Point-of-Care Diagnostic Testing for Emerging and Existing Poultry Viral Respiratory Pathogens Using Loop-Mediated Isothermal Amplification
Abstract
1. Introduction
2. The Genesis of Loop-Mediated Isothermal Amplification
3. The Basic Principle and Primers Used in the LAMP Assay
4. Advantages of the LAMP Assay
5. Challenges with Developing LAMP Assays
6. Detection of Poultry Viral Respiratory Pathogens Using LAMP
6.1. Iltovirus gallidalpha1 (Formerly Called Infectious Laryngotracheitis Virus)
6.2. Infectious Bronchitis Virus
6.3. Avian Metapneumovirus
6.4. Newcastle Disease Virus
6.5. Avian Influenza
| Feature | PCR/RT-PCR | LAMP/RT-LAMP | References |
|---|---|---|---|
| Amplification | Thermal cycling amplification of RNA or DNA | Isothermal amplification of RNA or DNA | [30,87] |
| Sensitivity | High | High and comparable to PCR | [30] |
| Specificity | High with proper primer design | High when primers are properly optimized | [88] |
| Time | 2–3 h | 30–60 min | [88,89] |
| Prone to presence of irrelevant DNA | More prone | Less prone | [30] |
| Equipment and cost | High cost, as it requires a thermal cycler and a regular supply of reagents | Low cost, as it requires minimal equipment | [90] |
| Method of detection | Gel electrophoresis or fluorescence | Color change, turbidity or fluorescence | [3,32,88,91] |
| Suitability for field conditions | Typically used in a laboratory setting | Suitable for field conditions or on-site diagnostic facilities | [89] |
7. LAMP/RT-LAMP Validation and Testing of Clinical Samples for Poultry Respiratory Pathogens
8. LAMP/RT-LAMP Assay Combined with Other Assays for Diagnosis of Some Poultry Respiratory Viruses
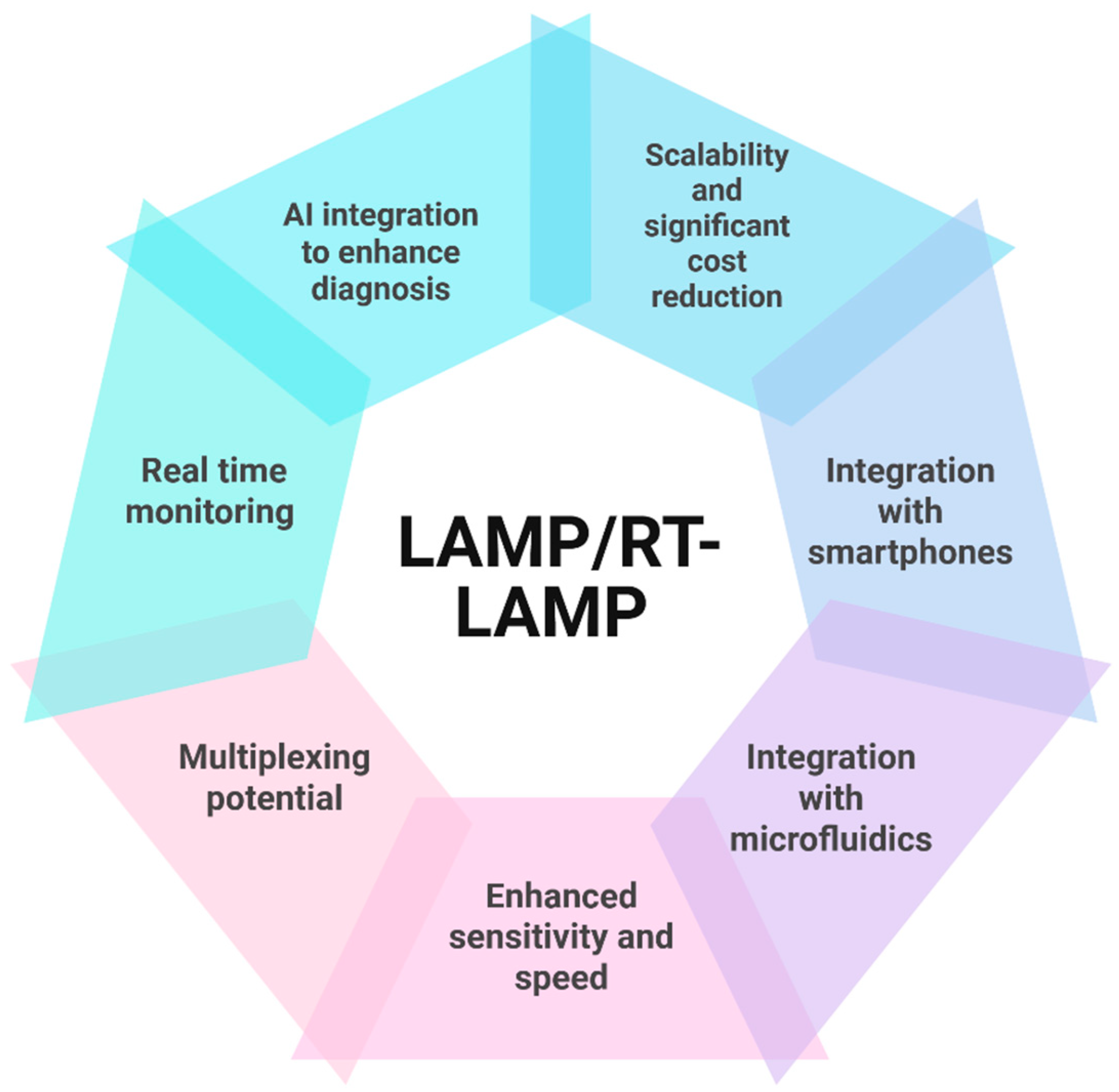
9. The Future of LAMP Assays
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yehia, N.; Salem, H.M.; Mahmmod, Y.; Said, D.; Samir, M.; Mawgod, S.A.; Sorour, H.K.; AbdelRahman, M.A.; Selim, S.; Saad, A.M. Common viral and bacterial avian respiratory infections: An updated review. Poult. Sci. 2023, 102, 102553. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.M.J.; Jeggo, M.H. Veterinary diagnostic laboratories in developing countries: The challenge of credibility. Rev. Sci. Tech. Off. Int. Epiz. 1998, 17, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Cea-Callejo, P.; Arca-Lafuente, S.; Gomez-Lucia, E.; Doménech, A.; Biarnés, M.; Blanco, A.; Benítez, L.; Madrid, R. An affordable detection system based on RT-LAMP and DNA-nanoprobes for avian metapneumovirus. Appl. Microbiol. Biotechnol. 2024, 108, 414. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Bau, H.H. Molecular Detection of Respiratory Tract Viruses in Chickens at the Point of Need by Loop-Mediated Isothermal Amplification (LAMP). Viruses 2024, 16, 1248. [Google Scholar] [CrossRef]
- Branda, F.; Ciccozzi, M.; Scarpa, F. Tracking the spread of avian influenza A (H5N1) with alternative surveillance methods: The example of wastewater data. Lancet Infect. Dis. 2024, 24, e604–e605. [Google Scholar] [CrossRef]
