Chemical Composition, Biocompatibility, and Anti-Candida albicans Activity of Schinus weinmanniifolia Mart. ex Engl.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Collection and Preparation of Aqueous Extract of Leaves of Schinus weinmanniifolia Mart. ex Engl. (AES)
2.2. Chemical Characterization
2.3. Antioxidant Activity
2.3.1. Radical Scavenging 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
2.3.2. Radical Scavenging 2,2-Azino-bis (3-Ethylbenzothiazoline-6-sulfonic Acid) (ABTS)
2.4. Antifungal Activity
2.4.1. Microorganisms
2.4.2. Minimum Inhibitory Concentration (MIC)
2.4.3. Growth Kinetics of C. albicans
2.5. Mechanisms of Action in Planktonic Cells of C. albicans
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Germ Tube Formation (GTF)
2.5.3. Yeast-to-Hyphal Transition
2.6. Biocompatibility
2.6.1. Hemolytic Activity
2.6.2. Mutagenicity Test
2.7. Selectivity Index (SI)
2.8. Statistical Analysis
3. Results
3.1. Chemical Characterization
3.2. Antioxidant Activity
3.3. Antifungal Activity
3.3.1. Minimum Inhibitory Concentration (MIC)
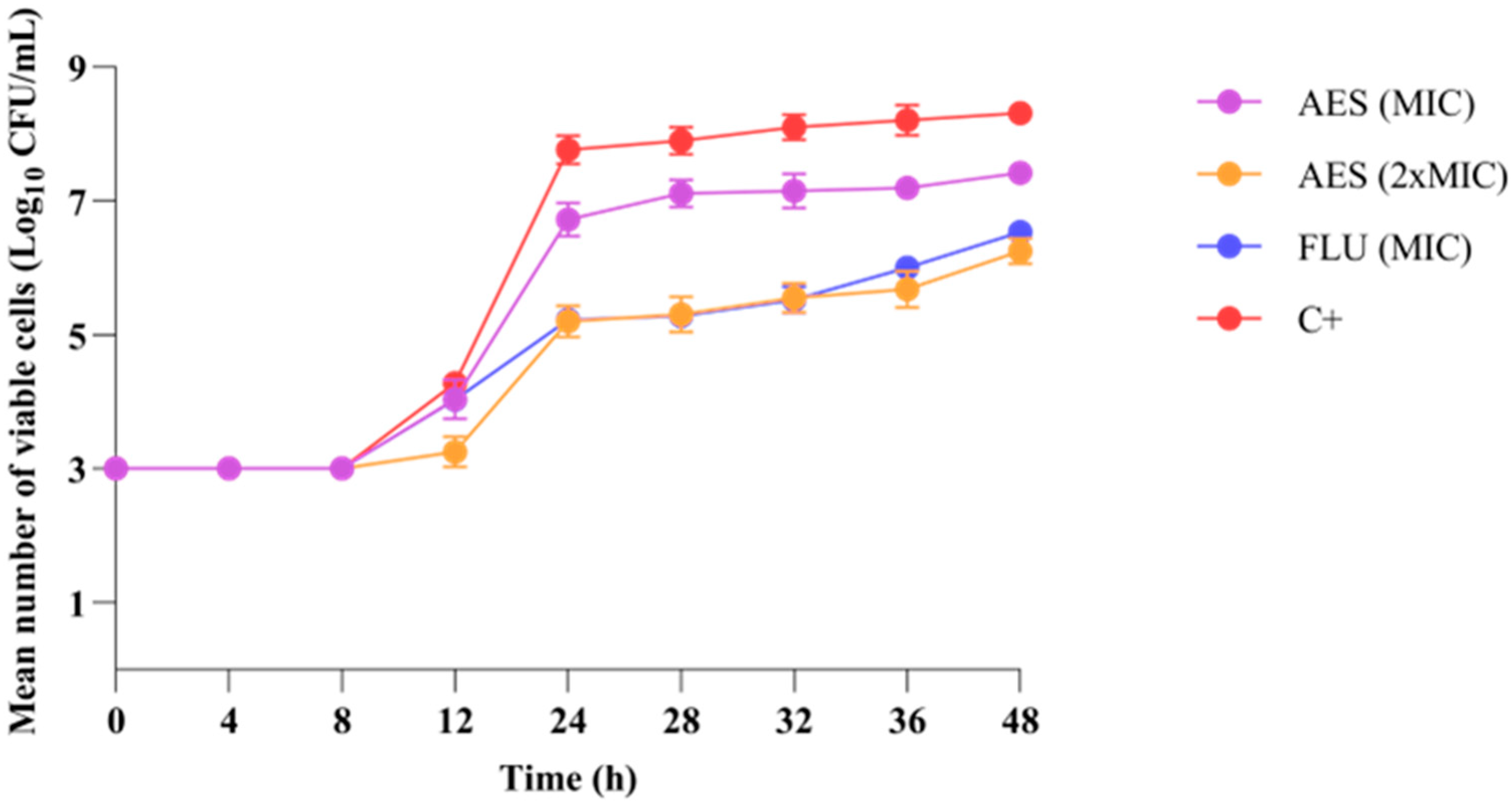
3.3.2. Growth Kinetics of C. albicans
3.4. Mechanisms of Action in Planktonic Cells of C. albicans
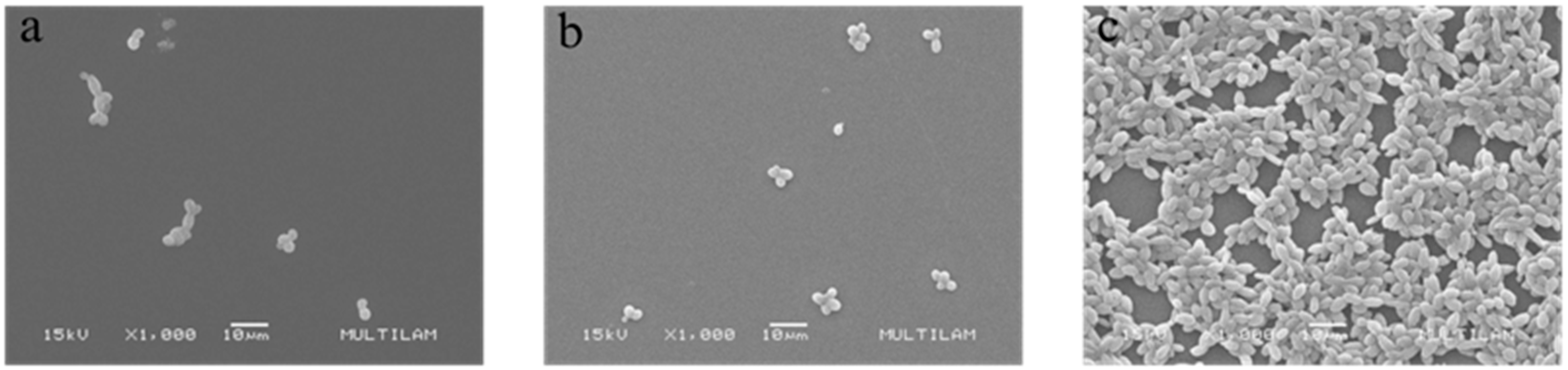
3.4.1. Scanning Electron Microscopy (SEM)
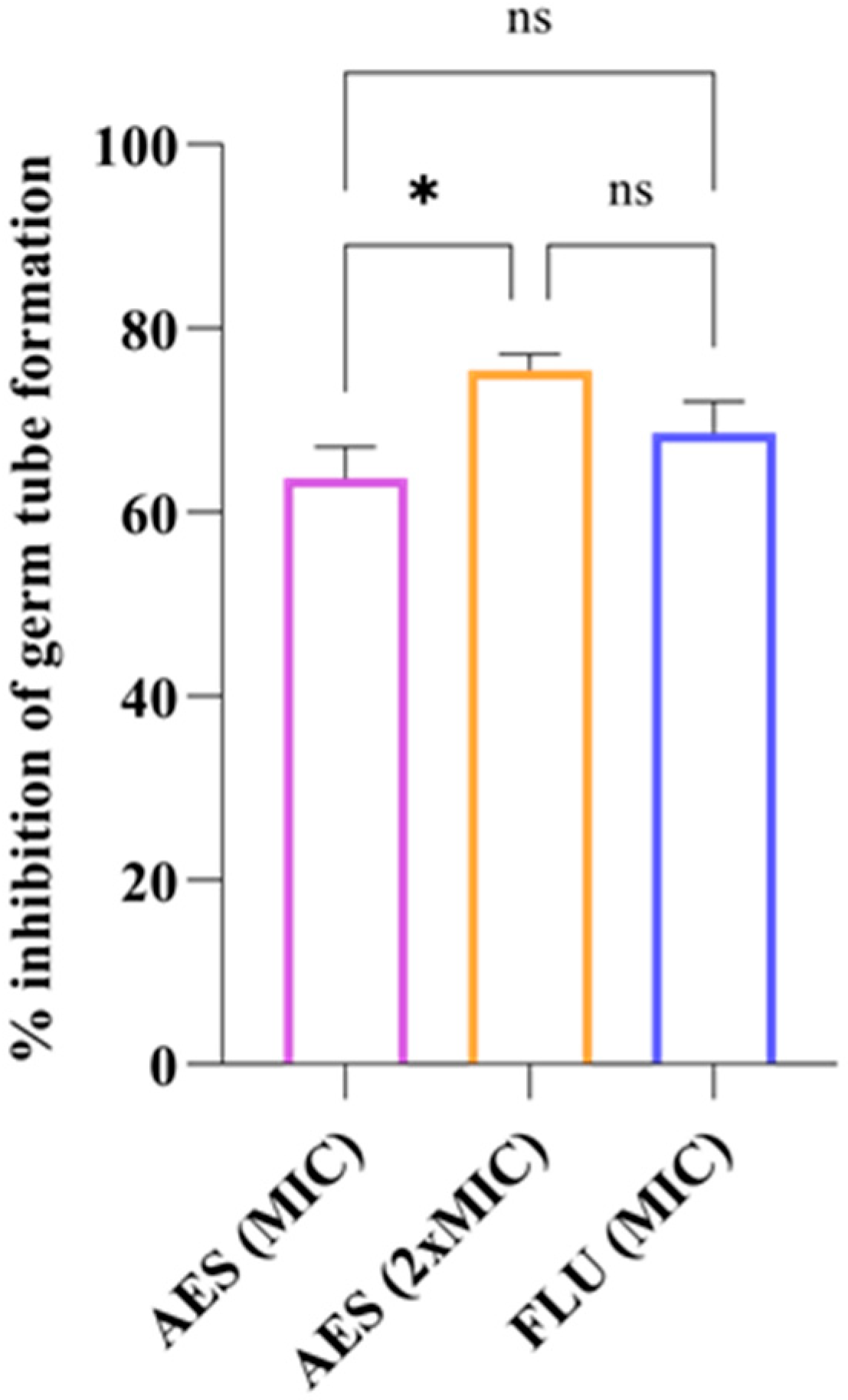
3.4.2. Germ Tube Formation
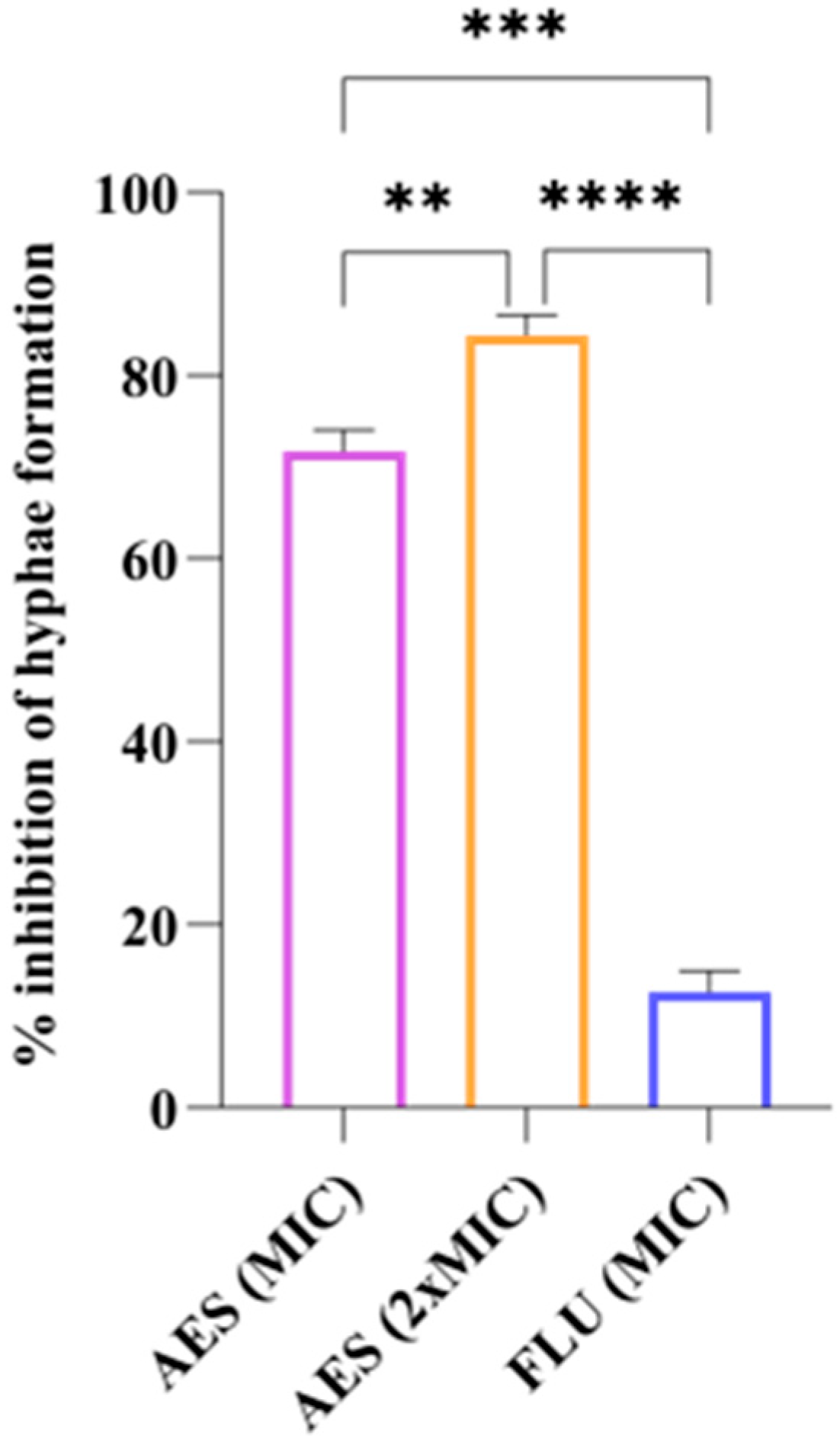
3.4.3. Yeast-to-Hyphal Transition
3.5. Biocompatibility
3.5.1. Hemolytic Activity
3.5.2. Mutagenicity Test
3.6. Selectivity Index (SI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| ABTS | 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| AES | Schinus weinmanniifolia Mart. ex Engl. |
| ANVISA | Agência Nacional de Vigilância Sanitária |
| ATCC | American Type Culture Collection |
| BHT | Butylated hydroxytoluene |
| BPC | Base peak chromatogram |
| CFU | Colony Forming Unit |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FLU | Fluconazole |
| GRP | Germination reduction percentage |
| GTF | Germ tube formation |
| HAT | Hydrogen atom transfer |
| HRMS | High-resolution mass spectrometry |
