Staphylococcus aureus Superantigen-Like Protein SSL1: A Toxic Protease
Abstract
1. Introduction
2. Results
2.1. Corneal Virulence of S. aureus Mutants
2.2. Corneal Toxicity of Culture Supernatants
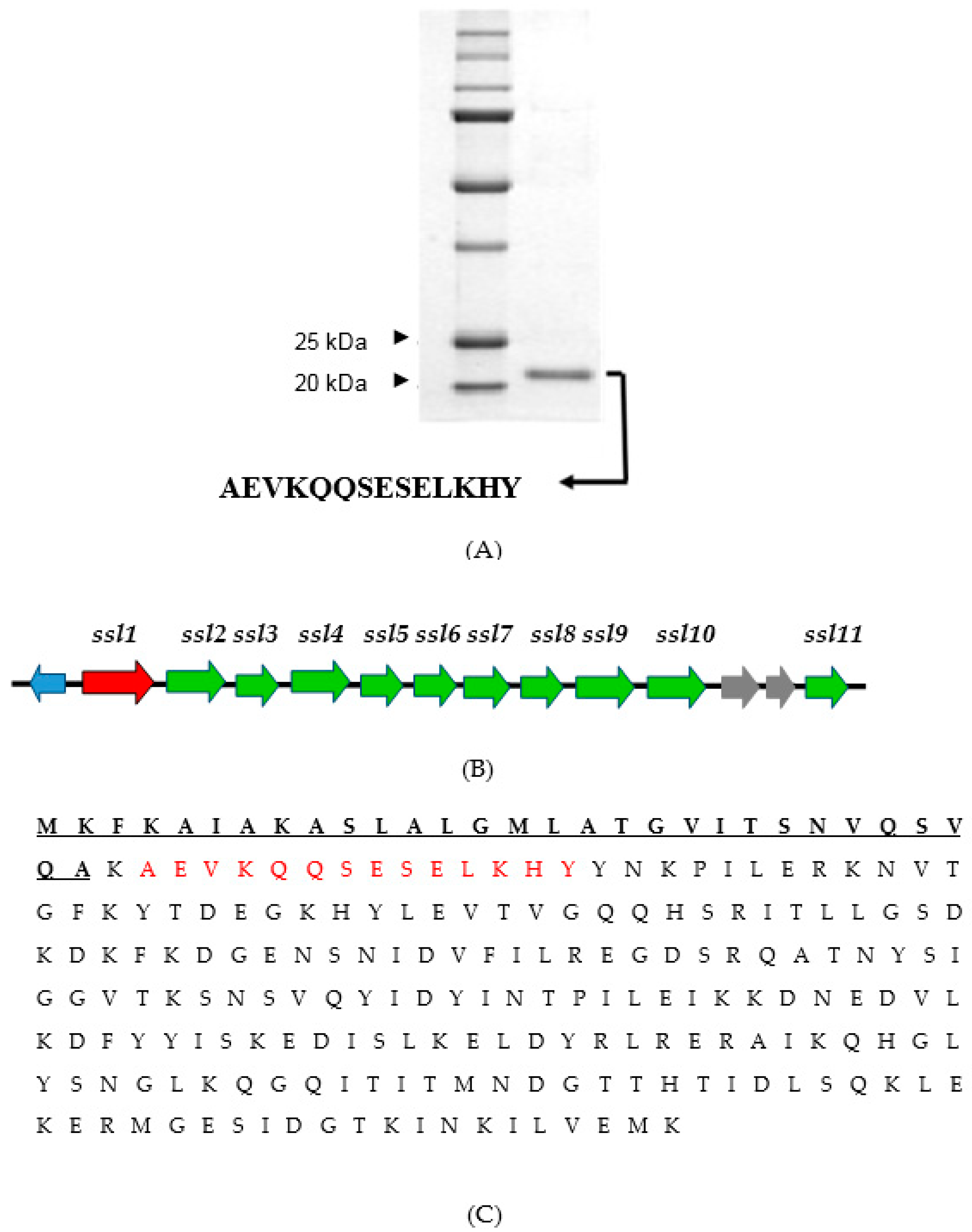
2.3. Identification of the Virulence Factor
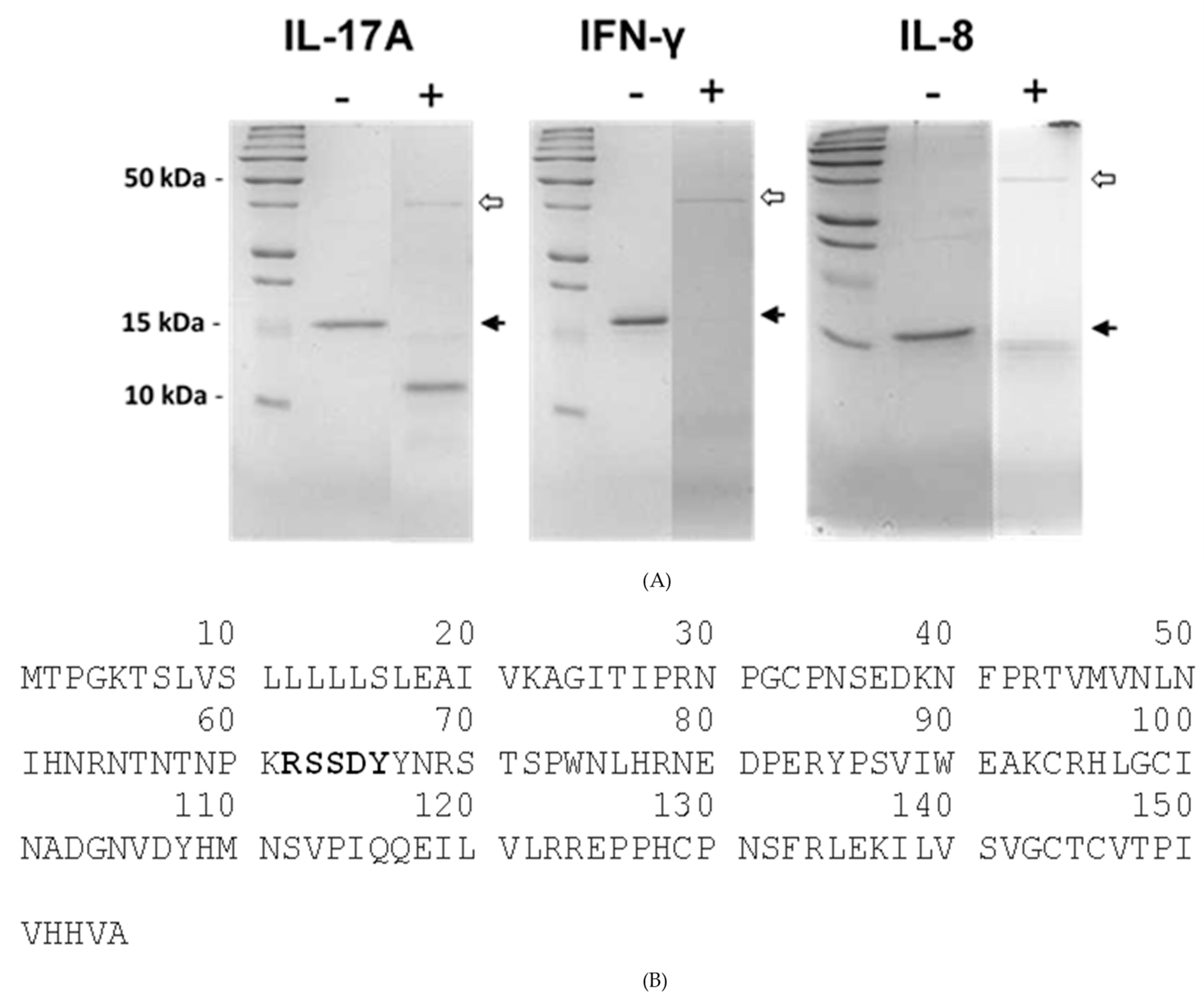
2.4. Recombinant SSL1: Immunogenicity and Toxicity
2.5. Recombinant SSL1: Polymerization
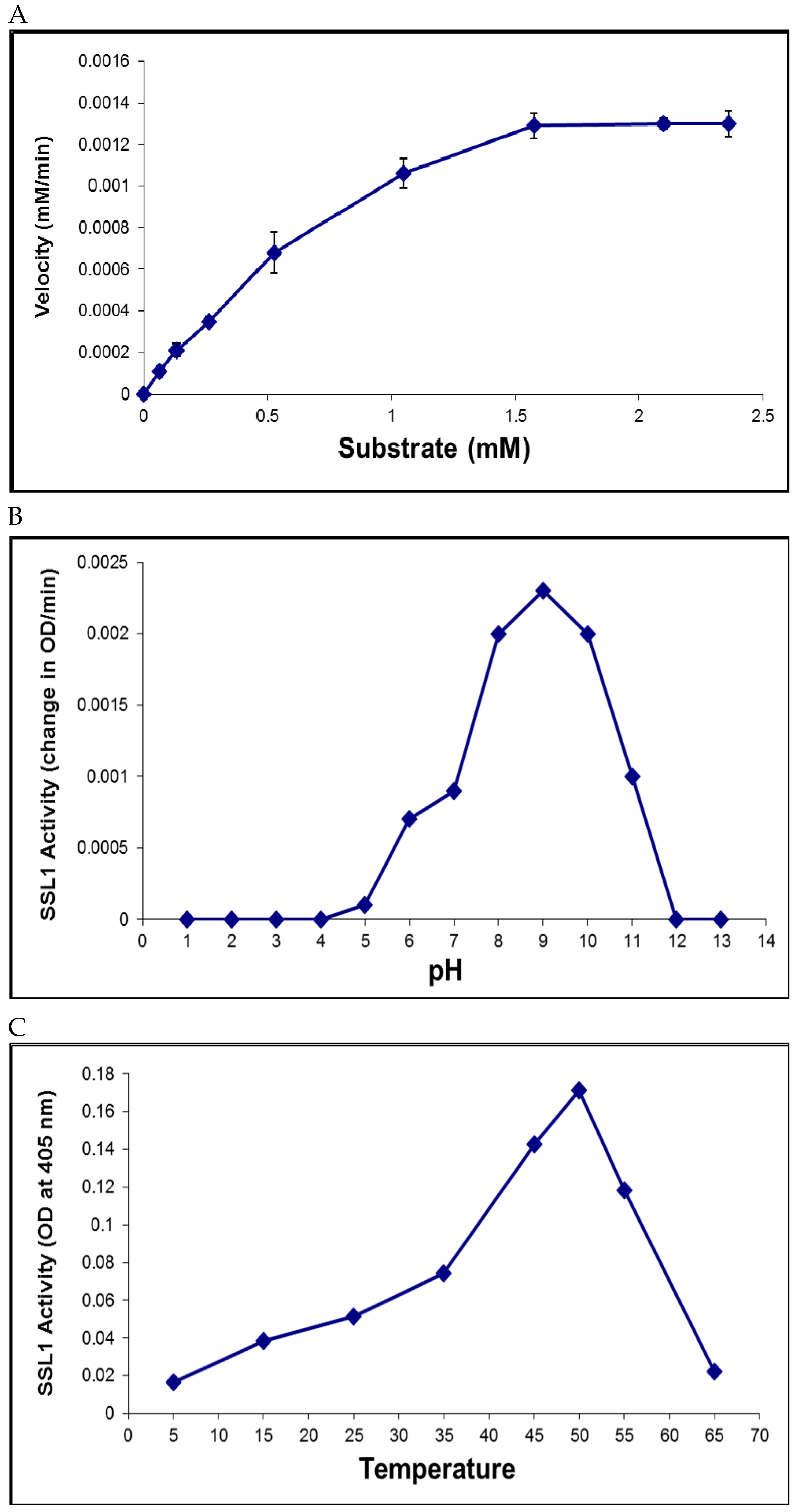
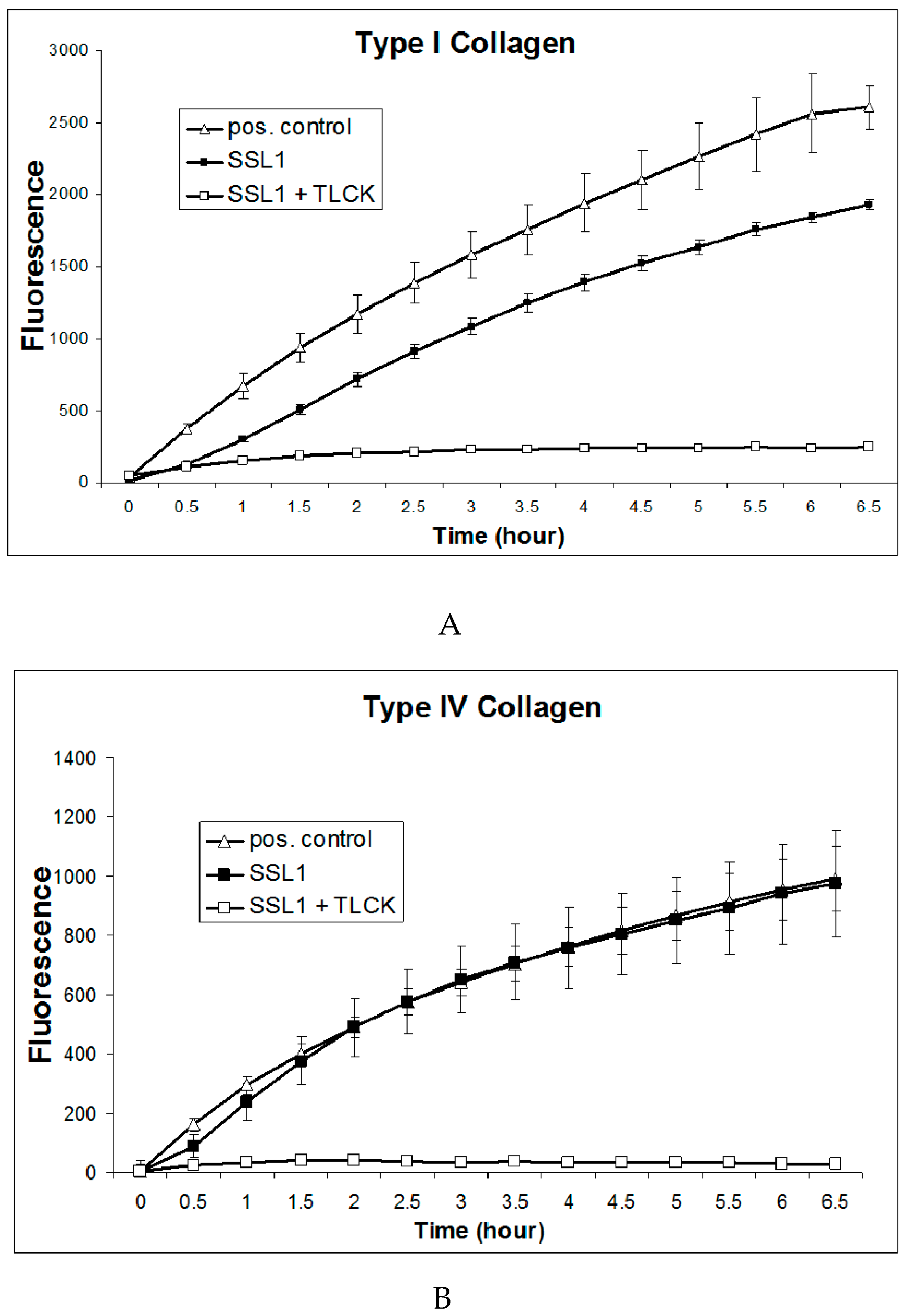
2.6. Recombinant SSL1: Enzymatic Properties
2.7. Corneal Virulence of the SSL1 Deficient Mutant
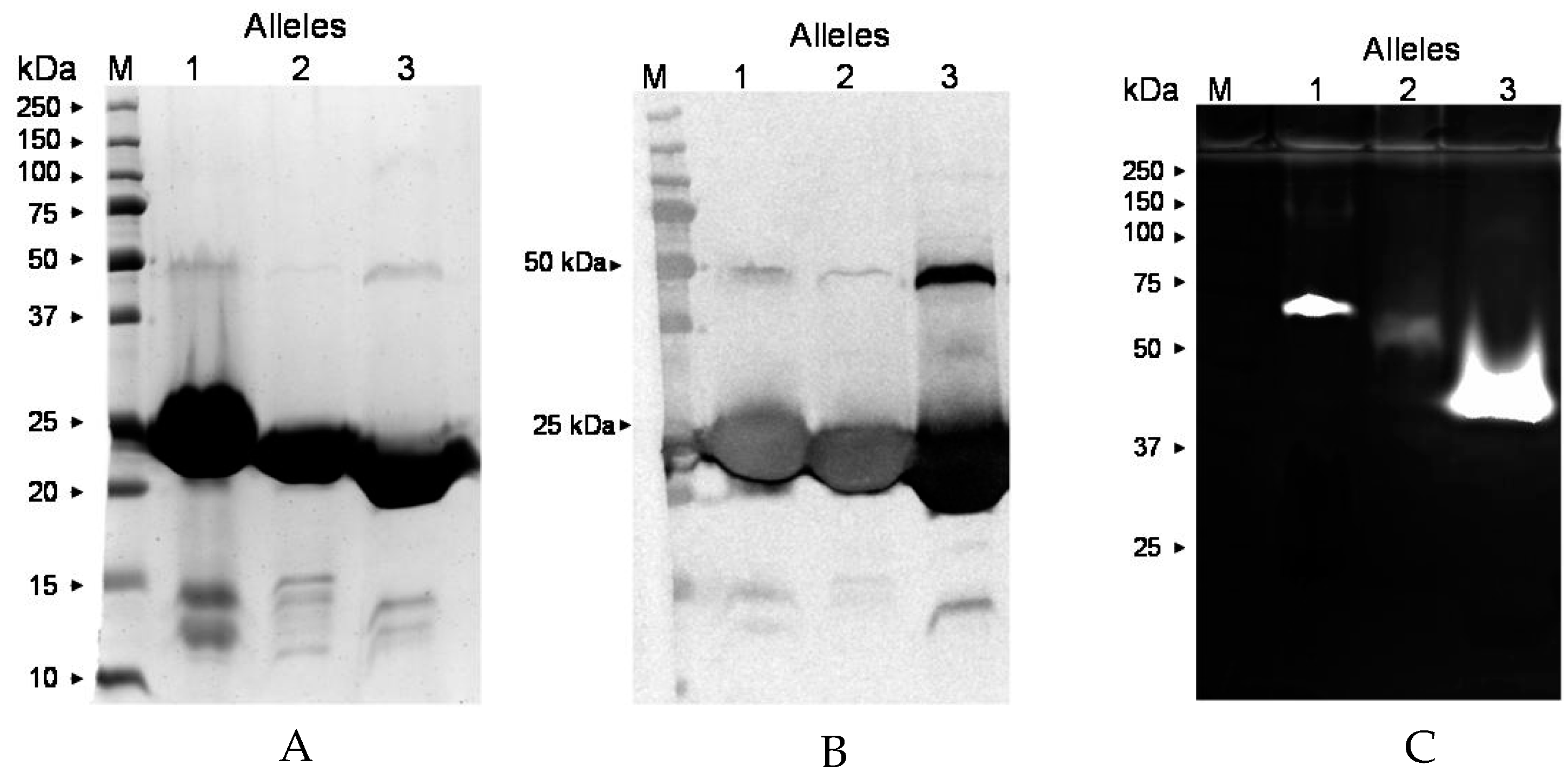
2.8. ssl1 Alleles
3. Discussion
4. Materials and Methods
4.1. Bacteria and Growth Conditions
4.2. PAGE and Gelatin Zymography
4.3. Identification of S. aureus Virulence Factor
4.4. Recombinant Protein Production
4.5. Production of Polyclonal Antibody
4.6. In Vitro Degradation of Host Proteins
4.7. Enzyme Activity Assay, Kinetics, and Optimal Conditions
4.8. ssl1 Allele Typing of S. aureus Clinical Isolates
4.9. Construction of Isogenic Mutants
4.10. Corneal Toxicity Model
4.11. Slit Lamp Examination Scoring and Quantification of Viable Bacteria
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, R.J. The pathogenesis of Staphylococcus aureus eye infections. Pathogens 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Freidlin, J.; Acharya, N.; Lietman, T.M.; Cevallos, V.; Whitcher, J.P.; Margolis, T.P. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am. J. Ophthalmol. 2007, 144, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Mah, F.S.; Davidson, R.; Holland, E.J.; Hovanesian, J.; John, T.; Kanellopoulos, J.; Shamie, N.; Starr, C.; Vroman, D.; Kim, T. Current knowledge about and recommendations for ocular methicillin-resistant Staphylococcus aureus. J. Cataract Refract. Surg. 2014, 40, 1894–1908. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Núñez, M.X. Antibiotic susceptibility of staphylococci isolates from patients with chronic conjunctivitis: Including associated factors and clinical evaluation. J. Ocul. Pharmacol. Ther. 2013, 29, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Suzuki, T.; Yamaguchi, S.; Inoue, T.; Ohashi, Y. Genotypic characterization of Staphylococcus aureus isolates from cases of keratitis and healthy conjunctival sacs. Cornea 2014, 33, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Jeng, B.H.; Gritz, D.C.; Kumar, A.B.; Holsclaw, D.S.; Porco, T.C.; Smith, S.D.; Whitcher, J.P.; Margolis, T.P.; Wong, I.G. Epidemiology of ulcerative keratitis in Northern California. Arch. Ophthalmol. 2010, 128, 1022–1028. [Google Scholar] [CrossRef]