- Amoia, C.F.; Hakizimana, J.N.; Chengula, A.A.; Munir, M.; Misinzo, G.; Weger-Lucarelli, J. Genomic Diversity and Geographic Distribution of Newcastle Disease Virus Genotypes in Africa: Implications for Diagnosis, Vaccination, and Regional Collaboration. Viruses 2024, 16, 795. [Google Scholar] [CrossRef]
- Rabiei, M.; Low, W.Y.; Ren, Y.; Cahyono, M.I.; Doan, P.T.K.; Dharmayanti, I.; Grande, E.D.; Hemmatzadeh, F. Indicators of the molecular pathogenesis of virulent Newcastle disease virus in chickens revealed by transcriptomic profiling of spleen. Sci. Rep. 2021, 11, 17570. [Google Scholar] [CrossRef]
- Perozo, F.; Merino, R.; Afonso, C.; Villegas, P.; Calderon, N. Biological and phylogenetic characterization of virulent Newcastle disease virus circulating in Mexico. Avian Dis. 2008, 52, 472–479. [Google Scholar] [CrossRef]
- Brown, V.R.; Bevins, S.N. A review of virulent Newcastle disease viruses in the United States and the role of wild birds in viral persistence and spread. Vet. Res. 2017, 48, 68. [Google Scholar] [CrossRef]
- Giacinti, J.A.; Signore, A.V.; Jones, M.E.; Bourque, L.; Lair, S.; Jardine, C.; Stevens, B.; Bollinger, T.; Goldsmith, D.; British Columbia Wildlife AIV Surveillance Program (BC WASP). Avian influenza viruses in wild birds in Canada following incursions of highly pathogenic H5N1 virus from Eurasia in 2021–2022. mBio 2024, 15, e03203–e03223. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Legnardi, M.; Mescolini, G.; Tucciarone, C.M.; Lupini, C.; Quaglia, G.; Catelli, E.; Cecchinato, M. Avian Metapneumovirus subtype B around Europe: A phylodynamic reconstruction. Vet. Res. 2020, 51, 88. [Google Scholar] [CrossRef] [PubMed]
- Goraichuk, I.V.; Torchetti, M.K.; Killian, M.L.; Kapczynski, D.R.; Sary, K.; Kulkarni, A.; Suarez, D.L. Introduction of Avian metapneumovirus subtype A to the United States: Molecular insights and implications. Front. Microbiol. 2024, 15, 1428248. [Google Scholar] [CrossRef] [PubMed]
- Ketan Ganar, K.G.; Moushumee Das, M.D.; Sugandha Sinha, S.S.; Sachin Kumar, S.K. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef]
- Peacock, T.P.; James, J.; Sealy, J.E.; Iqbal, M. A global perspective on H9N2 avian influenza virus. Viruses 2019, 11, 620. [Google Scholar] [CrossRef]
- Abozeid, H.H. Global Emergence of Infectious Bronchitis Virus Variants: Evolution, Immunity, and Vaccination Challenges. Transbound. Emerg. Dis. 2023, 2023, 1144924. [Google Scholar] [CrossRef]
- Kariithi, H.M.; Christy, N.; Decanini, E.L.; Lemiere, S.; Volkening, J.D.; Afonso, C.L.; Suarez, D.L. Detection and genome sequence analysis of avian metapneumovirus subtype A viruses circulating in commercial chicken flocks in Mexico. Vet. Sci. 2022, 9, 579. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Bai, H.; Mauk, M.G.; Anis, E.; Bau, H.H. Molecular detection of infectious Laryngotracheitis virus in chickens with a microfluidic chip. Animals 2021, 11, 3203. [Google Scholar] [CrossRef]