| IC50 | Half-maximal inhibitory concentration |
| MDA | Marker malondialdehyde |
| MI | Mutagenic Index |
| MIC | Minimum Inhibitory Concentration |
| PBS | Phosphate-buffered saline |
| RVVC | Recurrent Vulvovaginal Candidiasis |
| SEM | Scanning electron microscopy |
| SET | Single electron transfer |
| SI | Selectivity index |
| TAC | Total Antioxidant Capacity |
| VVC | Vulvovaginal Candidiasis |
| YNB | Yeast Nitrogen Base |
Appendix A
Appendix A.1
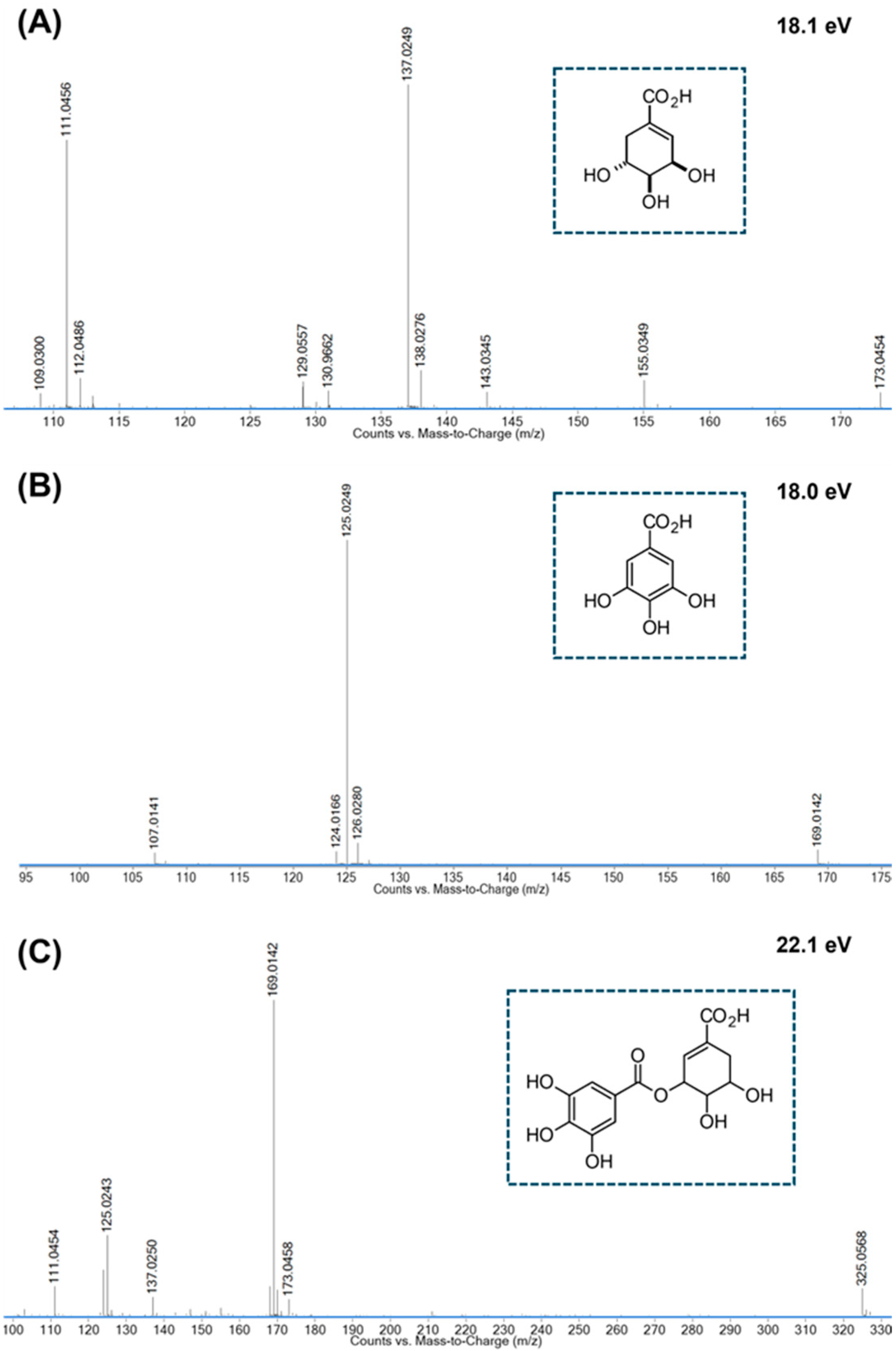
Appendix A.2
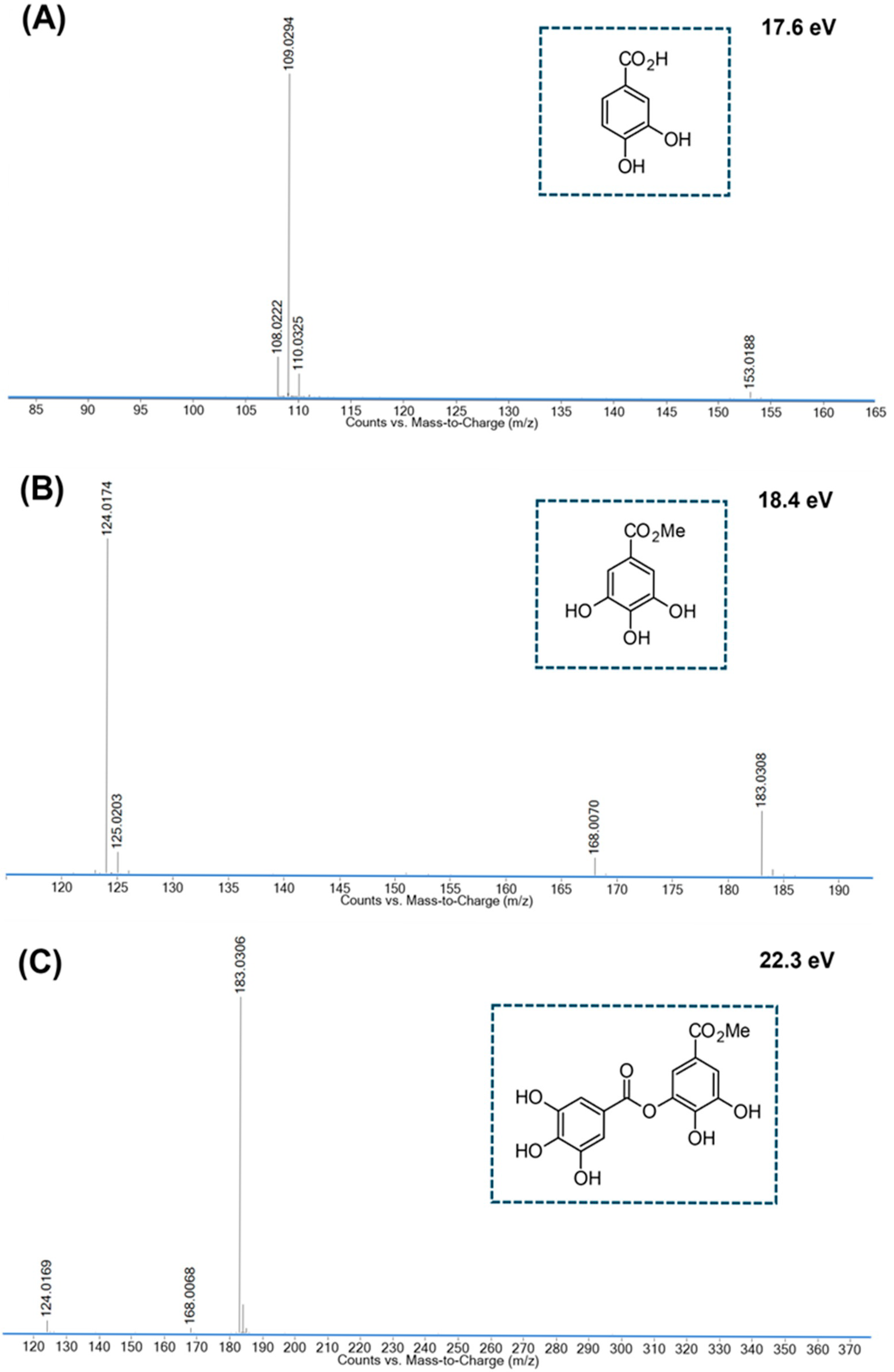
Appendix A.3
References
- Parambath, S.; Dao, A.; Kim, H.Y.; Zawahir, S.; Alastruey Izquierdo, A.; Tacconelli, E.; Govender, N.; Oladele, R.; Colombo, A.; Sorrell, T.; et al. Candida albicans-A Systematic Review to Inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae045. [Google Scholar] [CrossRef]
- WHO. Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 5 November 2024).
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global Burden of Recurrent Vulvovaginal Candidiasis: A Systematic Review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- David, H.; Solomon, A.P. Molecular Association of Candida albicans and Vulvovaginal Candidiasis: Focusing on a Solution. Front. Cell. Infect. Microbiol. 2023, 13, 1245808. [Google Scholar] [CrossRef] [PubMed]
- Health Inequalities in Latin America and the Caribbean. A Baseline for the Global Strategy for Women’s, Children’s and Adolescents’ Health; IDB, PAHO, UNAIDS, UNICEF, UNWOMEN, USAID, World Bank: Washington, DC, USA, 2023; ISBN 978-92-75-12628-8. [Google Scholar]
- PAHO and UNICEF. Health Inequalities in Latin America and the Caribbean: A Sustainable Development Goal Baseline Assessment for Women, Children and Adolescents; PAHO and UNICEF: New York, NY, USA, 2022; ISBN 978-92-75-12574-8. [Google Scholar]
- Traditional Midwives: Saving Lives by Combining the Knowledge of Ancestral and Western Medicines—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/stories/traditional-midwives-saving-lives-combining-knowledge-ancestral-and-western-medicines (accessed on 10 November 2024).
