Molecular and Microscopic Challenges in Detecting Plasmodium cynomolgi Co-Infections with Plasmodium vivax: A Case Report
Abstract
1. Introduction
2. Case Presentation
3. Methodology
3.1. Isolation of DNA
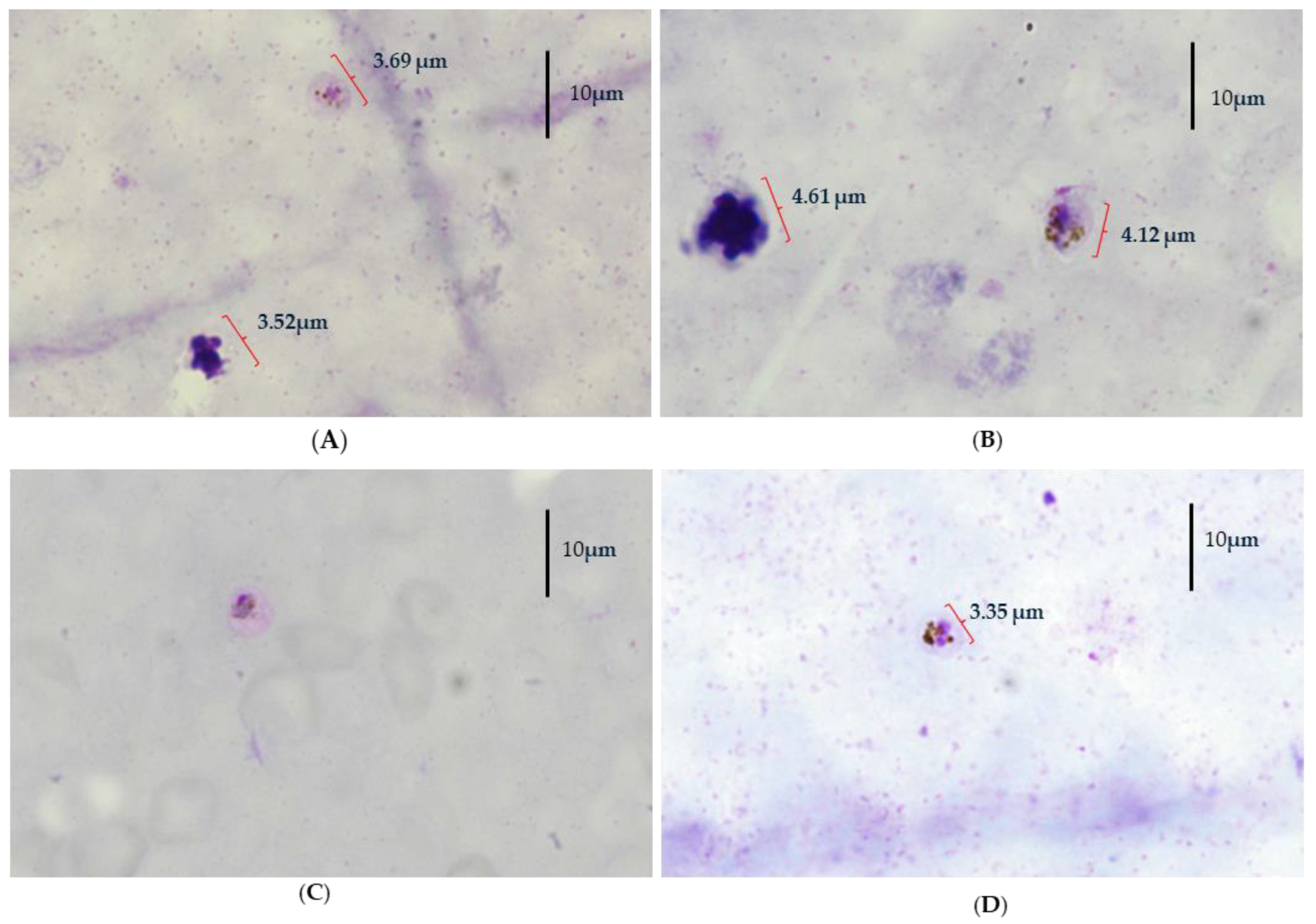
3.2. Blood Film Malaria Parasite (BFMP)
3.3. Nested PCR and Sequencing
3.4. abTES™ Real-Time PCR and Sequencing
3.5. Real-Time COI Plasmodium PCR (RT-COI 1R/5R)
3.6. Nested Multiplex Malaria PCR (NM-PCR)
3.7. Nested Genus Malaria PCR (NG-PCR)
3.8. Sequence Analysis and Phylogenetic Tree Construction
4. Result
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mewara, A.; Sreenivasan, P.; Khurana, S. Primate Malaria of Human Importance. Trop. Parasitol. 2023, 13, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Imwong, M.; Madmanee, W.; Suwannasin, K.; Kunasol, C.; Peto, T.J.; Tripura, R.; von Seidlein, L.; Nguon, C.; Davoeung, C.; Day, N.P.J.; et al. Asymptomatic Natural Human Infections with the Simian Malaria Parasites Plasmodium cynomolgi and Plasmodium knowlesi. J. Infect. Dis. 2019, 219, 695–702. [Google Scholar] [CrossRef]
- Putaporntip, C.; Kuamsab, N.; Pattanawong, U.; Yanmanee, S.; Seethamchai, S.; Jongwutiwes, S. Plasmodium cynomolgi Co-Infections among Symptomatic Malaria Patients, Thailand. Emerg. Infect. Dis. 2021, 27, 590–593. [Google Scholar] [CrossRef]
- Permana, D.H.; Hasmiwati; Suryandari, D.A.; Rozi, I.E.; Syahrani, L.; Setiadi, W.; Irawati, N.; Rizaldi; Wangsamuda, S.; Yusuf, Y.; et al. The Potential for Zoonotic Malaria Transmission in Five Areas of Indonesia Inhabited by Non-Human Primates. Parasites Vectors 2023, 16, 267. [Google Scholar] [CrossRef]
- Ministry of Health (MOH), Malaysia. Laporan Tahunan KKM 2023(pdf). Available online: https://www.moh.gov.my/index.php/pages/view/58?mid=19 (accessed on 20 May 2025).
- Singh, B.; Sung, L.K.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.G.; Cox-Singh, J.; Thomas, A.; Conway, D.J. A Large Focus of Naturally Acquired Plasmodium knowlesi Infections in Human Beings. Lancet 2004, 363, 1017–1024. [Google Scholar] [CrossRef]