- Padzil, F.; Mariatulqabtiah, A.R.; Tan, W.S.; Ho, K.L.; Isa, N.M.; Lau, H.Y.; Abu, J.; Chuang, K.-P. Loop-mediated isothermal amplification (LAMP) as a promising point-of-care diagnostic strategy in avian virus research. Animals 2021, 12, 76. [Google Scholar] [CrossRef]
- Atceken, N.; Munzer Alseed, M.; Dabbagh, S.R.; Yetisen, A.K.; Tasoglu, S. Point-of-Care diagnostic platforms for loop-mediated isothermal amplification. Adv. Eng. Mater. 2023, 25, 2201174. [Google Scholar] [CrossRef]
- Becherer, L.; Borst, N.; Bakheit, M.; Frischmann, S.; Zengerle, R.; von Stetten, F. Loop-mediated isothermal amplification (LAMP)—Review and classification of methods for sequence-specific detection. Anal. Methods 2020, 12, 717–746. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Bai, Y.; Zhang, P.; Yao, S.; Liu, J.; Zhang, T. Establishment of reverse transcription recombinase–aided amplification-lateral-flow dipstick and real-time fluorescence–based reverse transcription recombinase–aided amplification methods for detection of the Newcastle disease virus in chickens. Poult. Sci. 2020, 99, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Zelník, V. Use of nucleic acids amplification methods in Marek’s disease diagnosis and pathogenesis studies. Acta Virol. 2021, 65, 27–32. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Leonardi, A.A.; Calabrese, G.; Luca, G.D.; Coniglio, M.A.; Irrera, A.; Conoci, S. Nucleic acids analytical methods for viral infection diagnosis: State-of-the-art and future perspectives. Biomolecules 2021, 11, 1585. [Google Scholar] [CrossRef]
- Saiki, R.K.; Gelfand, D.H.; Stoffel, S.; Scharf, S.J.; Higuchi, R.; Horn, G.T.; Mullis, K.B.; Erlich, H.A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988, 239, 487–491. [Google Scholar] [CrossRef]
- Li, Q.; Xue, C.; Qin, J.; Zhou, Q.; Chen, F.; Bi, Y.; Cao, Y. An improved reverse transcription loop-mediated isothermal amplification assay for sensitive and specific detection of Newcastle disease virus. Arch. Virol. 2009, 154, 1433–1440. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Nested polymerase chain reaction (PCR). Cold Spring Harb. Protoc. 2019, 2019. [Google Scholar] [CrossRef]
- Wong, G.; Wong, I.; Chan, K.; Hsieh, Y.; Wong, S. A rapid and low-cost PCR thermal cycler for low resource settings. PLoS ONE 2015, 10, e0131701. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-C.; Wang, F.-X.; Zhang, S.-Q.; Song, N.; Li, J.-X.; Yang, Z.-Q.; Wen, Y.-J.; Wu, H. Comparative evaluation of conventional polymerase chain reaction (PCR), with loop-mediated isothermal amplification and SYBR green I-based real-time PCR for the quantitation of porcine circovirus-1 DNA in contaminated samples destined for vaccine production. J. Virol. Methods 2013, 191, 1–8. [Google Scholar] [PubMed]
