Clinical Features of Pulmonary Nocardiosis and Diagnostic Value of Metagenomic Next-Generation Sequencing: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical and Sample Collection
2.3. Conventional Microbiological Tests
2.4. Metagenomic Next-Generation Sequencing and Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
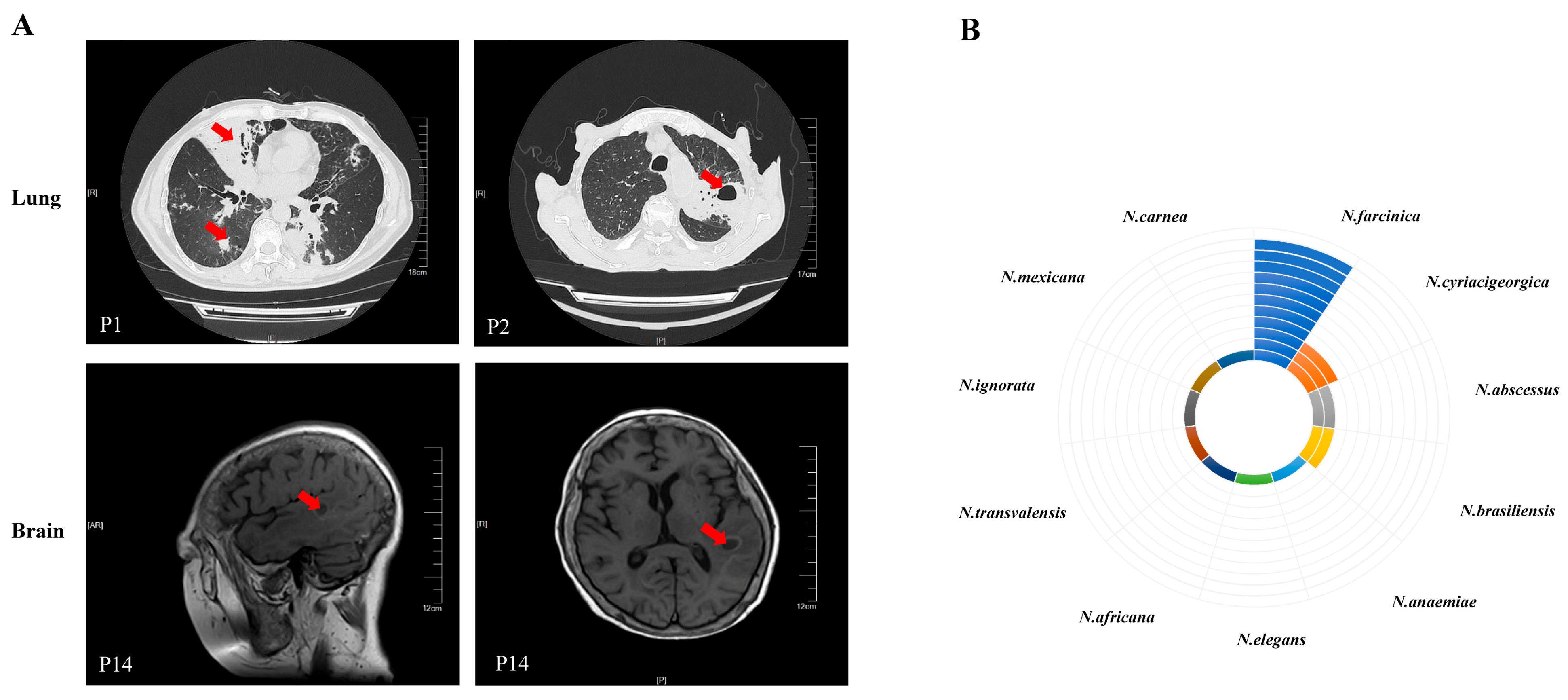
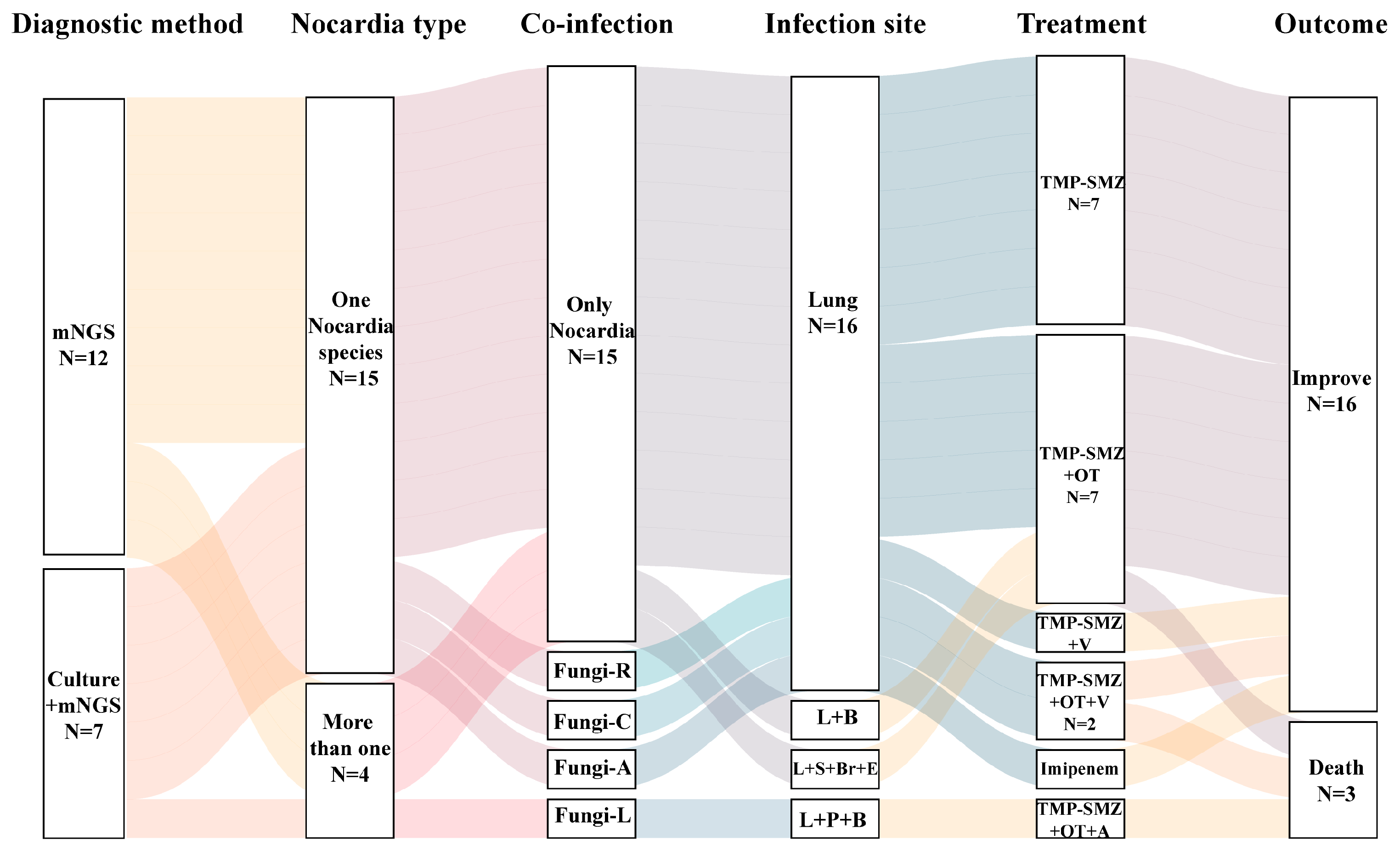
3.2. Microbiological Findings
3.3. Treatment and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, R.; Reyes, S.; Menéndez, R. Pulmonary nocardiosis: Risk factors, clinical features, diagnosis and prognosis. Curr. Opin. Pulm. Med. 2008, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Fatahi-Bafghi, M. Nocardiosis from 1888 to 2017. Microb. Pathog. 2018, 114, 369–384. [Google Scholar] [CrossRef]
- Wilson, J.W. Nocardiosis: Updates and clinical overview. Mayo Clin. Proc. 2012, 87, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Minero, M.; Marín, M.; Cercenado, E.; Rabadán, P.; Bouza, E.; Muñoz, P. Nocardiosis at the turn of the century. Medicine 2009, 88, 250–261. [Google Scholar] [CrossRef]
- Brown-Elliott, B.; Brown, J.; Conville, P.; Wallace, R. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin. Microbiol. Rev. 2006, 19, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ma, B.; Wei, X.; Li, Y. Detection of nocardia by 16S Ribosomal RNA Gene PCR and Metagenomic Next-Generation Sequencing (mNGS). Front. Cell. Infect. Microbiol. 2021, 11, 768613. [Google Scholar] [CrossRef]
- Miao, Q.; Ma, Y.; Wang, Q.; Pan, J.; Zhang, Y.; Jin, W.; Yao, Y.; Su, Y.; Huang, Y.; Wang, M.; et al. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, S231–S240. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, M.; Qiu, L.; Chen, M.; Tan, C.; Tu, C.; Zheng, X.; Liu, J. Clinical characteristics of hospitalized patients with Nocardia genus detection by metagenomic next generation sequencing in a tertiary hospital from southern China. BMC Infect. Dis. 2023, 23, 772. [Google Scholar] [CrossRef]
- Xu, Y.; Lian, Q.Y.; Chen, A.; Zhang, J.H.; Xu, X.; Huang, D.X.; He, J.X.; Ju, C.R. Clinical characteristics and treatment strategy of nocardiosis in lung transplant recipients: A single-center experience. IDCases 2023, 32, e01758. [Google Scholar] [CrossRef]
- Gao, L.; Yang, T.; Zhang, X.; Lei, W.; Huang, J.A. Rapid detection of pulmonary nocardiosis by metagenomic next generation sequencing. Diagn. Microbiol. Infect. Dis. 2023, 106, 115928. [Google Scholar] [CrossRef]