- Vythilingam, I.; Tan, C.H.; Matusop, A.; Chan, S.T.; Lee, K.S.; Singh, B. Natural Transmission of Plasmodium knowlesi to Humans by Anopheles latens in Sarawak, Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Vythilingam, I.; Noorazian, Y.M.; Huat, T.C.; Jiram, A.I.; Yusri, Y.M.; Azahari, A.H.; Norparina, I.; Noorrain, A.; Lokmanhakim, S. Plasmodium knowlesi in Humans, Macaques and Mosquitoes in Peninsular Malaysia. Parasites Vectors 2008, 1, 26. [Google Scholar] [CrossRef]
- Yusuf, N.M.; Zulkefli, J.; Jiram, A.I.; Vythilingam, I.; Hisam, S.; Devi, R.; Salehhuddin, A.; Ali, N.M.; Isa, M.; Alias, N.; et al. Plasmodium spp. in Macaques, Macaca fascicularis, in Malaysia, and Their Potential Role in Zoonotic Malaria Transmission. Parasite 2022, 29, 32. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, P.R.; Key, S.; Jumail, A.; Surendra, H.; Ferguson, H.M.; Drakeley, C.J.; Fornace, K. Epidemiology of the Zoonotic Malaria Plasmodium knowlesi in Changing Landscapes. Adv. Parasitol. 2021, 113, 225–286. [Google Scholar] [CrossRef]
- Shahari, S.; Abdullah, M.L.B.; Rohimly, A.A.B.I.; Ashrat, N.B.; Amir, A.; Atroosh, W.M.M.; Fong, M.Y.; Lau, Y.L. The Prevalence of Simian Malaria in Wild Long-Tailed Macaques Throughout Peninsular Malaysia. Sci. Rep. 2024, 14, 6023. [Google Scholar] [CrossRef]
- Hussin, N.; Lim, Y.A.L.; Goh, P.P.; William, T.; Jelip, J.; Mudin, R.N. Updates on Malaria Incidence and Profile in Malaysia from 2013 to 2017. Malar. J. 2020, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Coatney, G.R.; Collins, W.E.; Warren, M.; Contacos, P.G. The Primate Malarias; US National Institute of Allergy and Infectious Diseases: Washington, DC, USA, 1971. [Google Scholar]
- Singh, B.; Daneshvar, C. Human Infections and Detection of Plasmodium knowlesi. Clin. Microbiol. Rev. 2013, 26, 165–184. [Google Scholar] [CrossRef]
- Ta, T.H.; Hisam, S.; Lanza, M.; Jiram, A.I.; Ismail, N.; Rubio, J.M. First Case of a Naturally Acquired Human Infection with Plasmodium cynomolgi. Malar. J. 2014, 13, 68. [Google Scholar] [CrossRef]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.; Thaithong, S.; Brown, K.N. High Sensitivity of Detection of Human Malaria Parasites by the Use of Nested Polymerase Chain Reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health (MOH), Malaysia. Management Guidelines of Malaria in Malaysia. Available online: https://www.moh.gov.my/moh/resources/Penerbitan/Garis%20Panduan/Pengurusan%20KEsihatan%20&%20kawalan%20pykit/Management_Guidelines_of_Malaria_in_Malaysia_(Final).pdf (accessed on 20 May 2025).