- Asbell, P.A.; Sanfilippo, C.M.; Pillar, C.M.; DeCory, H.H.; Sahm, D.F.; Morris, T.W. Antibiotic resistance among ocular pathogens in the United States: Five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015, 133, 1445–1454. [Google Scholar] [CrossRef]
- Naicker, P.R.; Karayem, K.; Hoek, K.G.P.; Harvey, J.; Wasserman, E. Biofilm formation in invasive Staphylococcus aureus isolates is associated with the clonal lineage. Microb. Pathog. 2016, 90, 41–49. [Google Scholar] [CrossRef]
- Mootz, J.M.; Benson, M.A.; Heim, C.E.; Crosby, H.A.; Kavanaugh, J.S.; Dunman, P.M.; Kielian, T.; Torres, V.J.; Horswill, A.R. Rot is a key regulator of Staphylococcus aureus biofilm formation. Mol. Microbiol. 2015, 96, 388–404. [Google Scholar] [CrossRef]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.D.; Proft, T. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 2008, 225, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Kuinkel, B.K.; Wu, Y.; Tabor, D.E.; Mok, H.; Sellman, B.R.; Jenkins, A.; Yu, L.; Jafri, H.S.; Rude, T.H.; Ruffin, F.; et al. Characterization of alpha-toxin hla gene variants, alpha-toxin expression levels, and levels of antibody to alpha-toxin in hemodialysis and postsurgical patients with Staphylococcus aureus bacteremia. J. Clin. Microbiol. 2015, 53, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Prevost, G.; Couppie, P.; Prevost, P.; Gayet, S.; Petiau, P.; Cribier, B.; Monteil, H.; Piemont, Y. Epidemiological data on Staphylococcus aureus strains producing synergohymenotropic toxins. J. Med. Microbiol. 1995, 42, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Dajcs, J.J.; Austin, M.S.; Sloop, G.D.; Moreau, J.M.; Hume, E.B.H.; Thompson, H.W.; McAleese, F.M.; Foster, T.J.; O’Callaghan, R.J. Corneal pathogenesis of Staphylococcus aureus strain Newman. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1109–1115. [Google Scholar]
