First Serologic Analysis of Antibodies Against African Swine Fever Virus Detected in Domestic Pig Farms in South Korea from 2019 to 2024
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cell Cultures
2.2. Sampling
2.3. Antibody Detection Using Serological Testing
2.3.1. ELISA
2.3.2. IPT Assay
2.4. Spatial Clustering Analysis
2.5. Statistical Analysis
3. Results
3.1. Proportion of ASFV-Seropositive Pigs on Pig Farms Determined by ELISA
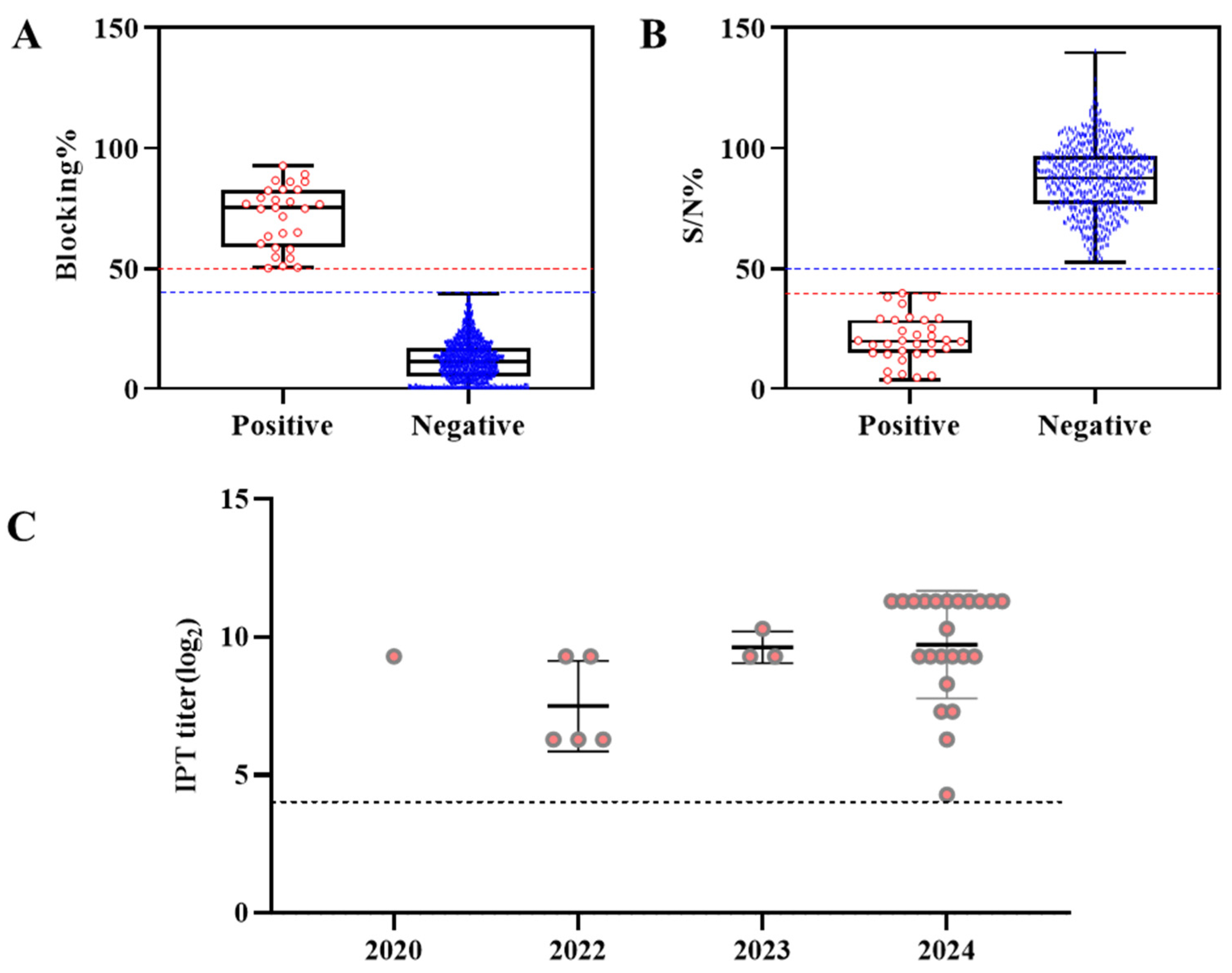
3.2. Comparison of the ELISA and IPT Results
3.3. Spatial Clustering Analysis with Seropositive Pigs on Pig Farms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASF | African Swine Fever |
| ASFV | African Swine Fever Virus |
| IPT | Immunoperoxidase Test |
| DBSCAN | Density-Based Spatial Clustering of Applications with Noise |
| WOAH | World Organisation for Animal Health |
| qPCR | Quantitative Real-Time PCR |
| PCR | Polymerase Chain Reaction |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| bELISA | Ingezim PPA COMPAC |
| cELISA | ID. Screen ASF Competition |
| R0 | The Basic Reproduction Number |
| Vero cell | African Green Monkey Kidney Cell |
| CISA-INIA | Centro de Investigación en Sanidad Animal—Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria |
| ATCC | American Type Culture Collection |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal Bovine Serum |
| MAFRA | Ministry of Agriculture, Food, and Rural Affairs |
| APQA | Animal and Plant Quarantine Agency |
| SOP | Standard Operating Procedure |
| BSL-3 | Biosafety Level 3 |
| x% | Blocking Percentage |
| S/N% | Competition Percentage |
| κ value | Hierarchical Kappa-Type Coefficient |
References
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wardley, R.C.; de M. Andrade, C.; Black, D.N.; de Castro Portugal, F.L.; Enjuanes, L.; Hess, W.R.; Mebus, C.; Ordas, A.; Rutili, D.; Sanchez Vizcaino, J.; et al. African Swine Fever virus. Brief Rev. Arch. Virol. 1983, 76, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef]
- Montgomery, R.E. On a Form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Sanchez-Vizcaino, J.M.; Mur, L.; Martinez-Lopez, B. African swine fever (ASF): Five years around Europe. Vet. Microbiol. 2013, 165, 45–50. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, K.H.; Lee, S.K.; Kim, D.Y.; Nah, J.J.; Kim, H.J.; Kim, H.J.; Hwang, J.Y.; Sohn, H.J.; Choi, J.G.; et al. Outbreak of African swine fever in South Korea, 2019. Transbound. Emerg. Dis. 2020, 67, 473–475. [Google Scholar] [CrossRef]
- Kim, G.; Park, J.E.; Kim, S.J.; Kim, Y.; Kim, W.; Kim, Y.K.; Jheong, W. Complete genome analysis of the African swine fever virus isolated from a wild boar responsible for the first viral outbreak in Korea, 2019. Front. Vet. Sci. 2022, 9, 1080397. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.; Son, K.; Choi, Y.; Jeong, H.S.; Kim, Y.K.; Park, J.E.; Hong, Y.J.; Lee, S.I.; Wang, S.J.; et al. Wild boar harbouring African swine fever virus in the demilitarized zone in South Korea, 2019. Emerg. Microbes Infect. 2020, 9, 628–630. [Google Scholar] [CrossRef]
