Congenital Parvovirus B19 During the 2024 European Resurgence: A Prospective Single-Centre Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection and Measurements
2.4. Objectives and Outcomes
2.5. Statistical Analysis
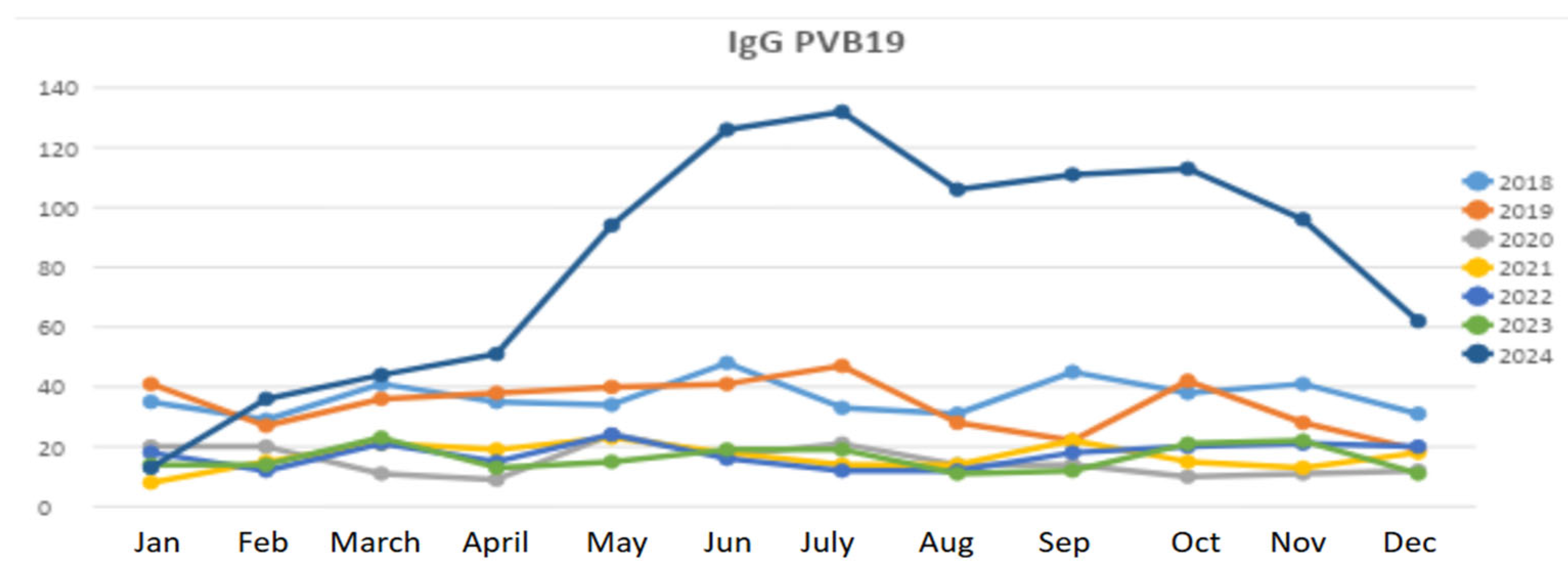
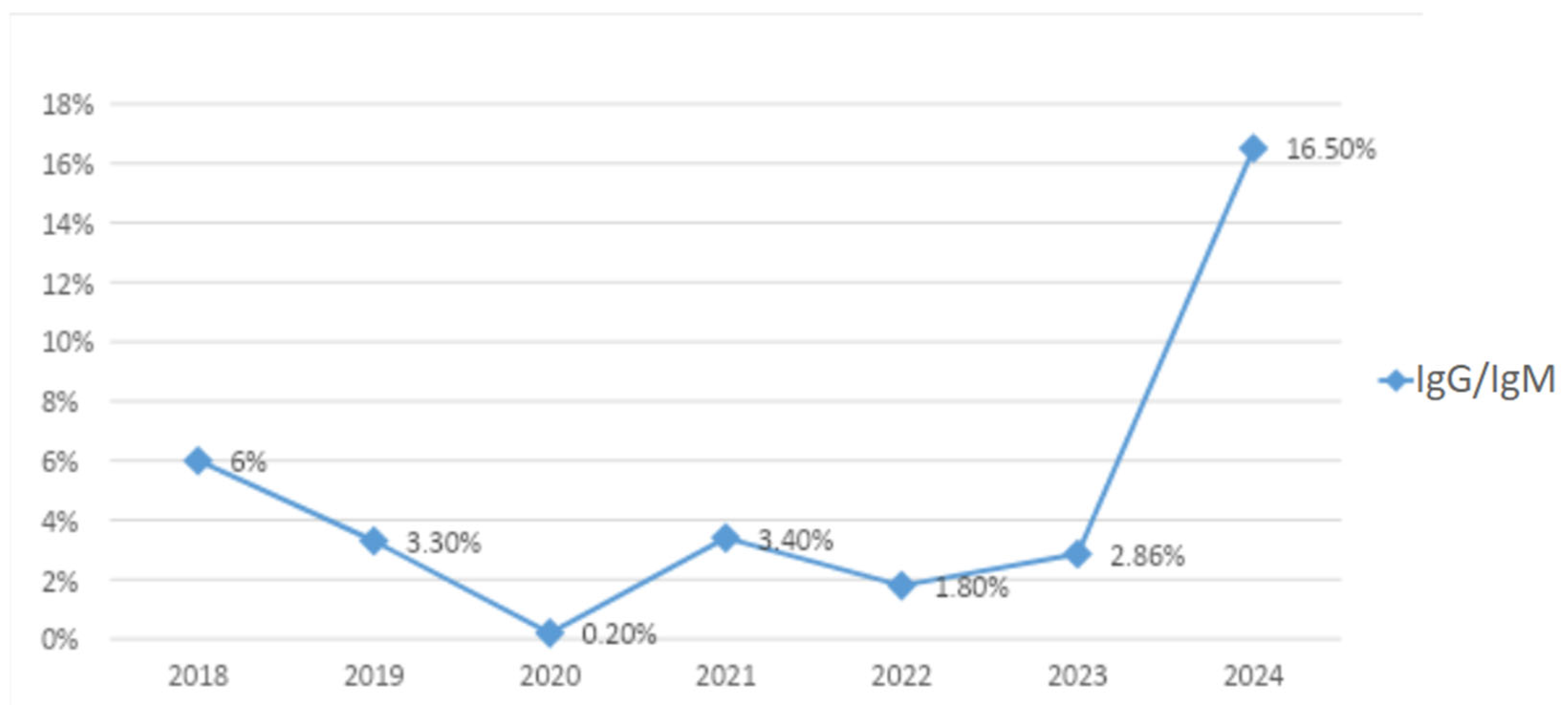
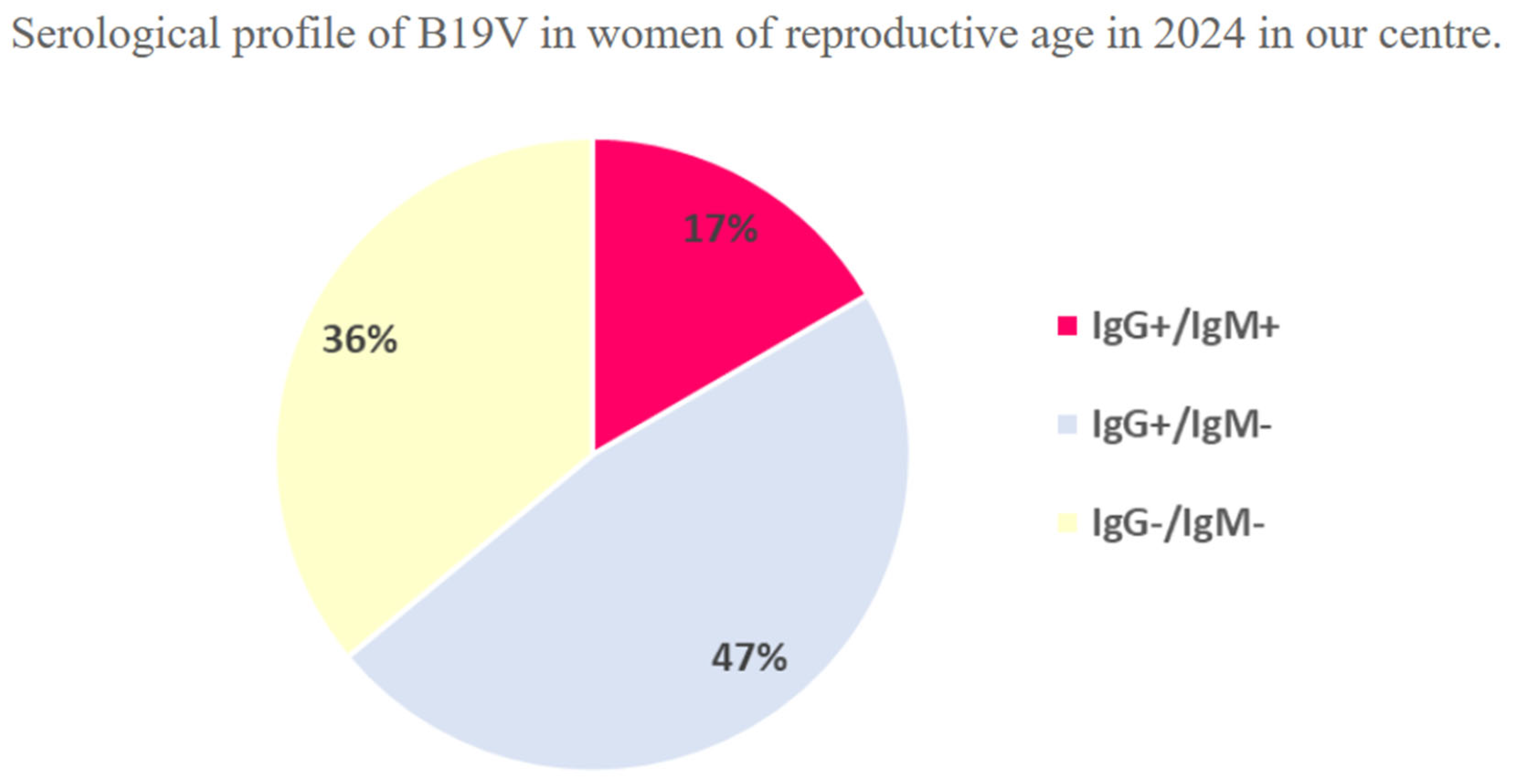
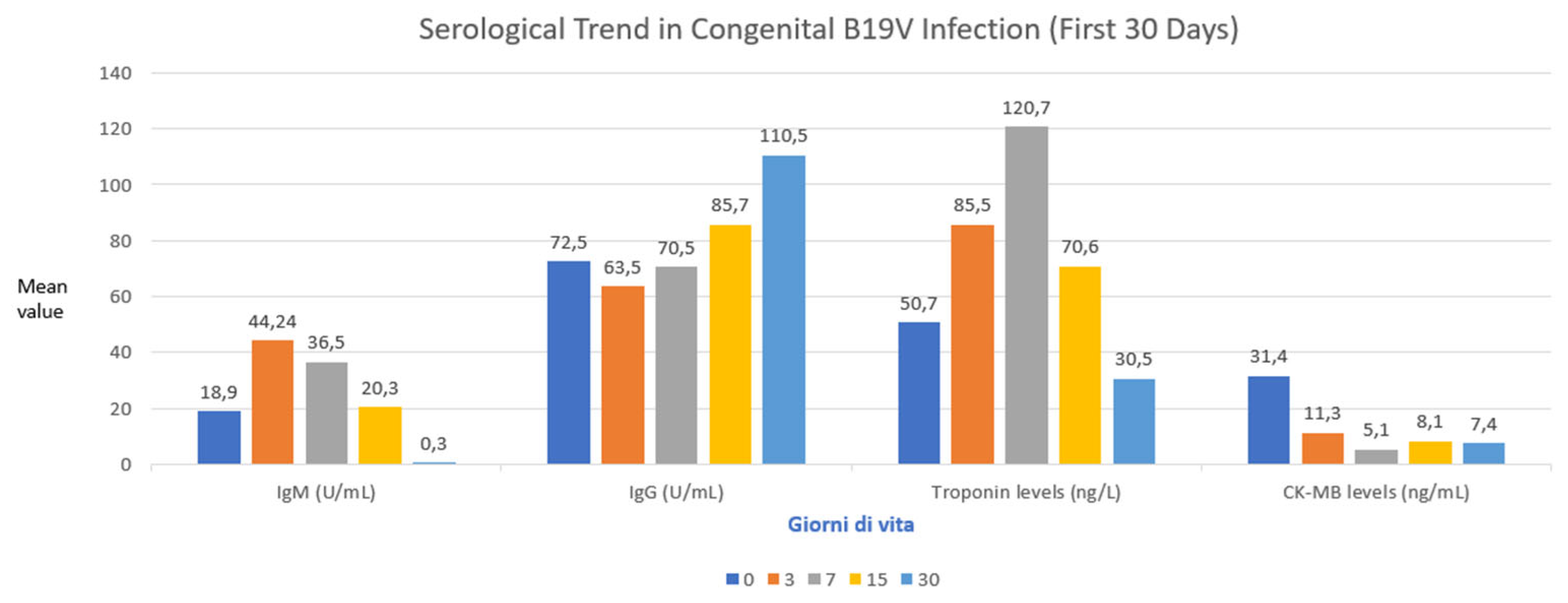
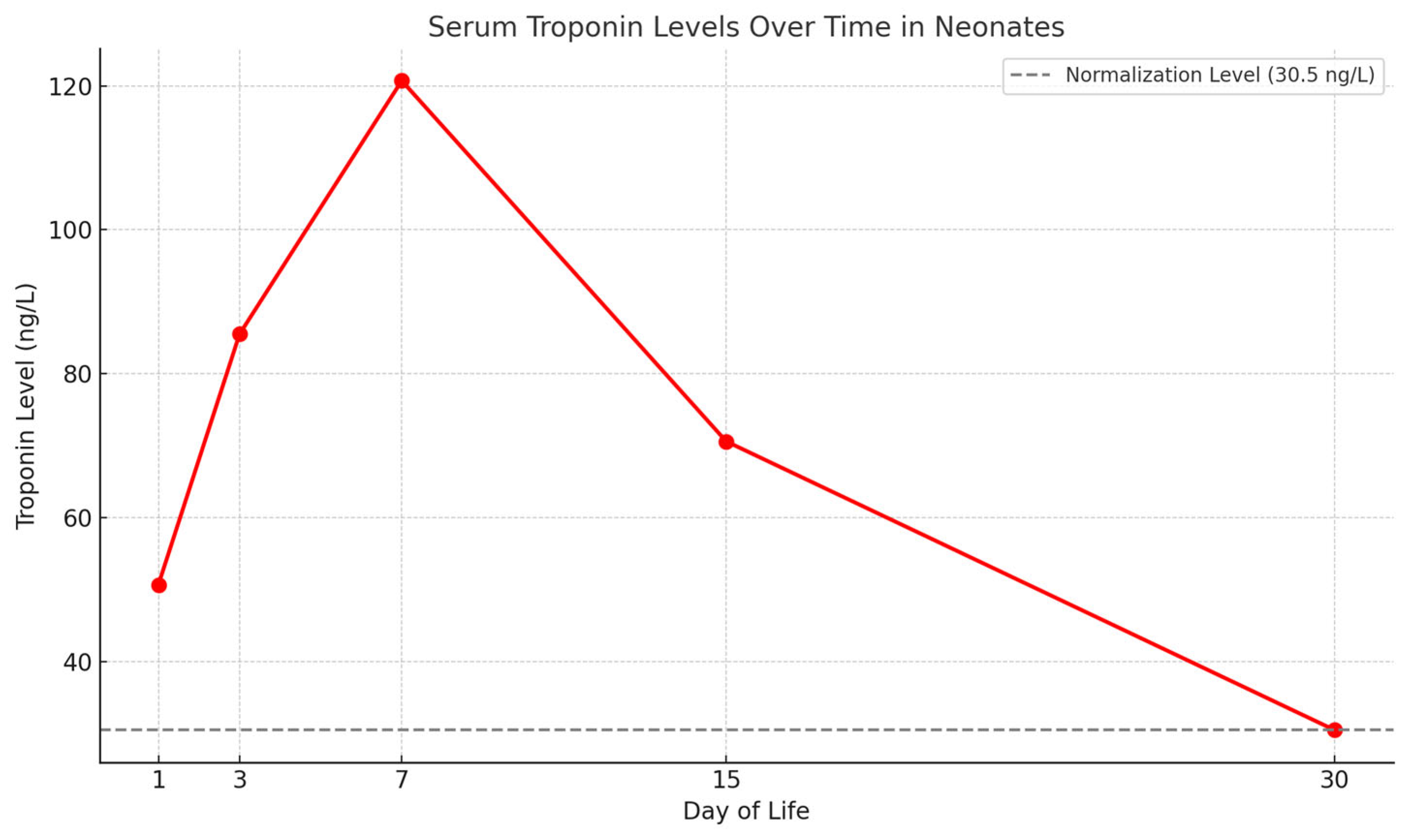
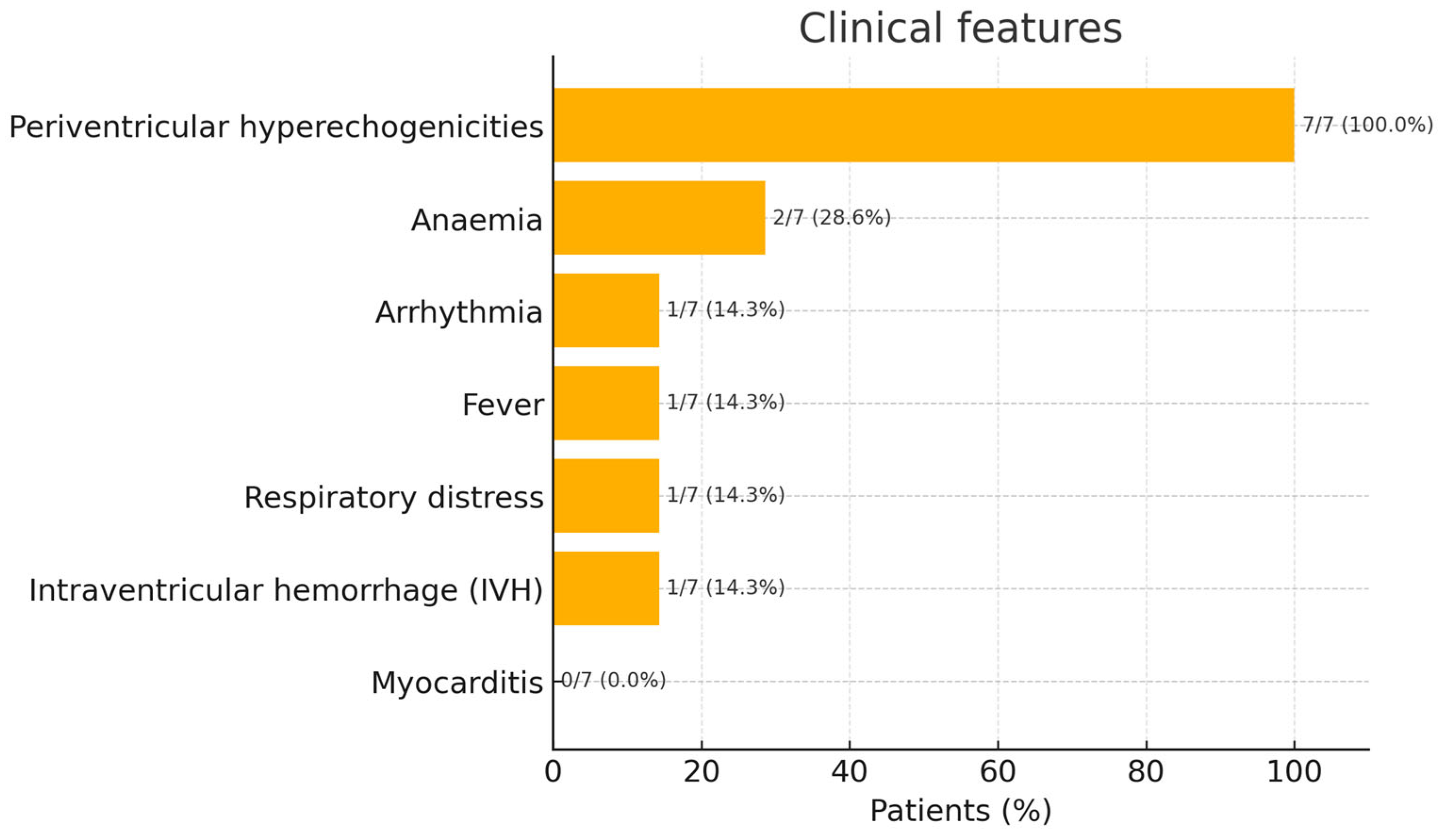
3. Results
Patient Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B19V | Parvovirus B19 |
| CK-MB | Creatine Kinase–Myocardial Band |
| CRP | C-reactive Protein |
| DNA | Deoxyribonucleic Acid |
| ECG | Electrocardiogram |
| GA | Gestational Age |
| gEq/mL | Genome Equivalents per Millilitre |
| Hb | Haemoglobin |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IVH | Intraventricular Haemorrhage |
| LMP | Last Menstrual Period |
| NICU | Neonatal Intensive Care Unit |
| PCR | Polymerase Chain Reaction |
| PCT | Procalcitonin |
| US | Ultrasound |
References