- Jiao, M.; Ma, X.; Li, Y.; Wang, H.; Liu, Y.; Guo, W.; Lv, J. Metagenomic next-generation sequencing provides prognostic warning by identifying mixed infections in nocardiosis. Front. Cell. Infect. Microbiol. 2022, 12, 894678. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yuan, M.; Shi, Y.; Su, X. Clinical Performance of BAL Metagenomic Next-Generation Sequence and Serum (1,3)-β-D-Glucan for Differential Diagnosis of Pneumocystis jirovecii Pneumonia and Pneumocystis jirovecii Colonisation. Front. Cell. Infect. Microbiol. 2021, 11, 784236. [Google Scholar] [CrossRef]
- Pendleton, K.M.; Huffnagle, G.B.; Dickson, R.P. The significance of Candida in the human respiratory tract: Our evolving understanding. Pathog. Dis. 2017, 75, ftx029. [Google Scholar] [CrossRef] [PubMed]
- Margalit, I.; Muhsen, K.; Ben Ari, Y.; Ben-Zvi, H.; Shostak, Y.; Krause, I.; Goldberg, E. Nocardia colonization in contrast to nocardiosis: A comparison of patients’ clinical characteristics. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2020, 39, 759–763. [Google Scholar] [CrossRef]
- Dumitrascu, A.G.; Rojas, C.A.; Stancampiano, F.; Johnson, E.M.; Harris, D.M.; Chirila, R.M.; Omer, M.; Hata, D.J.; Meza-Villegas, D.M.; Heckman, M.G.; et al. Invasive Nocardiosis Versus Colonization at a Tertiary Care Center: Clinical and Radiological Characteristics. Mayo Clin. Proc. Innov. Qual. Outcomes 2023, 7, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Sousa, C.; Redondo, M.; Carvalho, T.; Ribeiro, M.; Ramos, A.; Cruz-Martins, N.; Amorim, A. Nocardia spp. isolation in chronic lung diseases: Are there differences between patients with pulmonary nocardiosis and Nocardia colonization? J. Appl. Microbiol. 2022, 133, 3239–3249. [Google Scholar] [CrossRef]
- Lynch, J.; Reid, G.; Clark, N. Nocardia spp.: A Rare Cause of Pneumonia Globally. Semin. Respir. Crit. Care Med. 2020, 41, 538–554. [Google Scholar] [CrossRef]
- Lafont, E.; Conan, P.; Rodriguez-Nava, V.; Lebeaux, D. Invasive Nocardiosis: Disease Presentation, Diagnosis and Treatment—Old Questions, New Answers? Infect. Drug Resist. 2020, 13, 4601–4613. [Google Scholar] [CrossRef]
- Giménez-Arufe, V.; Gutiérrez-Urbón, J.M.; Blanco-Aparicio, M.; Míguez-Rey, E.; Martín-Herranz, M.I. Pulmonary nocardiosis treated with tedizolid. Farm. Hosp. 2019, 43, 208–210. [Google Scholar] [CrossRef]