- African Swine Fever (Infection with African Swine Fever Virus); World Organisation for Animal Health: Paris, France, 2024.
- Gallardo, C.; Fernandez-Pinero, J.; Arias, M. African swine fever (ASF) diagnosis, an essential tool in the epidemiological investigation. Virus Res. 2019, 271, 197676. [Google Scholar] [CrossRef]
- Sanchez-Vizcaino, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, C.; Gomez-Sebastian, S.; Moreno, N.; Nunez, M.C.; Mulumba-Mfumu, L.K.; Quembo, C.J.; Heath, L.; Etter, E.M.; Jori, F.; Escribano, J.M.; et al. African swine fever virus serodiagnosis: A general review with a focus on the analyses of African serum samples. Virus Res. 2013, 173, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Soler, A.; Nieto, R.; Carrascosa, A.L.; De Mia, G.M.; Bishop, R.P.; Martins, C.; Fasina, F.O.; Couacy-Hymman, E.; Heath, L.; et al. Comparative evaluation of novel African swine fever virus (ASF) antibody detection techniques derived from specific ASF viral genotypes with the OIE internationally prescribed serological tests. Vet. Microbiol. 2013, 162, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Reis, A.L.; Kalema-Zikusoka, G.; Malta, J.; Soler, A.; Blanco, E.; Parkhouse, R.M.; Leitao, A. Recombinant antigen targets for serodiagnosis of African swine fever. Clin. Vaccine Immunol. 2009, 16, 1012–1020. [Google Scholar] [CrossRef]
- Gallardo, C.; Nieto, R.; Soler, A.; Pelayo, V.; Fernandez-Pinero, J.; Markowska-Daniel, I.; Pridotkas, G.; Nurmoja, I.; Granta, R.; Simon, A.; et al. Assessment of African Swine Fever Diagnostic Techniques as a Response to the Epidemic Outbreaks in Eastern European Union Countries: How To Improve Surveillance and Control Programs. J. Clin. Microbiol. 2015, 53, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.C.; Reoyo, A.T.; Fernandez-Pinero, J.; Iglesias, I.; Munoz, M.J.; Arias, M.L. African swine fever: A global view of the current challenge. Porc. Health Manag. 2015, 1, 21. [Google Scholar] [CrossRef]
- Escribano, J.M.; Galindo, I.; Alonso, C. Antibody-mediated neutralization of African swine fever virus: Myths and facts. Virus Res. 2013, 173, 101–109. [Google Scholar] [CrossRef]
- Kollnberger, S.D.; Gutierrez-Castaneda, B.; Foster-Cuevas, M.; Corteyn, A.; Parkhouse, R.M.E. Identification of the principal serological immunodeterminants of African swine fever virus by screening a virus cDNA library with antibody. J. Gen. Virol. 2002, 83 Pt 6, 1331–1342. [Google Scholar] [CrossRef]
- Alcaraz, C.; De Diego, M.; Pastor, M.J.; Escribano, J.M. Comparison of a radioimmunoprecipitation assay to immunoblotting and ELISA for detection of antibody to African swine fever virus. J. Vet. Diagn. Investig. 1990, 2, 191–196. [Google Scholar] [CrossRef]
- Lim, J.-S.; Vergne, T.; Kim, E.; Guinat, C.; Dellicour, S.; Andraud, M. A spatially-heterogeneous impact of fencing on the African swine fever wavefront in the Korean wild boar population. Vet. Res. 2024, 55, 163. [Google Scholar] [CrossRef]