- Ushikubo, H. Principle of LAMP method—A simple and rapid gene amplification method. Uirusu 2004, 54, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-mediated isothermal amplification (LAMP): The better sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef]
- Parida, M.; Sannarangaiah, S.; Dash, P.K.; Rao, P.; Morita, K. Loop mediated isothermal amplification (LAMP): A new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 2008, 18, 407–421. [Google Scholar] [CrossRef]
- Asadi, R.; Mollasalehi, H. The mechanism and improvements to the isothermal amplification of nucleic acids, at a glance. Anal. Biochem. 2021, 631, 114260. [Google Scholar] [CrossRef]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, B.; Wang, M.; Ou, Y.; Wang, J.; Li, S. Enhancement of Polymerase Activity of the Large Fragment in DNA Polymerase I from Geobacillus stearothermophilus by Site-Directed Mutagenesis at the Active Site. BioMed Res. Int. 2016, 2016, 2906484. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Y.; Li, S.; Zhuo, C.; Xu, S.; Huang, L.; Yang, L.; Liao, K. Real-time fluorescence loop mediated isothermal amplification for the detection of Acinetobacter baumannii. PLoS ONE 2013, 8, e66406. [Google Scholar] [CrossRef]
- Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP): Recent progress in research and development. J. Infect. Chemother. 2013, 19, 404–411. [Google Scholar] [CrossRef]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.A.; Zhang, Y.; Evans, T.C., Jr. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. Biotechniques 2015, 58, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.L.; Wong, B.W.; Ma, E.H.; Chan, K.H.; Chow, L.M.; Abeyewickreme, W.; Tangpukdee, N.; Yuen, K.Y.; Guan, Y.; Looareesuwan, S. Sensitive and inexpensive molecular test for falciparum malaria: Detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin. Chem. 2006, 52, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, S.; Cheung, C.Y.; Barr, I.; Chan, K.H.; Chen, H.; Guan, Y.; Peiris, J.M.; Poon, L.L. Loop-mediated isothermal amplification for influenza A (H5N1) virus. Emerg. Infect. Dis. 2007, 13, 899–901. [Google Scholar] [CrossRef]
- Chaouch, M. Loop-mediated isothermal amplification (LAMP): An effective molecular point-of-care technique for the rapid diagnosis of coronavirus SARS-CoV-2. Rev. Med. Virol. 2021, 31, e2215. [Google Scholar] [CrossRef]
- Ehtisham-Ul-Haque, S.; Zaman, M.; Kiran, M.; Rafique, M.; Qamar, M.; Younus, M. Current loop-mediated isothermal amplification (LAMP) technologies for the detection of poultry pathogens. World’s Poult. Sci. J. 2018, 74, 287–300. [Google Scholar] [CrossRef]
- Németh, M.Z.; Kovács, G.M. A comprehensive guide to loop-mediated isothermal amplification, an emerging diagnostic tool for plant pathogenic fungi. Front. Plant Sci. 2025, 16, 1568657. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Bustin, S.A.; Mueller, R.; Nolan, T. Parameters for successful PCR primer design. Quant. Real-Time PCR Methods Protoc. 2020, 2065, 5–22. [Google Scholar]
