
- Lee, Y.J.; Lee, C. Porcine Deltacoronavirus Induces Caspase-Dependent Apoptosis through Activation of the Cytochrome C-Mediated Intrinsic Mitochondrial Pathway. Virus Res. 2018, 253, 112–123. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, X.; Wang, S.; Zhu, M.; He, W.; Pan, Z.; Su, S. Antiviral Effect of Lithium Chloride and Diammonium Glycyrrhizinate on Porcine Deltacoronavirus In Vitro. Pathogens 2019, 8, 144. https://doi.org/10.3390/pathogens8030144
Zhai X, Wang S, Zhu M, He W, Pan Z, Su S. Antiviral Effect of Lithium Chloride and Diammonium Glycyrrhizinate on Porcine Deltacoronavirus In Vitro. Pathogens. 2019; 8(3):144. https://doi.org/10.3390/pathogens8030144
Chicago/Turabian StyleZhai, Xiaofeng, Shilei Wang, Mengyan Zhu, Wei He, Zhongzhou Pan, and Shuo Su. 2019. "Antiviral Effect of Lithium Chloride and Diammonium Glycyrrhizinate on Porcine Deltacoronavirus In Vitro" Pathogens 8, no. 3: 144. https://doi.org/10.3390/pathogens8030144
APA StyleZhai, X., Wang, S., Zhu, M., He, W., Pan, Z., & Su, S. (2019). Antiviral Effect of Lithium Chloride and Diammonium Glycyrrhizinate on Porcine Deltacoronavirus In Vitro. Pathogens, 8(3), 144. https://doi.org/10.3390/pathogens8030144