- Ito, S.; Bosch, J.; Jeong, H.; Aguilar-Vega, C.; Park, J.; Martinez-Aviles, M.; Sanchez-Vizcaino, J.M. Spatio-Temporal Epidemiology of the Spread of African Swine Fever in Wild Boar and the Role of Environmental Factors in South Korea. Viruses 2022, 14, 2779. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, A.L.; Bustos, M.J.; de Leon, P. Methods for growing and titrating African swine fever virus: Field and laboratory samples. Curr. Protoc. Cell Biol. 2011, 53, 26 14 1–26 14 25. [Google Scholar] [CrossRef] [PubMed]
- Krug, P.W.; Holinka, L.G.; O’Donnell, V.; Reese, B.; Sanford, B.; Fernandez-Sainz, I.; Gladue, D.P.; Arzt, J.; Rodriguez, L.; Risatti, G.R.; et al. The progressive adaptation of a georgian isolate of African swine fever virus to vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. J. Virol. 2015, 89, 2324–2332. [Google Scholar] [CrossRef]
- Awosanya, E.J.; Olugasa, B.; Ogundipe, G.; Grohn, Y.T. Sero-prevalence and risk factors associated with African swine fever on pig farms in southwest Nigeria. BMC Vet. Res. 2015, 11, 133. [Google Scholar] [CrossRef]
- Carmina, G.; Nieto, R.; Arias, M. Indirect Immunoperoxidase Test (IPT) for Detection of Antibodies Against African Swine Fever Virus (ASFV) on African Green Monkey Cell Lines (Vero, MS). Methods Mol. Biol. 2022, 2503, 147–158. [Google Scholar]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. (Eds.) A Density-Based Algorithm for Discovering Clusters in Large Spatial Databases with Noise. In Proceedings of the 2nd International Conference on Knowledge Discovery and Data Mining (KDD-96), Portland, OR, USA, 2–4 August 1996; pp. 226–231. [Google Scholar]
- Keuling, O.; Massei, G. Does hunting affect the behavior of wild pigs? Hum. Wildl. Interact. 2021, 15, 11. [Google Scholar]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical methods for research workers. In Breakthroughs in Statistics: Methodology and Distribution; Springer: Berlin/Heidelberg, Germany, 1970; pp. 66–70. [Google Scholar]
- Chae, J.B.; Kang, J.G.; Kim, H.C.; Chong, S.T.; Lee, I.Y.; Shin, N.S.; Chae, J.S. Identification of Tick Species Collected from Wild Boars and Habitats of Wild Boars and Domestic Pigs in the Republic of Korea. Korean J. Parasitol. 2017, 55, 185–191. [Google Scholar] [CrossRef]
- Coelho, I.M.P.; Paiva, M.T.; da Costa, A.J.A.; Nicolino, R.R. African Swine Fever: Spread and seasonal patterns worldwide. Prev. Vet. Med. 2024, 235, 106401. [Google Scholar] [CrossRef]
- Gallardo, C.; Soler, A.; Nurmoja, I.; Cano-Gomez, C.; Cvetkova, S.; Frant, M.; Wozniakowski, G.; Simon, A.; Perez, C.; Nieto, R.; et al. Dynamics of African swine fever virus (ASFV) infection in domestic pigs infected with virulent, moderate virulent and attenuated genotype II ASFV European isolates. Transbound. Emerg. Dis. 2021, 68, 2826–2841. [Google Scholar] [CrossRef]