- Zhi, N.; Zádori, Z.; Brown, K.E.; Tijssen, P. Construction and sequencing of an infectious clone of the human parvovirus B19. Virology 2004, 318, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qiu, J. Human Parvovirus B19: A Mechanistic Overview of Infection and DNA Replication. Future Virol. 2015, 10, 155–167. [Google Scholar] [CrossRef]
- Heegaard, E.D.; Brown, K.E. Human Parvovirus B19. Clin. Microbiol. Rev. 2002, 15, 485–505. [Google Scholar] [CrossRef]
- Tassis, B.; Testa, L.; Pampo, F.; Boito, S.; Tiso, G.; Accurti, V.; Cetin, I.; Persico, N. The 2024 Outbreak of Parvovirus B19 as a Global Obstetrical Threat Insights From an Obstetrics Referral Center in Northern Italy. J. Med. Virol. 2024, 96, e70046. [Google Scholar] [CrossRef] [PubMed]
- d’Humières, C.; Fouillet, A.; Verdurme, L.; Lakoussan, S.-B.; Gallien, Y.; Coignard, C.; Hervo, M.; Ebel, A.; Soares, A.; Visseaux, B.; et al. An Unusual Outbreak of Parvovirus B19 Infections, France, 2023 to 2024. Euro Surveill. 2024, 29, 2400339. [Google Scholar] [CrossRef] [PubMed]