- Steinbrink, J.; Leavens, J.; Kauffman, C.; Miceli, M. Manifestations and outcomes of nocardia infections: Comparison of immunocompromised and nonimmunocompromised adult patients. Medicine 2018, 97, e12436. [Google Scholar] [CrossRef]
- Restrepo, A.; Clark, N. Nocardia infections in solid organ transplantation: Guidelines from the Infectious Diseases Community of Practice of the American Society of Transplantation. Clin. Transplant. 2019, 33, e13509. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chu, P.; Guo, J.; Xie, Y.; Lu, J. Nontuberculous mycobacterial and Nocardia infections mimicking pulmonary tuberculosis: A retrospective study of a general hospital patient population in China. J. Med. Microbiol. 2020, 69, 1145–1150. [Google Scholar] [CrossRef]
- Jiao, M.; Deng, X.; Yang, H.; Dong, J.; Lv, J.; Li, F. Case Report: A Severe and Multi-Site Nocardia farcinica Infection Rapidly and Precisely Identified by Metagenomic Next-Generation Sequencing. Front. Med. 2021, 8, 669552. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhuang, S.; He, L.; Wang, S.; Zhao, M.; Lyu, X. Brain Abscess Caused by Nocardia brevicatena in an Immunocompetent Patient: A Case Report. Infect. Drug Resist. 2022, 15, 7693–7697. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Z.; Xia, H.; Li, Q.; Bai, H. A case report on mixed pulmonary infection of Nocardia nova, Mycobacterium tuberculosis, and Aspergillus fumigatus based on metagenomic next-generation sequencing. Front. Public Health 2022, 10, 927338. [Google Scholar] [CrossRef]
- Huang, T.; Chen, Y.; Zhang, J.; He, R.; Qu, D.; Ye, Q.; Chen, X. Rapid and accurate diagnosis of brain abscess caused by Nocardia asiatica with a combination of Ziehl-Neelsen staining and metagenomics next-generation sequencing. Eur. J. Neurol. 2021, 28, 355–357. [Google Scholar] [CrossRef]
- Forero Morales, M.P.; Hopprich, R.; Wise, R.; Shephard, L.; Weldhagen, G.F. Cost-effective implementation of a custom MALDI-TOF library for the identification of South Australian Nocardia isolates. Pathology 2018, 50, 753–757. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.Y.; Deng, J.; Zhuang, K.W.; Tang, Y.; Wu, N.; Zhang, W.L.; Liao, Q.F.; Xiao, Y.L.; Kang, M. Application of MALDI-TOF mass spectrometry for identification of Nocardia species. BMC Microbiol. 2024, 24, 358. [Google Scholar] [CrossRef]
- Weng, S.; Zhang, H.; Ai, J.; Gao, Y.; Liu, Y.; Xu, B.; Zhang, W. Rapid Detection of Nocardia by Next-Generation Sequencing. Front. Cell. Infect. Microbiol. 2020, 10, 13. [Google Scholar] [CrossRef]