- Bhatnagar, N. Dacie, and Lewis Practical Haematology (12th Edition) by B. J. Bain, I. Bates, and M. A. Laffan, Elsevier, London, 2017. Br. J. Haematol. 2017, 178, 652. [Google Scholar] [CrossRef]
- Lee, K.S.; Divis, P.C.S.; Zakaria, S.K.; Matusop, A.; Julin, R.A.M.; Conway, D.J.; Cox-Singh, J.; Singh, B. Plasmodium knowlesi: Reservoir Hosts and Tracking the Emergence in Humans and Macaques. PLoS Pathog. 2011, 7, e1002015. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Van de Peer, Y.; De Wachter, R. TREECON for Windows: A Software Package for the Construction and Drawing of Evolutionary Trees for the Microsoft Windows Environment. Bioinformatics 1994, 10, 569–570. [Google Scholar] [CrossRef]
- Kantele, A.; Jokiranta, T.S. Review of Cases with the Emerging Fifth Human Malaria Parasite, Plasmodium knowlesi. Clin. Infect. Dis. 2011, 52, 1356–1362. [Google Scholar] [CrossRef]
- Chin, W.; Contacos, P.G.; Coatney, G.R.; Kimball, H.R. A Naturally Acquired Quotidian-Type Malaria in Man Transferable to Monkeys. Science 1965, 149, 865. [Google Scholar] [CrossRef]
- Contacos, P.G.; Lunn, J.S.; Coatney, G.R.; Kilpatrick, J.W.; Jones, F.E. Quartan-Type Malaria Parasite of New World Monkeys Transmissible to Man. Science 1963, 142, 676. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.E.; William, T.; Grigg, M.J.; Yeo, T.W.; Anstey, N.M. Limitations of Microscopy to Differentiate Plasmodium Species in a Region Co-Endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar. J. 2013, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.M.; Post, R.J.; van Leeuwen, W.M.; Henry, M.C.; Lindergard, G.; Hommel, M. Alternative Polymerase Chain Reaction Method to Identify Plasmodium Species in Human Blood Samples: The Semi-Nested Multiplex Malaria PCR (SnM-PCR). Trans. R. Soc. Trop. Med. Hyg. 2002, 96 (Suppl. S1), S199–S204. [Google Scholar] [CrossRef] [PubMed]
- Lazrek, Y.; Florimond, C.; Volney, B.; Discours, M.; Mosnier, E.; Houzé, S.; Pelleau, S.; Musset, L. Molecular detection of human Plasmodium species using a multiplex real time PCR. Sci Rep 2023, 13, 11388. [Google Scholar] [CrossRef]


| Parameter | Reference Range (Adult Male) | Patient Value | Remarks |
|---|---|---|---|