- Nordholm, A.C.; Trier Møller, F.; Fischer Ravn, S.; Flink Sørensen, L.; Moltke-Prehn, A.; Elskær Mollerup, J.; Funk, T.; Sperling, L.; Jeyaratnam, U.; Træholt Franck, K.; et al. Epidemic of Parvovirus B19 and Disease Severity in Pregnant People, Denmark, January to March 2024. Euro Surveill. 2024, 29, 2400299. [Google Scholar] [CrossRef] [PubMed]
- Farcet, M.R.; Karbiener, M.; Aberham, C.; Powers, N.; Aue, D.; Kreil, T.R. Parvovirus B19 Rebound Outbreak 2024 and Implications for Blood- and Plasma-Product Safety. Transfusion 2024, 64, 2218–2221. [Google Scholar] [CrossRef]
- Ornoy, A.; Ergaz, Z. Parvovirus B19 Infection during Pregnancy and Risks to the Fetus. Birth Defects Res. 2017, 109, 311–323. [Google Scholar] [CrossRef]
- Kielaite, D.; Paliulyte, V. Parvovirus (B19) Infection during Pregnancy: Possible Effect on the Course of Pregnancy and Rare Fetal Outcomes. A Case Report and Literature Review. Medicina 2022, 58, 664. [Google Scholar] [CrossRef]
- Bonvicini, F.; Puccetti, C.; Salfi, N.C.M.; Guerra, B.; Gallinella, G.; Rizzo, N.; Zerbini, M. Gestational and Fetal Outcomes in B19 Maternal Infection: A Problem of Diagnosis. J. Clin. Microbiol. 2011, 49, 3514–3518. [Google Scholar] [CrossRef]