- Mehta, H.; Shamoo, Y. Pathogenic Nocardia: A diverse genus of emerging pathogens or just poorly recognized? PLoS Pathog. 2020, 16, e1008280. [Google Scholar] [CrossRef]
- Martínez-Barricarte, R. Isolated Nocardiosis, an Unrecognized Primary Immunodeficiency? Front. Immunol. 2020, 11, 590239. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Cui, Q.; Wu, W.; Li, G.; Chen, D.; Xiang, L.; Qu, J.; Shi, D.; Lu, B. Epidemiology and Antimicrobial Resistance Profiles of the Nocardia Species in China, 2009 to 202. Microbiol. Spectr. 2022, 10, e0156021. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Q.; Yan, J.; Liao, X.; Long, S.; Zheng, M.; Zhang, Y.; Yang, X.; Shi, G.; Zhao, Y.; et al. The species distribution and antimicrobial resistance profiles of Nocardia species in China: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2023, 17, e0011432. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, J.; Yang, C.; Yang, L.; Chen, J.; Li, X.; Wang, Y.; Feng, J. Prevalence of Fungal and Bacterial Co-Infection in Pulmonary Fungal Infections: A Metagenomic Next Generation Sequencing-Based Study. Front. Cell. Infect. Microbiol. 2021, 11, 749905. [Google Scholar] [CrossRef] [PubMed]
- Onaiwu, C.; Velagapudi, M.; Sarsam, L.; Utley, L.; Bricker, L.; Bendi, V.; Vivekanandan, R. Rare Multidrug-Resistant Pulmonary Nocardiosis in AIDS. Cureus 2017, 9, e1839. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, R.; Wang, Y.; Zhou, Y.; Xu, S.; Duan, Y.; Lv, W.; Wang, S.; Hou, M.; Chen, Y.; et al. Economic impact of metagenomic next-generation sequencing versus traditional bacterial culture for postoperative central nervous system infections using a decision analysis mode: Study protocol for a randomized controlled trial. mSystems 2023, 8, e0058123. [Google Scholar] [CrossRef]
- Song, X.; Jiang, H.; Zong, L.; Shi, D.; Zhu, H. The clinical value of mNGS of bronchoalveolar lavage fluid versus traditional microbiological tests for pathogen identification and prognosis of severe pneumonia (NT-BALF): Study protocol for a prospective multi-center randomized clinical trial. Trials 2024, 25, 276. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, X.; Du, P.; Li, G.; Li, L.; Li, Z. Susceptibility profiles of Nocardia spp. to antimicrobial and antituberculotic agents detected by a microplate Alamar Blue assay. Sci. Rep. 2017, 7, 43660. [Google Scholar] [CrossRef]