- Dajcs, J.J.; Thibodeaux, B.A.; Girgis, D.O.; O’Callaghan, R.J. Corneal virulence of Staphylococcus aureus in an experimental model of keratitis. DNA Cell Biol. 2002, 21, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, T.; Zaidi, T.; Yoong, P.; Pier, G.B. Staphylococcus aureus corneal infections: Effect of the Panton-Valentine leukocidin (PVL) and antibody to PVL on virulence and pathology. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Recsei, P.; Kreiswirth, B.; O’Reilly, M.; Schlievert, P.; Gruss, A.; Novick, R.P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol. Gen. Genet. MGG 1986, 202, 58–61. [Google Scholar] [CrossRef]
- Pantrangi, M.; Singh, V.K.; Wolz, C.; Shukla, S.K. Staphylococcal superantigen-like genes, ssl5 and ssl8, are positively regulated by Sae and negatively by Agr in the Newman strain. FEMS Microbiol. Lett. 2010, 308, 175–184. [Google Scholar] [CrossRef]
- Langley, R.; Wines, B.; Willoughby, N.; Basu, I.; Proft, T.; Fraser, J.D. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc alpha RI binding and serum killing of bacteria. J. Immunol. 2005, 174, 2926–2933. [Google Scholar] [CrossRef]
- Patel, D.; Wines, B.D.; Langley, R.J.; Fraser, J.D. Specificity of staphylococcal superantigen-like protein 10 toward the human IgG1 Fc domain. J. Immunol. 2010, 184, 6283–6292. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Hamada, E.; Kamoshida, G.; Yokoyama, R.; Takii, T.; Onozaki, K.; Tsuji, T. Staphylococcal superantigen-like protein 10 (SSL10) binds to human immunoglobulin G (IgG) and inhibits complement activation via the classical pathway. Mol. Immunol. 2010, 47, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.C.; Wines, B.D.; Baker, H.; Langley, R.J.; Baker, E.N.; Fraser, J.D. The crystal structure of staphylococcal superantigen-like protein 11 in complex with sialyl Lewis X reveals the mechanism for cell binding and immune inhibition. Mol. Microbiol. 2007, 66, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Bardoel, B.W.; Vos, R.; Bouman, T.; Aerts, P.C.; Bestebroer, J.; Huizinga, E.G.; Brondijk, T.H.C.; van Strijp, J.A.G.; de Haas, C.J.C. Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3. J. Mol. Med. 2012, 90, 1109–1120. [Google Scholar] [CrossRef]