- Bascietto, F.; Liberati, M.; Murgano, D.; Buca, D.; Iacovelli, A.; Flacco, M.E.; Manzoli, L.; Familiari, A.; Scambia, G.; D’Antonio, F. Outcome of Fetuses with Congenital Parvovirus B19 Infection: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 52, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, F.P.; Guimarães, C.d.M.; Peixoto, A.B.; Pontes, K.F.M.; Bonasoni, M.P.; Tonni, G.; Araujo Júnior, E. Parvovirus B19 Infection and Pregnancy: Review of the Current Knowledge. J. Pers. Med. 2024, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Kagan, K.O.; Hoopmann, M.; Geipel, A.; Sonek, J.; Enders, M. Prenatal Parvovirus B19 Infection. Arch. Gynecol. Obstet. 2024, 310, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, J.; Hazebroek, M.; Merken, J.; Debing, Y.; Dennert, R.; Brunner-La Rocca, H.-P.; Heymans, S. Relevance of Cardiac Parvovirus B19 in Myocarditis and Dilated Cardiomyopathy: Review of the Literature. Eur. J. Heart Fail. 2016, 18, 1430–1441. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Liu, F.; Han, M.; Xu, D.; Zang, Y. Prevalence of Human Parvovirus B19 DNA in Cardiac Tissues of Patients with Congenital Heart Diseases Indicated by Nested PCR and in Situ Hybridization. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2004, 31, 20–24. [Google Scholar] [CrossRef]
- Keramari, S.; Poutoglidis, A.; Chatzis, S.; Keramaris, M.; Savopoulos, C.; Kaiafa, G. Parvovirus B19-Associated Myocarditis: A Literature Review of Pediatric Cases. Cureus 2022, 14, e21726. [Google Scholar] [CrossRef]
- Molina, K.M.; Garcia, X.; Denfield, S.W.; Fan, Y.; Morrow, W.R.; Towbin, J.A.; Frazier, E.A.; Nelson, D.P. Parvovirus B19 Myocarditis Causes Significant Morbidity and Mortality in Children. Pediatr. Cardiol. 2013, 34, 390–397. [Google Scholar] [CrossRef]
- Liotti, F.M.; Marchetti, S.; D’Onghia, S.; Romano, L.; Ricci, R.; Sanguinetti, M.; Santangelo, R.; Posteraro, B. Compelling Increase in Parvovirus B19 Infections: Analysis of Molecular Diagnostic Trends (2019–2024). Viruses 2025, 17, 523. [Google Scholar] [CrossRef]
- Gigi, C.E.; Anumba, D.O.C. Parvovirus B19 Infection in Pregnancy–A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 358–362. [Google Scholar] [CrossRef]