- Moehling, T.J.; Choi, G.; Dugan, L.C.; Salit, M.; Meagher, R.J. LAMP diagnostics at the point-of-care: Emerging trends and perspectives for the developer community. Expert Rev. Mol. Diagn. 2021, 21, 43–61. [Google Scholar] [CrossRef]
- Wang, D.-G.; Brewster, J.D.; Paul, M.; Tomasula, P.M. Two methods for increased specificity and sensitivity in loop-mediated isothermal amplification. Molecules 2015, 20, 6048–6059. [Google Scholar] [CrossRef] [PubMed]
- Meagher, R.J.; Priye, A.; Light, Y.K.; Huang, C.; Wang, E. Impact of primer dimers and self-amplifying hairpins on reverse transcription loop-mediated isothermal amplification detection of viral RNA. Analyst 2018, 143, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Crego-Vicente, B.; del Olmo, M.D.; Muro, A.; Fernández-Soto, P. Multiplexing LAMP Assays: A Methodological Review and Diagnostic Application. Int. J. Mol. Sci. 2024, 25, 6374. [Google Scholar] [CrossRef] [PubMed]
- Zen, L.; Lai, M.Y.; Lau, Y.L. Elimination of contamination in loop-mediated isothermal amplification assay for detection of human malaria. Trop. Biomed. 2020, 37, 1124–1128. [Google Scholar]
- Liu, W.; Huang, S.; Liu, N.; Dong, D.; Yang, Z.; Tang, Y.; Ma, W.; He, X.; Ao, D.; Xu, Y. Establishment of an accurate and fast detection method using molecular beacons in loop-mediated isothermal amplification assay. Sci. Rep. 2017, 7, 40125. [Google Scholar] [CrossRef]
- Feng, W.; Newbigging, A.M.; Le, C.; Pang, B.; Peng, H.; Cao, Y.; Wu, J.; Abbas, G.; Song, J.; Wang, D.-B. Molecular diagnosis of COVID-19: Challenges and research needs. Anal. Chem. 2020, 92, 10196–10209. [Google Scholar] [CrossRef]
- Shirato, K.; Semba, S.; El-Kafrawy, S.A.; Hassan, A.M.; Tolah, A.M.; Takayama, I.; Kageyama, T.; Notomi, T.; Kamitani, W.; Matsuyama, S. Development of fluorescent reverse transcription loop-mediated isothermal amplification (RT-LAMP) using quenching probes for the detection of the Middle East respiratory syndrome coronavirus. J. Virol. Methods 2018, 258, 41–48. [Google Scholar] [CrossRef]
- Turbawaty, D.K.; Sudjadi, A.; Lismayanti, L.; Rostini, T.; Logito, V. The Performance of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Virus Using the Colorimetric Reverse-Transcription Loop Mediated Isothermal Amplification (RT-LAMP) Method in Saliva Specimens of Suspected COVID-19 Patients. Int. J. Gen. Med. 2024, 17, 3329–3335. [Google Scholar] [CrossRef]
- Parikh, B.A.; Anderson, N.W. Quantitative Viral Load Monitoring: How far are We from Commutable Results? Adv. Mol. Pathol. 2023, 6, 77–86. [Google Scholar] [CrossRef]
- ICTV. Genus: Iltovirus. 2025. Available online: https://ictv.global/report/chapter/herpesviridae/herpesviridae/iltovirus (accessed on 9 June 2025).