- Kwon, O.-K.; Kim, D.-W.; Heo, J.-H.; Kim, J.-Y.; Nah, J.-J.; Choi, J.-D.; Lee, D.-W.; Cho, K.-H.; Hong, S.-K.; Kim, Y.-H.; et al. Genomic Epidemiology of African Swine Fever Virus Identified in Domestic Pig Farms in South Korea during 2019–2021. Transbound. Emerg. Dis. 2024, 2024, 9077791. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Hong, S.K.; Kim, D.Y.; Sohn, H.J.; Yoo, D.S.; Kang, H.E.; Kim, Y.H. Disease Course of Korean African Swine Fever Virus in Domestic Pigs Exposed Intraorally, Intranasally, Intramuscularly, and by Direct Contact with Infected Pigs. Viruses 2024, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Hong, S.K.; Kim, D.Y.; Jang, M.K.; Kim, J.H.; Lee, H.; Kim, E.M.; Park, J.H.; Suh, T.Y.; Choi, J.G.; et al. Pathogenicity and Pathological Characteristics of African Swine Fever Virus Strains from Pig Farms in South Korea from 2022 to January 2023. Pathogens 2023, 12, 1158. [Google Scholar] [CrossRef]
- Gallardo, C.; Soler, A.; Nieto, R.; Cano, C.; Pelayo, V.; Sanchez, M.A.; Pridotkas, G.; Fernandez-Pinero, J.; Briones, V.; Arias, M. Experimental Infection of Domestic Pigs with African Swine Fever Virus Lithuania 2014 Genotype II Field Isolate. Transbound. Emerg. Dis. 2017, 64, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef]
- Igolkin, A.; Mazloum, A.; Zinyakov, N.; Chernyshev, R.; Schalkwyk, A.V.; Shotin, A.; Lavrentiev, I.; Gruzdev, K.; Chvala, I. Detection of the first recombinant African swine fever virus (genotypes I and II) in domestic pigs in Russia. Mol. Biol. Rep. 2024, 51, 1011. [Google Scholar] [CrossRef]
- Lee, K.; Vu, T.T.H.; Yeom, M.; Nguyen, V.D.; Than, T.T.; Nguyen, V.T.; Jeong, D.G.; Ambagala, A.; Le, V.P.; Song, D. Molecular characterization of emerging recombinant African swine fever virus of genotype I and II in Vietnam, 2023. Emerg. Microbes Infect. 2024, 13, 2404156. [Google Scholar] [CrossRef]

| Province | Region | Collection Date | Num. of Sera | Antibody Detection 1 | % Sero- Positivity | |||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | %SE 2 | %SP 3 | |||||
| Incheon | Ganghwa | September 2019 | 36 | 0 | 36 | - | 100 | - |
| Gyeonggi | Gimpo | September 2019 to August 2024 | 235 | 0 | 234 | - | 99.57 (1) | - |
| Yeoncheon | September to October 2019 | 35 | 0 | 35 | - | 100 | - | |
| Paju | September 2019 to January 2024 | 212 | 0 | 212 | - | 100 | - | |
| Pocheon | January to April 2023 | 440 | 0 | 440 | - | 100 | - | |
| Yangju | December 2024 | 120 | 0 | 120 | - | 100 | - | |
| Gangwon | Goseong | August 2021 | 65 | 0 | 65 | - | 100 | - |
| Yanggu | August 2022 | 25 | 2 | 23 | 100 | 100 | 8.0 | |
| Yangyang | February 2023 | 30 | 3 | 23 | 100 | 82.61 (4) | 10.0 | |
| Inje | August to October 2021 | 40 | 0 | 40 | - | 100 | - | |
| Cheorwon | November 2022 to May 2024 | 265 | 5 | 260 | 100 | 100 | 1.89 | |
| Chuncheon | September 2022 | 30 | 0 | 30 | - | 100 | - | |
| Hongcheon | August 2021 to November 2024 | 209 | 8 | 198 | 100 | 98.48 (3) | 3.83 | |
| Hwacheon | October 2020 to October 2024 | 266 | 12 | 253 | 91.67 (1) | 99.60 (1) | 4.51 | |
| Gyeongsangbuk | Andong | July 2024 | 18 | 1 | 16 | 100 | 93.75 (1) | 5.56 |