- Jansåker, F.; Frimodt-Møller, N.; Li, X.; Sundquist, K. Novel Risk Factors Associated with Common Vaginal Infections: A Nationwide Primary Health Care Cohort Study. Int. J. Infect. Dis. 2022, 116, 380–386. [Google Scholar] [CrossRef]
- del Carmen Morales-Ramírez, K.; Avila-Sosa, R.; Cid-Pérez, T.S.; Avelino-Flores, F.; Duarte-Escalante, E.; Munguía-Pérez, R. Environmental and Social Determinants Related to Candidiasis. In Candida Albicans—Epidemiology and Treatment; IntechOpen: London, UK, 2024; ISBN 978-0-85466-247-0. [Google Scholar]
- Ortiz-Prado, E.; Izquierdo-Condoy, J.S.; Vasconez-González, J.E.; Dávila, G.; Correa, T.; Fernández-Naranjo, R. The Pharmaceutical Market for Biological Products in Latin America: A Comprehensive Analysis of Regional Sales Data. J. Law Med. Ethics 2023, 51, 39–61. [Google Scholar] [CrossRef]
- Satora, M.; Grunwald, A.; Zaremba, B.; Frankowska, K.; Żak, K.; Tarkowski, R.; Kułak, K. Treatment of Vulvovaginal Candidiasis-An Overview of Guidelines and the Latest Treatment Methods. J. Clin. Med. 2023, 12, 5376. [Google Scholar] [CrossRef]
- Bonfim, A.P.; Sakita, K.M.; Faria, D.R.; Arita, G.S.; Vendramini, F.A.V.R.; Capoci, I.R.G.; Braga, A.G.; Santos, R.d.S.; Bruschi, M.L.; Becker, T.C.A.; et al. Preclinical Approaches in Vulvovaginal Candidiasis Treatment with Mucoadhesive Thermoresponsive Systems Containing Propolis. PLoS ONE 2020, 15, e0243197. [Google Scholar] [CrossRef]
- Fernandes, L.; Barco-Tejada, A.; Blázquez, E.; Araújo, D.; Ribeiro, A.; Silva, S.; Cussó, L.; Costa-de-Oliveira, S.; Rodrigues, M.E.; Henriques, M. Development and Evaluation of Microencapsulated Oregano Essential Oil as an Alternative Treatment for Candida albicans Infections. ACS Appl. Mater. Interfaces 2024, 16, 40628–40640. [Google Scholar] [CrossRef]
- Heydarian Moghadam, F.; Tansaz, M.; Aminimoghaddam, S.; Hajimehdipoor, H. The Effect of Boswellia Vaginal Gel on Oxidative Stress and Expression of Apoptotic Biomarkers in Vaginal Discharge of Women with Vaginitis. Res. J. Pharmacogn. 2022, 9, 29–36. [Google Scholar] [CrossRef]
- Paterniti, I.; Casili, G.; Filippone, A.; Lanza, M.; Ardizzone, A.; Capra, A.P.; Campolo, M.; Esposito, E. A New Approach for the Treatment of Recurrent Vulvovaginal Candidiasis with a Combination of Pea Protein, Grape Seed Extract, and Lactic Acid Assessed In Vivo. J. Fungi 2022, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; Aspinall, S.; Beynon, A.; Ash, A.; Lymbery, A. Traditional Medicinal Plants in the Treatment of Gastrointestinal Parasites in Humans: A Systematic Review and Meta-Analysis of Clinical and Experimental Evidence. Phytother. Res. 2023, 37, 3675–3687. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; Abd El-Ghffar, E.A.; Eldahshan, O.A.; Singab, A.N.B. The Genus Schinus (Anacardiaceae): A Review on Phytochemicals and Biological Aspects. Nat. Prod. Res. 2021, 36, 4833–4851. [Google Scholar] [CrossRef]
- Schinus terebinthifolia. Available online: https://fitoterapiabrasil.com.br/planta-medicinal/schinus-terebinthifolia (accessed on 6 November 2024).
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical Composition and Anticancer and Antioxidant Activities of Schinus molle L. and Schinus terebinthifolius Raddi Berries Essential Oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef]
- Silva, M.d.M.; Iriguchi, E.K.K.; Kassuya, C.A.L.; Vieira, M.d.C.; Foglio, M.A.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Souza, K.d.P.; Formagio, A.S.N. Schinus terebinthifolius: Phenolic Constituents and In Vitro Antioxidant, Antiproliferative and In Vivo Anti-Inflammatory Activities. Rev. Bras. Farmacogn. 2017, 27, 445–452. [Google Scholar] [CrossRef]
- Cutro, A.C.; Castelli, M.V.; López, S.N.; Rosales, M.A.; Hollmann, A.; Rodriguez, S.A. Chemical Composition of Schinus Essential Oil and Antimicrobial Action against Staphylococcus aureus. Nat. Prod. Res. 2021, 35, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- da Silva Dannenberg, G.; Funck, G.D.; Mattei, F.J.; da Silva, W.P.; Fiorentini, Â.M. Antimicrobial and Antioxidant Activity of Essential Oil from Pink Pepper Tree (Schinus terebinthifolius Raddi) In Vitro and in Cheese Experimentally Contaminated with Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2016, 36, 120–127. [Google Scholar] [CrossRef]
- El-Massry, K.F.; El-Ghorab, A.H.; Shaaban, H.A.; Shibamoto, T. Chemical Compositions and Antioxidant/Antimicrobial Activities of Various Samples Prepared from Schinus terebinthifolius Leaves Cultivated in Egypt. J. Agric. Food Chem. 2009, 57, 5265–5270. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.C.; Franciscato, L.M.S.S.; Mendes, S.S.; Barizon, F.M.A.; Gonçalves, D.D.; Barbosa, L.N.; Faria, M.G.I.; Valle, J.S.; Casalvara, R.F.A.; Gonçalves, J.E.; et al. Essential Oil from the Leaves, Fruits and Twigs of Schinus terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential. Molecules 2024, 29, 469. [Google Scholar] [CrossRef]
- Uliana, M.P.; Fronza, M.; da Silva, A.G.; Vargas, T.S.; de Andrade, T.U.; Scherer, R. Composition and Biological Activity of Brazilian Rose Pepper (Schinus terebinthifolius Raddi) Leaves. Ind. Crops Prod. 2016, 83, 235–240. [Google Scholar] [CrossRef]
- Ceruks, M.; Romoff, P.; Fávero, O.A.; Lago, J.H.G. Constituíntes Fenólicos Polares de Schinus terebinthifolius Raddi (Anacardiaceae). Quím. Nova 2007, 30, 597–599. [Google Scholar] [CrossRef]
- Pott, A.; Pott, V.J.; de Souza, T.W. Plantas Daninhas de Pastagem na Região dos Cerrados; Embrapa Gado de Corte: Campo Grande, MS, Brazil, 2006; ISBN 978-85-297-0202-5. [Google Scholar]
- Schinus weinmanniifolia Engl.|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:71076-1 (accessed on 6 November 2024).