- Hichijo, A.; Morine, M. A Case of Fetal Parvovirus B19 Myocarditis That Caused Terminal Heart Failure. Case Rep. Obstet. Gynecol. 2014, 2014, 463571. [Google Scholar] [CrossRef]
- Chaulin, A. Clinical and Diagnostic Value of Highly Sensitive Cardiac Troponins in Arterial Hypertension. Vasc. Health Risk Manag. 2021, 17, 431–443. [Google Scholar] [CrossRef]
- Ricchiuti, V.; Apple, F.S. RNA Expression of Cardiac Troponin T Isoforms in Diseased Human Skeletal Muscle. Clin. Chem. 1999, 45, 2129–2135. [Google Scholar] [CrossRef]
- Hickman, P.E.; Potter, J.M.; Aroney, C.; Koerbin, G.; Southcott, E.; Wu, A.H.B.; Roberts, M.S. Cardiac Troponin May Be Released by Ischemia Alone, without Necrosis. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Nadimpalli, S.S.; Miller, R.S.; Kamath, V.M.; Farkouh, C.R.; Nhan-Chang, C.-L.; Rathe, J.A.; Collins, A.; Duchon, J.M.; Neu, N.; Simpson, L.L.; et al. Congenital Parvovirus B19 Infection: Persistent Viremia and Red Blood Cell Aplasia. Open Forum Infect. Dis. 2015, 2, ofv049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betta, P.; Leonardi, R.; Mattia, C.; Saporito, A.; Gentile, S.; Trovato, L.; Palermo, C.I.; Scalia, G. Congenital