- Gowthaman, V.; Kumar, S.; Koul, M.; Dave, U.; Murthy, T.G.K.; Munuswamy, P.; Tiwari, R.; Karthik, K.; Dhama, K.; Michalak, I. Infectious laryngotracheitis: Etiology, epidemiology, pathobiology, and advances in diagnosis and control—A comprehensive review. Vet. Q. 2020, 40, 140–161. [Google Scholar] [CrossRef]
- García, M.; Spatz, S.; Guy, J.S. Infectious laryngotracheitis. In Diseases of Poultry; Wiley: Hoboken, NJ, USA, 2013; pp. 161–179. [Google Scholar]
- Xie, Q.-m.; Ji, J.; Pickens, T.T.; Du, L.-q.; Cao, Y.-c.; Li, H.-m.; Wang, L.-g.; Ma, J.-y.; Bi, Y.-z. Rapid detection of infectious laryngotracheitis virus isolates by loop-mediated isothermal amplification. J. Virol. Methods 2010, 165, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-C.; Giambrone, J.J.; Macklin, K.S. Comparison of a TaqMan real-time polymerase chain reaction assay with a loop-mediated isothermal amplification assay for detection of Gallid herpesvirus 1. J. Vet. Diagn. Investig. 2012, 24, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, J.; Cao, Y.; Kang, R.; Lin, Y.; Wei, Y.; Liao, D.; Li, X.; Ye, Y.; Pan, M. A modified loop-mediated isothermal amplification method for detecting avian infectious laryngotracheitis virus. Anim. Biotechnol. 2021, 32, 766–773. [Google Scholar] [CrossRef]
- ICTV. 2025. Available online: https://ictv.global/report/chapter/coronaviridae/coronaviridae/gammacoronavirus (accessed on 9 June 2025).
- Falchieri, M.; Coward, V.J.; Reid, S.M.; Lewis, T.; Banyard, A.C. Infectious bronchitis virus: An overview of the “chicken coronavirus”. J. Med. Microbiol. 2024, 73, 001828. [Google Scholar] [CrossRef]
- Ennaji, Y.; Khataby, K.; Ennaji, M.M. Infectious bronchitis virus in poultry: Molecular epidemiology and factors leading to the emergence and reemergence of novel strains of infectious bronchitis virus. In Emerging and Reemerging Viral Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–44. [Google Scholar]
- Legnardi, M.; Tucciarone, C.M.; Franzo, G.; Cecchinato, M. Infectious bronchitis virus evolution, diagnosis and control. Vet. Sci. 2020, 7, 79. [Google Scholar] [CrossRef]
- Chen, H.-t.; Zhang, J.; Ma, Y.-p.; Ma, L.-n.; Ding, Y.-z.; Liu, X.-t.; Cai, X.-p.; Ma, L.-q.; Zhang, Y.-g.; Liu, Y.-s. Reverse transcription loop-mediated isothermal amplification for the rapid detection of infectious bronchitis virus in infected chicken tissues. Mol. Cell. Probes 2010, 24, 104–106. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Raja, A.; Dhinakar Raj, G.; Thangavelu, A.; Kumanan, K. Rapid detection of avian infectious bronchitis virus by reverse transcriptase-loop mediated isothermal amplification. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 815–820. [Google Scholar] [CrossRef]
- Wu, X.; Song, Z.; Zhai, X.; Zuo, L.; Mei, X.; Xiang, R.; Kang, Z.; Zhou, L.; Wang, H. Simultaneous and visual detection of infectious bronchitis virus and Newcastle disease virus by multiple LAMP and lateral flow dipstick. Poult. Sci. 2019, 98, 5401–5411. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Mauk, M.G.; Anis, E.; Bau, H.H. A closed-tube, single-step, real time, reverse transcription-loop-mediated isothermal amplification assay for infectious bronchitis virus detection in chickens. J. Virol. Methods 2020, 284, 113940. [Google Scholar] [CrossRef]
- Kaboudi, K.; Lachheb, J. Avian metapneumovirus infection in turkeys: A review on turkey rhinotracheitis. J. Appl. Poult. Res. 2021, 30, 100211. [Google Scholar] [CrossRef]
- ICTV. 2025. Available online: https://ictv.global/report/chapter/pneumoviridae/pneumoviridae/metapneumovirus (accessed on 9 June 2025).