- Mereles, F. Recursos Fitogeneticos: Plantas Útiles de las Cuencas del Tebicuary mí y Capiíbary, Paraguay Oriental; Departamento de Botánica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción: San Lorenzo, Paraguay, 2001. [Google Scholar]
- Velázquez, E.; Tournier, H.A.; Mordujovich de Buschiazzo, P.; Saavedra, G.; Schinella, G.R. Antioxidant Activity of Paraguayan Plant Extracts. Fitoterapia 2003, 74, 91–97. [Google Scholar] [CrossRef]
- Hernandes, C.; Taleb-Contini, S.H.; Bartolomeu, A.C.D.; Bertoni, B.W.; França, S.C.; Pereira, A.M.S. Chemical Composition and Antifungal Activity of the Essential Oils of Schinus weinmannifolius Collected in the Spring and Winter. Nat. Prod. Commun. 2014, 9, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.F.P.; Vicentini, M.; Moura, J.; Acosta, A.; Martinez, M.; Ferreira, B.F.P.; Vicentini, M.; Moura, J.; Acosta, A.; Martinez, M. Estudio Químico-Biológico del Extracto Crudo Etanolico de Hojas del Schinus weinmanniifolia Mart. Ex Engl (Molle’i) de la Localidad de Pirareta (Cordillera, Paraguay). Rep. Cient. FACEN 2023, 14, 11–24. [Google Scholar] [CrossRef]
- do Prado Schneidewind, F.C.C.; de Castilho, P.F.; Galvão, F.; de Andrade Dos Santos, J.V.; da Silva Dantas, F.G.; Negri, M.; da Silva Pinto, L.; Moraes, C.A.F.; Freitas, J.; de Souza, P.R.B.; et al. Effects of Bioconversion by Battus polydamas on the Chemical Composition of Aristolochia spp. and Evaluation of Antimicrobial Activity and Biocompatibility. Fitoterapia 2024, 175, 105949. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- M27 Ed4 Broth Dilution Antifungal Susceptibility, Yeasts. Available online: https://clsi.org/standards/products/microbiology/documents/m27/ (accessed on 4 November 2024).
- de Freitas, A.L.D.; Kaplum, V.; Rossi, D.C.P.; da Silva, L.B.R.; Melhem, M.d.S.C.; Taborda, C.P.; de Mello, J.C.P.; Nakamura, C.V.; Ishida, K. Proanthocyanidin Polymeric Tannins from Stryphnodendron adstringens Are Effective against Candida spp. Isolates and for Vaginal Candidiasis Treatment. J. Ethnopharmacol. 2018, 216, 184–190. [Google Scholar] [CrossRef]
- Sannomiya, M.; Rodrigues, C.M.; Oliveira, G.C.A.; Carvalho, J.C.S.; da Costa, L.S.; Spadari, C.d.C.; Ferreira, M.J.P.; Vilegas, W.; Ishida, K. Galloylquinic Acid Derivatives from Byrsonima fagifolia Leaf Extract and Potential Antifungal Activity. J. Ethnopharmacol. 2022, 297, 115534. [Google Scholar] [CrossRef]
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of Test Conditions on Antifungal Time-Kill Curve Results: Proposal for Standardized Methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef]
- Ramalho, S.R.; de Cássia Orlandi Sardi, J.; Júnior, E.C.; Marchetto, R.; Wender, H.; Vargas, L.F.P.; de Miranda, A.; Almeida, C.V.; de Oliveira Almeida, L.H.; de Oliveira, C.F.R.; et al. The Synthetic Antimicrobial Peptide IKR18 Displays Anti-Infectious Properties in Galleria mellonella I n Vivo Model. Biochim. Biophys. Acta (BBA) Gen. Subj. 2022, 1866, 130244. [Google Scholar] [CrossRef]
- Haghdoost, N.S.; Salehi, T.Z.; Khosravi, A.; Sharifzadeh, A. Antifungal Activity and Influence of Propolis against Germ Tube Formation as a Critical Virulence Attribute by Clinical Isolates of Candida albicans. J. Mycol. Médicale 2016, 26, 298–305. [Google Scholar] [CrossRef]
- Bravo-Chaucanés, C.P.; Vargas-Casanova, Y.; Chitiva-Chitiva, L.C.; Ceballos-Garzon, A.; Modesti-Costa, G.; Parra-Giraldo, C.M. Evaluation of Anti-Candida Potential of Piper Nigrum Extract in Inhibiting Growth, Yeast-Hyphal Transition, Virulent Enzymes, and Biofilm Formation. J. Fungi 2022, 8, 784. [Google Scholar] [CrossRef]
- Dhonnar, S.L.; More, R.A.; Adole, V.A.; Jagdale, B.S.; Sadgir, N.V.; Chobe, S.S. Synthesis, Spectral Analysis, Antibacterial, Antifungal, Antioxidant and Hemolytic Activity Studies of Some New 2,5-Disubstituted-1,3,4-Oxadiazoles. J. Mol. Struct. 2022, 1253, 132216. [Google Scholar] [CrossRef]
- Kado, N.Y.; Langley, D.; Eisenstadt, E. A Simple Modification of the Salmonella Liquid-Incubation Assay Increased Sensitivity for Detecting Mutagens in Human Urine. Mutat. Res. Lett. 1983, 121, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kummrow, F.; Rech, C.M.; Coimbrão, C.A.; Umbuzeiro, G.A. Blue Rayon-Anchored Technique/Salmonella Microsome Microsuspension Assay as a Tool to Monitor for Genotoxic Polycyclic Compounds in Santos Estuary. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2006, 609, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sikora, K.; Bauer, M.; Bartoszewska, S.; Neubauer, D.; Kamysz, W. Glycosylated Lipopeptides—Synthesis and Evaluation of Antimicrobial Activity and Cytotoxicity. Biomolecules 2023, 13, 172. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumara, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef]