- Koymans, K.J.; Bisschop, A.; Vughs, M.M.; van Kessel, K.P.M.; de Haas, C.J.C.; van Strijp, J.A.G. Staphylococcal superantigen-like protein 1 and 5 (SSL1 & SSL5) limit neutrophil chemotaxis and migration through MMP-inhibition. Int. J. Mol. Sci. 2016, 17, 1072. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Lindsay, J.A. Staphylococcus aureus innate immune evasion is lineage-specific: A bioinfomatics study. Infect. Genet. Evol. 2013, 19, 7–14. [Google Scholar] [CrossRef]
- Meek, K.M.; Boote, C. The use of X-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog. Retin. Eye Res. 2009, 28, 369–392. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Silva, M.L.; Araújo, R.L.; Ferrucci, D.L.; Mineo, T.; Thiesen, R.; Bandarra, M.B.; Laus, J.L. Expression of matrix metalloproteinases, type IV collagen, and interleukin-10 in rabbits treated with morphine after lamellar keratectomy. Vet. Ophthalmol. 2012, 15, 153–163. [Google Scholar] [CrossRef]
- Kida, Y.; Higashimoto, Y.; Inoue, H.; Shimizu, T.; Kuwano, K. A novel secreted protease from Pseudomonas aeruginosa activates NF-κB through protease-activated receptors. Cell. Microbiol. 2008, 10, 1491–1504. [Google Scholar] [CrossRef]
- Kida, Y.; Inoue, H.; Shimizu, T.; Kuwano, K. Serratia marcescens serralysin induces inflammatory responses through protease-activated receptor 2. Infect. Immun. 2007, 75, 164–174. [Google Scholar] [CrossRef]
- Dutta, D.; Dutta, A.; Bhattacharjee, A.; Basak, A.; Das, A.K. Cloning, expression, crystallization and preliminary X-ray diffraction studies of staphylococcal superantigen-like protein 1 (SSL1). Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Al-Shangiti, A.M.; Nair, S.P.; Chain, B.M. The interaction between staphylococcal superantigen-like proteins and human dendritic cells. Clin. Exp. Immunol. 2005, 140, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Al-Shangiti, A.M.; Naylor, C.E.; Nair, S.P.; Briggs, D.C.; Henderson, B.; Chain, B.M. Structural relationships and cell tropism of staphylococcal superantigen-like proteins. Infect. Immun. 2004, 72, 4261–4270. [Google Scholar] [CrossRef] [PubMed]
- Hanakawa, Y.; Stanley, J. Mechanisms of blister formation by staphylococcal toxins. J. Biochem. 2004, 136, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Arcus, V.L.; Langley, R.; Proft, T.; Fraser, J.D.; Baker, E.N. The three-dimensional structure of a superantigen-like protein, SET3, from a pathogenicity island of the Staphylococcus aureus genome. J. Biol. Chem. 2002, 277, 32274–32281. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.A.; Lilo, S.; Wasserman, G.A.; Thoendel, M.; Smith, A.; Horswill, A.R.; Fraser, J.; Novick, R.P.; Shopsin, B.; Torres, V.J. Staphylococcus aureus regulates the expression and production of the staphylococcal superantigen-like secreted proteins in a Rot-dependent manner. Mol. Microbiol. 2011, 81, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.A.; Lilo, S.; Nygaard, T.; Voyich, J.M.; Torres, V.J. Rot and SaeRS cooperate to activate expression of the staphylococcal superantigen-like exoproteins. J. Bacteriol. 2012, 194, 4355–4365. [Google Scholar] [CrossRef] [PubMed]
- Twining, S.S.; Kirschner, S.E.; Mahnke, L.A.; Frank, D.W. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2699–2712. [Google Scholar]
- Engel, L.S.; Hill, J.M.; Caballero, A.R.; Green, L.C.; O’Callaghan, R.J. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J. Biol. Chem. 1998, 273, 16792–16797. [Google Scholar] [CrossRef]
- Marquart, M.E.; Caballero, A.R.; Chomnawang, M.; Thibodeaux, B.A.; Twining, S.S.; O’Callaghan, R.J. Identification of a novel secreted protease from Pseudomonas aeruginosa that causes corneal erosions. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3761–3768. [Google Scholar] [CrossRef]
- Monk, I.R.; Shah, I.M.; Xu, M.; Tan, M.-W.; Foster, T.J. Transforming the untransformable: Application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Arana, A.M.; Bierdeman, M.A.; Balzli, C.L.; Tang, A.; Caballero, A.R.; Patel, R.; O’Callaghan, R.J. Staphylococcus alpha-toxin action on the rabbit iris: Toxic effects and their inhibition. Curr. Eye Res. 2015, 40, 830–838. [Google Scholar] [CrossRef] [PubMed]
 ) lacking alpha- and gamma-toxins, which produced substantial pathology as compared to normal eyes, was significantly less than that of the wild-type strain (
) lacking alpha- and gamma-toxins, which produced substantial pathology as compared to normal eyes, was significantly less than that of the wild-type strain ( ) (P ≤ 0.001). When their culture supernatants were injected directly into the corneal stroma, the SLE score of the double mutant at 24 h after injection was substantial compared to normal eyes, but significantly lower than that of the wild-type strain (P = 0.004). (B) Non-denaturing (no SDS) zymogram of the pooled fractions of concentrated culture supernatant of the S. aureus double mutant (Newman ΔhlaΔhlg) containing toxic activities to the rabbit cornea. (C) A zymogram with SDS showing standard proteins (Lane 1) and the extracted toxin (Lane 2).