- Mao, Q.; Ma, S.; Schrickel, P.L.; Zhao, P.; Wang, J.; Zhang, Y.; Li, S.; Wang, C. Review detection of Newcastle disease virus. Front. Vet. Sci. 2022, 9, 936251. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.M.; Nakajima, C.; Ohashi, K.; Onuma, M. Loop-mediated isothermal amplification for rapid detection of Newcastle disease virus. J. Clin. Microbiol. 2005, 43, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Ma, J.; Zardán Gómez de la Torre, T.; Bálint, A.d.m.; Donolato, M.; Hansen, M.F.; Svedlindh, P.; Strömberg, M. Rapid newcastle disease virus detection based on Loop-mediated isothermal amplification and optomagnetic readout. Acs Sens. 2016, 1, 1228–1234. [Google Scholar] [CrossRef]
- Simancas-Racines, A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Zambrano, A.K.; Simancas-Racines, D. Avian influenza: Strategies to manage an outbreak. Pathogens 2023, 12, 610. [Google Scholar] [CrossRef]
- AbuBakar, U.; Amrani, L.; Kamarulzaman, F.A.; Karsani, S.A.; Hassandarvish, P.; Khairat, J.E. Avian influenza virus tropism in humans. Viruses 2023, 15, 833. [Google Scholar] [CrossRef]
- Shivakoti, S.; Ito, H.; Murase, T.; Ono, E.; Takakuwa, H.; Yamashiro, T.; Otsuki, K.; Ito, T. Development of reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay for detection of avian influenza viruses in field specimens. J. Vet. Med. Sci. 2010, 72, 519–523. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, Z.; Liu, J.; Pang, Y.; Deng, X.; Xie, Z.; Xie, L.; Fan, Q.; Feng, J.; Khan, M.I. Visual detection of H3 subtype avian influenza viruses by reverse transcription loop-mediated isothermal amplification assay. Virol. J. 2011, 8, 337. [Google Scholar] [CrossRef]
- Bao, H.; Zhao, Y.; Wang, Y.; Xu, X.; Shi, J.; Zeng, X.; Wang, X.; Chen, H. Development of a reverse transcription loop-mediated isothermal amplification method for the rapid detection of subtype H7N9 avian influenza virus. BioMed Res. Int. 2014, 2014, 525064. [Google Scholar] [CrossRef]
- Nakauchi, M.; Takayama, I.; Takahashi, H.; Tashiro, M.; Kageyama, T. Development of a reverse transcription loop-mediated isothermal amplification assay for the rapid diagnosis of avian influenza A (H7N9) virus infection. J. Virol. Methods 2014, 204, 101–104. [Google Scholar] [CrossRef]
- Park, Y.-R.; Kim, E.-M.; Han, D.-H.; Kang, D.-Y.; Yeo, S.-G.; Park, C.-K. Reverse transcription loop-mediated isothermal amplification assay for the rapid and simultaneous detection of H5 and other subtypes of avian influenza viruses. Korean J. Vet. Serv. 2017, 40, 15–20. [Google Scholar]
- Jang, W.S.; Lim, D.H.; Nam, J.; Mihn, D.-C.; Sung, H.W.; Lim, C.S.; Kim, J. Development of a multiplex isothermal amplification molecular diagnosis method for on-site diagnosis of influenza. PLoS ONE 2020, 15, e0238615. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.S.; Devi, N.A.; Gan, K.B.; Then, S.-M. Design and development of polymerase chain reaction thermal cycler using proportional-integral temperature controller. Malays. J. Fundam. Appl. Sci. 2018, 14, 213–218. [Google Scholar]
- Kursa, O.; Woźniakowski, G.; Tomczyk, G.; Sawicka, A.; Minta, Z. Rapid detection of Mycoplasma synoviae by loop-mediated isothermal amplification. Arch. Microbiol. 2015, 197, 319–325. [Google Scholar] [CrossRef]