| Characteristic | Mean ± SD, or N (%) |
|---|---|
| Age (years) | 58 ± 15 |
| Gender | |
| Male | 11 (57.9) |
| Female | 8 (42.1) |
| Underlying diseases | |
| Acute leukemia | 4 (21.1) |
| Multiple myeloma | 1 (5.3) |
| Bronchiectasis | 2 (10.5) |
| Chronic obstructive pulmonary disease (COPD) | 2 (10.5) |
| Antineutrophil cytoplasmic antibody-associated vasculitis | 2 (10.5) |
| Diabetes mellitus type 2 | 2 (10.5) |
| Systemic lupus erythematosus | 1 (5.3) |
| Dermatomyositis | 1 (5.3) |
| Membranous nephropathy | 1 (5.3) |
| Hemolytic anemia | 1 (5.3) |
| None | 2 (10.5) |
| Immunosuppressive therapy | 14 (73.7) |
| White blood cell (WBC), 109/L | 9.07 ± 7.59 |
| Lymphocyte count (LY), 109/L | 1.22 ± 1.78 |
| Serum albumin (ALB), g/L | 32.48 ± 9.15 |
| Clinical symptoms | 18 (94.7) |
| Cough | 11 (57.9) |
| Sputum | 8 (42.1) |
| Fever | 13 (68.4) |
| No symptom | 1 (5.3) |
| Isolated pulmonary nocardiosis | 16 (84.2) |
| Disseminated course, extrapulmonary foci | 3 (15.8) |
| Brain | 1 (5.3) |
| Skin | 1 (5.3) |
| Blood | 2 (10.5) |
| Pleura | 1 (5.3) |
| Eye | 1 (5.3) |
| Imaging features | |
| Exudative shadow | 11 (57.9) |
| Nodular shadow | 8 (42.1) |
| Consolidation shadow | 6 (31.6) |
| Cavity formation | 4 (21.1) |
| Pleural effusion | 7 (36.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Fu, H.; Zhu, Q.; Ren, Y.; Liu, J.; Wu, Y.; Xu, J. Clinical Features of Pulmonary Nocardiosis and Diagnostic Value of Metagenomic Next-Generation Sequencing: A Retrospective Study. Pathogens 2025, 14, 656. https://doi.org/10.3390/pathogens14070656
Chen Y, Fu H, Zhu Q, Ren Y, Liu J, Wu Y, Xu J. Clinical Features of Pulmonary Nocardiosis and Diagnostic Value of Metagenomic Next-Generation Sequencing: A Retrospective Study. Pathogens. 2025; 14(7):656. https://doi.org/10.3390/pathogens14070656
Chicago/Turabian StyleChen, Yanbin, Hailong Fu, Qiongfang Zhu, Yalu Ren, Jia Liu, Yining Wu, and Jie Xu. 2025. "Clinical Features of Pulmonary Nocardiosis and Diagnostic Value of Metagenomic Next-Generation Sequencing: A Retrospective Study" Pathogens 14, no. 7: 656. https://doi.org/10.3390/pathogens14070656
APA StyleChen, Y., Fu, H., Zhu, Q., Ren, Y., Liu, J., Wu, Y., & Xu, J. (2025). Clinical Features of Pulmonary Nocardiosis and Diagnostic Value of Metagenomic Next-Generation Sequencing: A Retrospective Study. Pathogens, 14(7), 656. https://doi.org/10.3390/pathogens14070656