- Nurazah, Z. Metabolomics Unravel Differences Between Cameroon Dura and Deli Dura Oil Palm (Elaeis guineensis jacq.) Genetic Backgrounds Against Basal Stem rot. J. Oil Palm Res. 2017, 29, 227–241. [Google Scholar] [CrossRef][Green Version]
- Galvão, F.; Dos Santos, E.; Gomes da Silva Dantas, F.; Irlan da Silva Santos, J.; da Paz Costa Sauda, T.; Carvalho Dos Santos, A.; Carvalho Souza, R.I.; da Silva Pinto, L.; Ferreira Moraes, C.A.; Sangalli, A.; et al. Chemical Composition and Effects of Ethanolic Extract and Gel of Cochlospermum regium (Schrank) Pilg. Leaves on Inflammation, Pain, and Wounds. J. Ethnopharmacol. 2023, 302, 115881. [Google Scholar] [CrossRef]
- . Kahnt, A.; Iinuma, Y.; Blockhuys, F.; Mutzel, A.; Vermeylen, R.; Kleindienst, T.E.; Jaoui, M.; Offenberg, J.H.; Lewandowski, M.; Böge, O.; et al. 2-Hydroxyterpenylic Acid: An Oxygenated Marker Compound for α-Pinene Secondary Organic Aerosol in Ambient Fine Aerosol. Env. Sci. Technol. 2014, 48, 4901–4908. [Google Scholar] [CrossRef]
- Picheta, N.; Piekarz, J.; Burdan, O.; Satora, M.; Tarkowski, R.; Kułak, K. Phytotherapy of Vulvovaginal Candidiasis: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 3796. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Wahyuni, D.K.; Kharisma, V.D.; Murtadlo, A.A.A.; Rahmawati, C.T.; Syukriya, A.J.; Prasongsuk, S.; Subramaniam, S.; Wibowo, A.T.; Purnobasuki, H. The Antioxidant and Antimicrobial Activity of Ethanolic Extract in Roots, Stems, and Leaves of Three Commercial Cymbopogon Species. BMC Complement. Med. Ther. 2024, 24, 272. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Dantas-Medeiros, R.; Zanatta, A.C.; de Souza, L.B.F.C.; Fernandes, J.M.; Amorim-Carmo, B.; Torres-Rêgo, M.; Fernandes-Pedrosa, M.d.F.; Vilegas, W.; Araújo, T.A.d.S.; Michel, S.; et al. Antifungal and Antibiofilm Activities of B-Type Oligomeric Procyanidins from Commiphora leptophloeos Used Alone or in Combination with Fluconazole Against Candida Spp. Front. Microbiol. 2021, 12, 613155. [Google Scholar] [CrossRef]
- Vila, T.; Lopez-Ribot, J.L. Screening the Pathogen Box for Identification of Candida albicans Biofilm Inhibitors. Antimicrob. Agents Chemother. 2016, 61, e02006. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.d.N.; Ferreira, A.R.; Duarte, A.B.S.; Melo, A.K.V.; de Sousa, D.P.; Castro, R.D. de Breakpoints for the Classification of Anti-Candida Compounds in Antifungal Screening. BioMed Res. Int. 2021, 2021, 6653311. [Google Scholar] [CrossRef] [PubMed]
- Wakade, R.S.; Wellington, M.; Krysan, D.J. Temporal Dynamics of Candida albicans Morphogenesis and Gene Expression Reveals Distinctions between In Vitro and In Vivo Filamentation. mSphere 2024, 9, e00110-24. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119. [Google Scholar] [CrossRef]
- Salama, O.E.; Gerstein, A.C. Differential Response of Candida Species Morphologies and Isolates to Fluconazole and Boric Acid. Antimicrob. Agents Chemother. 2022, 66, e02406-21. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Jacobsen, I.D.; Wilson, D.; Wächtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans Dimorphism as a Therapeutic Target. Expert Rev. Anti-Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P.; Mencherini, T.; Sansone, F.; Del Gaudio, P.; Granata, I.; Porta, A.; Aquino, R.P. Screening of a Polar Extract of Paeonia rockii: Composition and Antioxidant and Antifungal Activities. J. Ethnopharmacol. 2011, 138, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, G.R.; Gontijo, A.V.L.; Salvador, M.J.; Tanaka, M.H.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Koga-Ito, C.Y. Effects of Acetone Fraction from Buchenavia tomentosa Aqueous Extract and Gallic Acid on Candida albicans Biofilms and Virulence Factors. Front. Microbiol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Costa-Lotufo, L.V.; Khan, M.T.H.; Ather, A.; Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; de Moraes, M.E.A.; de Moraes, M.O. Studies of the Anticancer Potential of Plants Used in Bangladeshi Folk Medicine. J. Ethnopharmacol. 2005, 99, 21–30. [Google Scholar] [CrossRef]