- Lin, S.; Yu, X.-W.; Wei, Y.; Yu, B.-L.; He, L.-K.; Yuan, G.; Zhang, Y.-X.; Tian, G.-B.; Ping, J.-H.; Wang, X.-R. Development of a reverse-transcription loop-mediated isothermal amplification assay to detect avian influenza viruses in clinical specimens. J. Integr. Agric. 2019, 18, 1428–1435. [Google Scholar]
- Rawnuck, T.; Reza, M.S.; Khan, M.J.R.; Khanam, R.A.; Munshi, S.U. A Comparative Study of LAMP and PCR in Relation to Time and Cost. J. Shaheed Suhrawardy Med. Coll. 2020, 12, 72–75. [Google Scholar] [CrossRef]
- Song, H.-S.; Kim, H.-S.; Kim, J.-Y.; Kwon, Y.-K.; Kim, H.-R. The Development of Novel Reverse Transcription Loop-Mediated Isothermal Amplification Assays for the Detection and Differentiation of Virulent Newcastle Disease Virus. Int. J. Mol. Sci. 2023, 24, 13847. [Google Scholar] [CrossRef]
- Luo, S.; Xie, Z.; Xie, L.; Liu, J.; Xie, Z.; Deng, X.; Huang, L.; Huang, J.; Zeng, T.; Khan, M.I. Reverse-transcription, loop-mediated isothermal amplification assay for the sensitive and rapid detection of H10 subtype avian influenza viruses. Virol. J. 2015, 12, 145. [Google Scholar] [CrossRef]
- Chen, H.-t.; Zhang, J.; Sun, D.-h.; Ma, L.-n.; Liu, X.-t.; Cai, X.-p.; Liu, Y.-s. Development of reverse transcription loop-mediated isothermal amplification for rapid detection of H9 avian influenza virus. J. Virol. Methods 2008, 151, 200–203. [Google Scholar] [CrossRef]
- Jang, W.S.; Lee, J.M.; Lee, E.; Park, S.; Lim, C.S. Loop-Mediated Isothermal Amplification and Lateral Flow Immunochromatography Technology for Rapid Diagnosis of Influenza A/B. Diagnostics 2024, 14, 967. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, T.; Liu, L.; Chen, S.; Li, J.; Zhang, Y.; Chen, J.; Yang, C.; Huang, J.; Huang, T. Rapid field visual detection of avian metapneumovirus by integrating MIRA pre-amplification with CRISPR-Cas13a to enhance sensitivity and specificity: Innovative technologies well-suited for real-time large-scale epidemiological surveillance. Int. J. Biol. Macromol. 2025, 318, 144966. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enyetornye, B.; Yondo, A.; Velayudhan, B.T. Point-of-Care Diagnostic Testing for Emerging and Existing Poultry Viral Respiratory Pathogens Using Loop-Mediated Isothermal Amplification. Pathogens 2025, 14, 657. https://doi.org/10.3390/pathogens14070657
Enyetornye B, Yondo A, Velayudhan BT. Point-of-Care Diagnostic Testing for Emerging and Existing Poultry Viral Respiratory Pathogens Using Loop-Mediated Isothermal Amplification. Pathogens. 2025; 14(7):657. https://doi.org/10.3390/pathogens14070657
Chicago/Turabian StyleEnyetornye, Ben, Aurelle Yondo, and Binu T. Velayudhan. 2025. "Point-of-Care Diagnostic Testing for Emerging and Existing Poultry Viral Respiratory Pathogens Using Loop-Mediated Isothermal Amplification" Pathogens 14, no. 7: 657. https://doi.org/10.3390/pathogens14070657
APA StyleEnyetornye, B., Yondo, A., & Velayudhan, B. T. (2025). Point-of-Care Diagnostic Testing for Emerging and Existing Poultry Viral Respiratory Pathogens Using Loop-Mediated Isothermal Amplification. Pathogens, 14(7), 657. https://doi.org/10.3390/pathogens14070657