) (P ≤ 0.001). When their culture supernatants were injected directly into the corneal stroma, the SLE score of the double mutant at 24 h after injection was substantial compared to normal eyes, but significantly lower than that of the wild-type strain (P = 0.004). (B) Non-denaturing (no SDS) zymogram of the pooled fractions of concentrated culture supernatant of the S. aureus double mutant (Newman ΔhlaΔhlg) containing toxic activities to the rabbit cornea. (C) A zymogram with SDS showing standard proteins (Lane 1) and the extracted toxin (Lane 2).
 ) lacking alpha- and gamma-toxins, which produced substantial pathology as compared to normal eyes, was significantly less than that of the wild-type strain (
) lacking alpha- and gamma-toxins, which produced substantial pathology as compared to normal eyes, was significantly less than that of the wild-type strain ( ) (P ≤ 0.001). When their culture supernatants were injected directly into the corneal stroma, the SLE score of the double mutant at 24 h after injection was substantial compared to normal eyes, but significantly lower than that of the wild-type strain (P = 0.004). (B) Non-denaturing (no SDS) zymogram of the pooled fractions of concentrated culture supernatant of the S. aureus double mutant (Newman ΔhlaΔhlg) containing toxic activities to the rabbit cornea. (C) A zymogram with SDS showing standard proteins (Lane 1) and the extracted toxin (Lane 2).
) (P ≤ 0.001). When their culture supernatants were injected directly into the corneal stroma, the SLE score of the double mutant at 24 h after injection was substantial compared to normal eyes, but significantly lower than that of the wild-type strain (P = 0.004). (B) Non-denaturing (no SDS) zymogram of the pooled fractions of concentrated culture supernatant of the S. aureus double mutant (Newman ΔhlaΔhlg) containing toxic activities to the rabbit cornea. (C) A zymogram with SDS showing standard proteins (Lane 1) and the extracted toxin (Lane 2).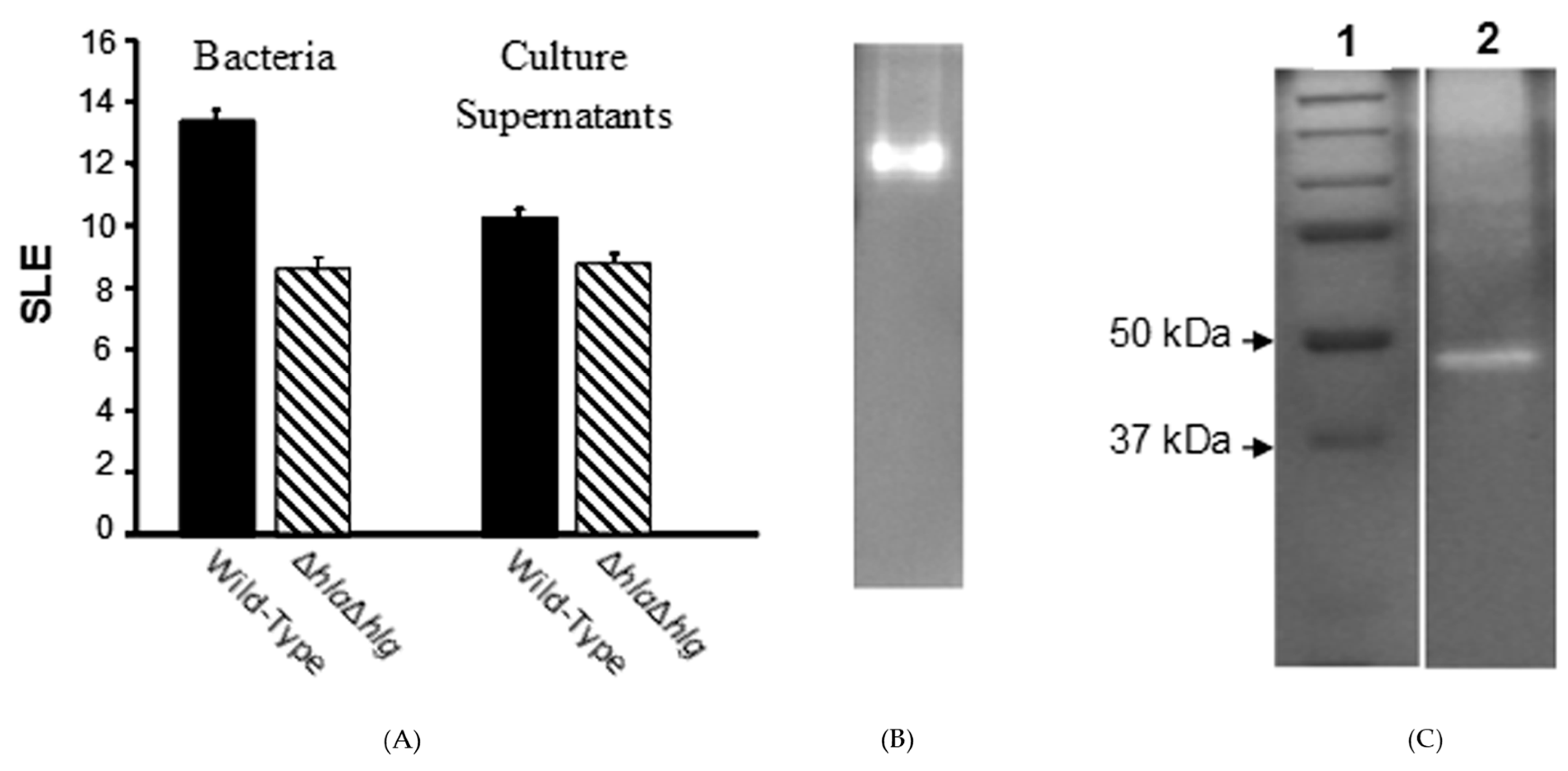

 ), the double mutant (
), the double mutant ( ), the triple mutant (
), the triple mutant ( ), or the rescue strain (