- Dantas, F.G.d.S.; de Castilho, P.F.; de Almeida-Apolonio, A.A.; de Araújo, R.P.; de Oliveira, K.M.P. Mutagenic Potential of Medicinal Plants Evaluated by the Ames Salmonella/Microsome Assay: A Systematic Review. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108338. [Google Scholar] [CrossRef]
- Ahmed, R.K.S.; Shameem, I. Role of Ābzan (Sitz Bath) in Gynaecological Disorders: A Comprehensive Review with Scientific Evidence. CELLMED 2022, 12, 5. [Google Scholar] [CrossRef]
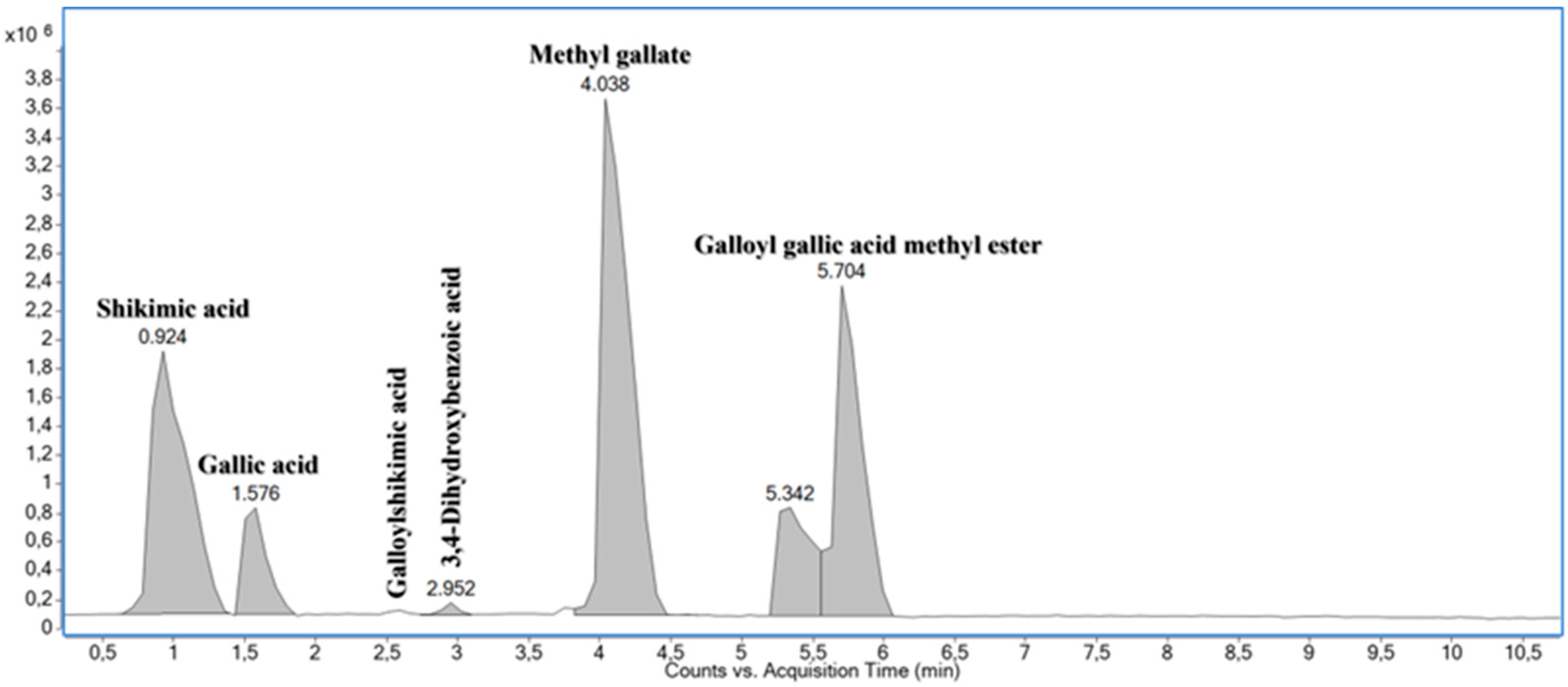
| No. | tR (min) | FM | Ionized Molecules and Relevant Fragment Ions (m/z) | Annotation | ||||
|---|---|---|---|---|---|---|---|---|
| MS | Error | MS | Error | MS/MS [+ (#) and − (*) modes] | [Reference(s)] | |||
| 1 | 0.924 | C7H10O5 | 347.0965 [2M-H]− 173.0450 [M-H]− | −3.8 0.0 *** | *173.04 → 155.04; 143.03; 137.02; 111.05 | Shikimic acid [47] | ||
| 2 | 1.576 | C7H6O5 | 171.0293 [M+H]+ | 0.0 ** | 169.0134 [M-H]− | −1.8 | #171.03 → 153.02; 135.01; 125.02; 109.03; 107.01; * 169.01 → 125.02; 124.02; 107.01 | Gallic acid [48] |
| 3 | 2.590 | C14H14O9 | 349.0539 [M+Na]+ 327.0731 [M+H]+ | +1.0 +4.6 | 325.0567 [M-H]− | +2.3 | #325.06 → 153.02; * 325.06 → 173.04; 170.02; 169.01; 168.01; 137.02; 125.04; 124.02; 111.04 | Galloylshikimic acid [46] |
| 4 | 2.952 | C7H6O4 | 155.0347 [M+H]+ | +1.7 | 153.0182 [M-H]− | −3.8 | #155.03 → 137.02; 109.03; * 153.02 → 110.03; 109.03; 108.02 | 3,4-Dihydroxybenzoic acid [33] |
| 5 | 4.038 | C8H8O5 | 185.0455 [M+H]+ | +2.7 | 367.0656 [2M-H]− 183.0300 [M-H]− | −2.5 +3.6 | #185.04 → 154.02; 153.02; 126.03; 125.02; 123.04; 107.01; * 183.03 → 168.01; 125.02; 124.02 | Methyl gallate [46,48] |
| 6 | 5.196–5.993 | C15H12O9 | 337.0570 [M+H]+ | +3.1 | 671.0865 [2M-H]− 335.0402 [M-H]− | −2.9 −0.3 | #337.06 → 153.02; 125.02; * 335.04 → 183.03; 168.01; 124.02 | Galloyl gallic acid methyl ester [48] |
| IC50 (µg/mL) | ||
|---|---|---|
| Sample | DPPH | ABTS |
| AES | 5.51 ± 0.25 b | 1.52 ± 0.06 c |
| AA | 3.91 ± 0.28 a | 1.92 ± 0.08 a |
| BHT | 5.53 ± 0.14 b | 6.62 ± 0.15 b |
| Microorganism | AES | FLU |
|---|---|---|
| Candida albicans | 1.95 | 0.125 |
| Candida tropicalis | 0.97 | 0.125 |
| Candida parapsilosis | 0.97 | 1 |
| Nakaseomyces glabrata | 0.48 | 8 |
| Pichia kudriavzeveii | 0.48 | - |
| Cryptococcus gattii | 62.5 | 8 |
| Cryptococcus neoformans | 0.97 | 8 |
| C. albicans CMRP3475 | 1.95 | 0.125 |
| C. albicans CMRP3476 | 1.95 | 0.125 |
| C. albicans CMRP3477 | 1.95 | 0.125 |
| C. albicans CMRP3478 | 1.95 | 0.125 |
| C. albicans CMRP3479 | 1.95 | 0.125 |
| AES | ||||
|---|---|---|---|---|
| [µg/plate] | TA98 | TA100 | ||
| S9(−) | S9(+) | S9(−) | S9(+) | |
| 0a | 52 ± 6 | 54 ± 8 | 88 ± 5 | 87 ± 5 |
| 50 | 48 ± 2 (0.9) | 59 ± 1 (1) | 123 ± 11 (1) * | 119 ± 3 (1) ** |
| 150 | 44 ± 1 (0.8) | 60 ± 3 (1) | 145 ± 4 (1) ** | 115 ± 4 (1) ** |
| 500 | 43 ± 1 (0.8) | 47 ± 6 (0.8) | 157 ± 8 (1) ** | 108 ± 4 (1) * |
| 1500 | 47 ± 3 (0.9) | 45 ± 4 (0.8) | 126 ± 3 (1) ** | 150 ± 5 (1) ** |
| 5000 | 47 ± 3 (0.9) | 51 ± 4 (0.9) | 117 ± 5 (1) ** | 138 ± 3 (1) ** |
| C+ | 260 ± 9 b | 293 ± 7 c | 677 ± 9 b | 708 ± 7 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, J.; Almeida-Apolonio, A.; Dantas, F.; Nogueira, C.; Pinto, L.; Moraes, C.; Fernandes, L.; Rodrigues, M.E.; Henriques, M.; Oliveira, K. Chemical Composition, Biocompatibility, and Anti-Candida albicans Activity of Schinus weinmanniifolia Mart. ex Engl. Pathogens 2025, 14, 799. https://doi.org/10.3390/pathogens14080799
Andrade J, Almeida-Apolonio A, Dantas F, Nogueira C, Pinto L, Moraes C, Fernandes L, Rodrigues ME, Henriques M, Oliveira K. Chemical Composition, Biocompatibility, and Anti-Candida albicans Activity of Schinus weinmanniifolia Mart. ex Engl. Pathogens. 2025; 14(8):799. https://doi.org/10.3390/pathogens14080799
Chicago/Turabian StyleAndrade, João, Adriana Almeida-Apolonio, Fabiana Dantas, Cláudio Nogueira, Luciano Pinto, Carlos Moraes, Liliana Fernandes, Maria Elisa Rodrigues, Mariana Henriques, and Kelly Oliveira. 2025. "Chemical Composition, Biocompatibility, and Anti-Candida albicans Activity of Schinus weinmanniifolia Mart. ex Engl." Pathogens 14, no. 8: 799. https://doi.org/10.3390/pathogens14080799
APA StyleAndrade, J., Almeida-Apolonio, A., Dantas, F., Nogueira, C., Pinto, L., Moraes, C., Fernandes, L., Rodrigues, M. E., Henriques, M., & Oliveira, K. (2025). Chemical Composition, Biocompatibility, and Anti-Candida albicans Activity of Schinus weinmanniifolia Mart. ex Engl. Pathogens, 14(8), 799. https://doi.org/10.3390/pathogens14080799