| Yeongdeok | January 2024 | 20 | 0 | 20 | - | 100 | - | |
| Yeongcheon | June to August 2024 | 166 | 1 | 165 | 100 | 100 | 0.60 | |
| Yecheon | July 2024 | 20 | 0 | 20 | - | 100 | - | |
| Total | 2232 | 32 | 2190 | 96.88 | 99.54 | 1.43 | ||
| Year | Num. of Outbreaks in Pig Farms 1 | Num. of Ab Positive /Num. of Samples | Phases of Pig Production (Antibody Detection) | |||||
|---|---|---|---|---|---|---|---|---|
| Sow | Gilt | Finishing /Growing | Nursery /Piglet | Boar | Unknown | |||
| 2019 | 14 | 0/238 | 0/66 | 0/6 | 0/81 | 0/35 | 0/0 | 0/50 |
| 2020 | 2 | 1/50 | 1/10 | 0/0 | 0/20 | 0/10 | 0/0 | 0/10 |
| 2021 | 5 | 0/145 | 0/15 | 0/10 | 0/80 | 0/40 | 0/0 | 0/0 |
| 2022 | 7 | 5/308 | 3/128 | 0/5 | 2/145 | 0/30 | 0/0 | 0/0 |
| 2023 | 10 | 3/690 | 0/205 | 0/50 | 3/265 | 0/170 | 0/0 | 0/0 |
| 2024 | 11 | 23/681 | 1/213 | 3/45 | 18/364 | 1/172 | 0/7 | 0/0 |
| Total | 49 | 1.43 2 (32/2232) | 0.78 (5/637) | 2.59 (3/116) | 2.41 (23/955) | 0.22 (1/457) | 0.00 (0/7) | 0.00 (0/60) |
| IPT | bELISA | cELISA | ||||
|---|---|---|---|---|---|---|
| Positive | Inconclusive 1 | Negative | Positive | Inconclusive 1 | Negative | |
| Positive | 25 | 7 | 0 | 29 | 2 | 1 |
| Negative | 3 | 2 | 2195 | 4 | 3 | 641 |
| Total | 2232 | 675 | ||||
| %SE | 100 (25/25) | 96.67 (29/30) | ||||
| %SP | 99.86 (2195/2198) | 99.38 (636/640) | ||||
| κ value | 0.94 | 0.92 | ||||
| Cluster ID | Num. of Wild Boar Points | Num. of Seropositive Pig Farms | Num. of Outbreak Pig Farms | Radius | Color on Map |
|---|---|---|---|---|---|
| Cluster 1 (N = 42) | 39 | 1 | 3 | 0.287 | ● |
| Cluster 2 (N = 32) | 28 | 2 | 4 | 0.218 | ● |
| Cluster 3 (N = 227) | 220 | 3 | 7 | 0.635 | ● |
| Cluster 4 (N = 15) | 10 | 2 | 5 | 0.165 | ● |
| Cluster 5 (N = 613) | 608 | 2 | 5 | 0.787 | ● |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-K.; Moon, M.; Cho, K.-H.; Kang, H.-E.; Lee, J.-S.; Kim, Y.-H. First Serologic Analysis of Antibodies Against African Swine Fever Virus Detected in Domestic Pig Farms in South Korea from 2019 to 2024. Pathogens 2025, 14, 581. https://doi.org/10.3390/pathogens14060581
Hong S-K, Moon M, Cho K-H, Kang H-E, Lee J-S, Kim Y-H. First Serologic Analysis of Antibodies Against African Swine Fever Virus Detected in Domestic Pig Farms in South Korea from 2019 to 2024. Pathogens. 2025; 14(6):581. https://doi.org/10.3390/pathogens14060581
Chicago/Turabian StyleHong, Seong-Keun, Mugyeom Moon, Ki-Hyun Cho, Hae-Eun Kang, Jong-Soo Lee, and Yeon-Hee Kim. 2025. "First Serologic Analysis of Antibodies Against African Swine Fever Virus Detected in Domestic Pig Farms in South Korea from 2019 to 2024" Pathogens 14, no. 6: 581. https://doi.org/10.3390/pathogens14060581
APA StyleHong, S.-K., Moon, M., Cho, K.-H., Kang, H.-E., Lee, J.-S., & Kim, Y.-H. (2025). First Serologic Analysis of Antibodies Against African Swine Fever Virus Detected in Domestic Pig Farms in South Korea from 2019 to 2024. Pathogens, 14(6), 581. https://doi.org/10.3390/pathogens14060581