), or the rescue strain ( ). The SLE score for eyes infected with the triple mutant was significantly lower than that of eyes infected with the wild-type Newman, the double mutant, or the rescue strain (*, P ≤ 0.001). (C) Photographs of rabbit eyes 24 h after intrastromal injection with 100 CFU of strain Newman, the double mutant (Newman ΔhlaΔhlg), the triple mutant (Newman ΔhlaΔhlgΔssl1), or the rescue strain (Newman ΔhlaΔhlgΔssl1/ssl1). These eyes contained similar log CFU per cornea (P = 0.19).
). The SLE score for eyes infected with the triple mutant was significantly lower than that of eyes infected with the wild-type Newman, the double mutant, or the rescue strain (*, P ≤ 0.001). (C) Photographs of rabbit eyes 24 h after intrastromal injection with 100 CFU of strain Newman, the double mutant (Newman ΔhlaΔhlg), the triple mutant (Newman ΔhlaΔhlgΔssl1), or the rescue strain (Newman ΔhlaΔhlgΔssl1/ssl1). These eyes contained similar log CFU per cornea (P = 0.19).
 ), the double mutant (
), the double mutant ( ), the triple mutant (
), the triple mutant ( ), or the rescue strain (
), or the rescue strain ( ). The SLE score for eyes infected with the triple mutant was significantly lower than that of eyes infected with the wild-type Newman, the double mutant, or the rescue strain (*, P ≤ 0.001). (C) Photographs of rabbit eyes 24 h after intrastromal injection with 100 CFU of strain Newman, the double mutant (Newman ΔhlaΔhlg), the triple mutant (Newman ΔhlaΔhlgΔssl1), or the rescue strain (Newman ΔhlaΔhlgΔssl1/ssl1). These eyes contained similar log CFU per cornea (P = 0.19).
). The SLE score for eyes infected with the triple mutant was significantly lower than that of eyes infected with the wild-type Newman, the double mutant, or the rescue strain (*, P ≤ 0.001). (C) Photographs of rabbit eyes 24 h after intrastromal injection with 100 CFU of strain Newman, the double mutant (Newman ΔhlaΔhlg), the triple mutant (Newman ΔhlaΔhlgΔssl1), or the rescue strain (Newman ΔhlaΔhlgΔssl1/ssl1). These eyes contained similar log CFU per cornea (P = 0.19).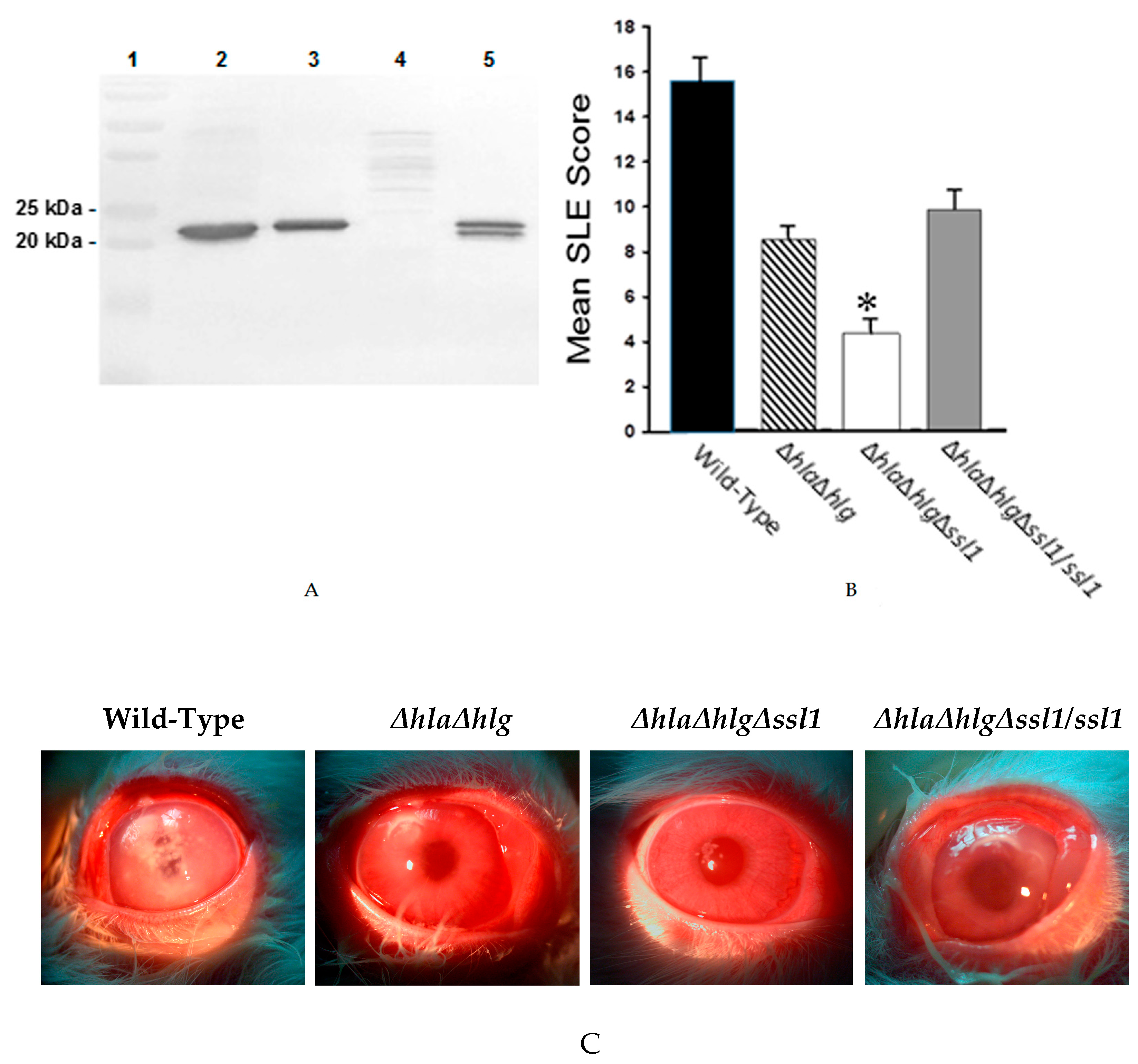
| Inhibitor | Target Protease Class | Concentration Used | % Activity of Control 1 |
|---|---|---|---|
| None | - | - | 100 |
| Chymostatin | Cys/Ser | 0.1 mM | 0 |
| AEBSF | Serine | 10 mM | 0 |