Parvovirus B19 During the 2024 European Resurgence: A Prospective Single-Centre Cohort Study. Pathogens 2025, 14, 798. https://doi.org/10.3390/pathogens14080798
Betta P, Leonardi R, Mattia C, Saporito A, Gentile S, Trovato L, Palermo CI, Scalia G. Congenital Parvovirus B19 During the 2024 European Resurgence: A Prospective Single-Centre Cohort Study. Pathogens. 2025; 14(8):798. https://doi.org/10.3390/pathogens14080798
Chicago/Turabian StyleBetta, Pasqua, Roberta Leonardi, Carmine Mattia, Alessandro Saporito, Silvia Gentile, Laura Trovato, Concetta Ilenia Palermo, and Guido Scalia. 2025. "Congenital Parvovirus B19 During the 2024 European Resurgence: A Prospective Single-Centre Cohort Study" Pathogens 14, no. 8: 798. https://doi.org/10.3390/pathogens14080798
APA StyleBetta, P., Leonardi, R., Mattia, C., Saporito, A., Gentile, S., Trovato, L., Palermo, C. I., & Scalia, G. (2025). Congenital Parvovirus B19 During the 2024 European Resurgence: A Prospective Single-Centre Cohort Study. Pathogens, 14(8), 798. https://doi.org/10.3390/pathogens14080798