| TLCK | Serine | 1 mM | 0 |
| EDTA | Metallo | 10 mM | 78 |
| Aprotinin | Serine | 0.001 mM | 83 |
| Antipain | Cys/Ser | 0.1 mM | 100 |
| Pepstatin | Aspartic | 0.001 mM | 101 |
| Leupeptin | Cys/Ser | 0.1 mM | 110 |
| Bestatin | Metallo | 0.1 mM | 119 |
| E-64 | Cysteine | 0.01 mM | 130 |
| Phosphoramidon | Metallo | 1 mM | 137 |
| Ocular Isolates | |||
|---|---|---|---|
| Strain | Allele Type 1 | Strain | Allele Type 1 |
| 05.4144 † | 2 | 82936 § | 2 |
| 06.6451 † | 1 | 83523 § | 2 |
| 11.14697 † | 5 | 85278 § | 2 |
| 13.9793 † | 3 | 85397 § | 2 |
| 190.164 ‡ | 7 | 91731 § | 2 |
| 279.274 ‡ | 3 | SD22125 § | 3 |
| 295.236 ‡ | 2 | CO19432 § | 1 |
| 43506 § | 2 | CO61879 § | 2 |
| 67993 § | 2 | CO63355 § | 2 |
| 70468 § | 2 | CO63825 § | 2 |
| Non-Ocular Isolates | |||
| Strain | Allele Type 1 | Strain | Allele Type 1 |
| MRSA 301 || | 3 | 76.137 ‡ | 2 |
| MRSA 306 || | 3 | 103.93 ‡ | 3 |
| 44.255 ‡ | 1 | 116.60 ‡ | 9 |
| 63.21 ‡ | 2 | ||
| Motif ID 1 | Amino Acids | Definition | E-Value |
|---|---|---|---|
| pf: SSL_OB | 41–123 | Staphylococcal superantigen-like OB-fold domain | 4.9 × 10−32 |
| pf: MAP | 142–209 | MAP domain | 0.014 |
| pf: Stap_Strp_tox_C | 147–206 | Staphylococcal/Streptococcal toxin, beta-grasp domain | 0.0002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, A.; Caballero, A.R.; Bierdeman, M.A.; Marquart, M.E.; Foster, T.J.; Monk, I.R.; O’Callaghan, R.J. Staphylococcus aureus Superantigen-Like Protein SSL1: A Toxic Protease. Pathogens 2019, 8, 2. https://doi.org/10.3390/pathogens8010002
Tang A, Caballero AR, Bierdeman MA, Marquart ME, Foster TJ, Monk IR, O’Callaghan RJ. Staphylococcus aureus Superantigen-Like Protein SSL1: A Toxic Protease. Pathogens. 2019; 8(1):2. https://doi.org/10.3390/pathogens8010002
Chicago/Turabian StyleTang, Aihua, Armando R. Caballero, Michael A. Bierdeman, Mary E. Marquart, Timothy J. Foster, Ian R. Monk, and Richard J. O’Callaghan. 2019. "Staphylococcus aureus Superantigen-Like Protein SSL1: A Toxic Protease" Pathogens 8, no. 1: 2. https://doi.org/10.3390/pathogens8010002
APA StyleTang, A., Caballero, A. R., Bierdeman, M. A., Marquart, M. E., Foster, T. J., Monk, I. R., & O’Callaghan, R. J. (2019). Staphylococcus aureus Superantigen-Like Protein SSL1: A Toxic Protease. Pathogens, 8(1), 2. https://doi.org/10.3390/pathogens8010002