| Total White Blood Cell Count (×103/µL) | 4.00–10.00 | 11.30 | Elevated |
| Hemoglobin (g/dL) | 13.0–17.0 | 15.7 | Normal |
| Platelet Count (×103/µL) | 150.0–400.0 | 237 | Normal |
| Primer/Probe | Sequence (5′–3′) | PCR | Final Conc. (µM) |
|---|---|---|---|
| JM-P-COI 2F | GGTGTGTACAAGGCAACAATAC | RT-COI R1/R5 | 0.20 |
| JM-P-COI 1R | CATATAACGGTAAGAAGGTTCGC | RT-COI R1 | 0.20 |
| JM-P-COI 5R | CAAAGTACGCGATCTCTTGTATG | RT-COI R5 | 0.20 |
| MALCOI 2 | Cy5–ATTGGCACCTCCATGTCGTCTCAT–BHQ2 | RT-COI R1/R5 | 0.15 |
| UNR PLF HUF NewPLFshort MARshort FARshort OVRshort VIRshort NewPLFshort NewRevshort | GACGGTATCTGATCGTCTTC AGTGTGTATCCAATCGAGTTTC GAGCCGCCTGGATACCGC CTATCAGCTTTTGATGTTAG TCCAATTGCCTTCTG GTTCCCCTAGAATAGTTACA AGGAATGCAAAGARCAG AAGGACTTCCAAGCC CTATCAGCTTTTGATGTTAG CCTTAACTTTCGTTCTTG | 1st NM-PCR 1st NM-PCR 1st NM-PCR 2nd NM-PCR 2nd NM-PCR 2nd NM-PCR 2nd NM-PCR 2nd NM-PCR NG-PCR NG-PCR | 0.10 0.10 0.01 0.15 0.10 0.15 0.10 0.10 0.30 0.30 |
| Target Gene | - | 18S rRNA | COI | |||
|---|---|---|---|---|---|---|
| Method | abTES™ | Nested PCR | NM-PCR | NG-PCR | RT-COIR1-PCR | RT-COIR5-PCR |
| PCR Result | P. vivax | P. vivax, P. cynomolgi | P. vivax | P. vivax | Positive | Positive |
| Sequencing Result | P. cynomolgi | P. vivax, P. cynomolgi | P. vivax | P. cynomolgi | P. vivax-like | P. cynomolgi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaacob, M.A.; Radin Hisam, R.S.; Parina Ismail, N.; Md Yusuf, N.A.; Rubio Muñoz, J.M.; Hashim, S.; Zhueng, T.J. Molecular and Microscopic Challenges in Detecting Plasmodium cynomolgi Co-Infections with Plasmodium vivax: A Case Report. Pathogens 2025, 14, 651. https://doi.org/10.3390/pathogens14070651
Yaacob MA, Radin Hisam RS, Parina Ismail N, Md Yusuf NA, Rubio Muñoz JM, Hashim S, Zhueng TJ. Molecular and Microscopic Challenges in Detecting Plasmodium cynomolgi Co-Infections with Plasmodium vivax: A Case Report. Pathogens. 2025; 14(7):651. https://doi.org/10.3390/pathogens14070651
Chicago/Turabian StyleYaacob, Mohd Adilin, Raden Shamilah Radin Hisam, Nor Parina Ismail, Noor Azian Md Yusuf, Jose Miguel Rubio Muñoz, Suhana Hashim, and Tam Jenn Zhueng. 2025. "Molecular and Microscopic Challenges in Detecting Plasmodium cynomolgi Co-Infections with Plasmodium vivax: A Case Report" Pathogens 14, no. 7: 651. https://doi.org/10.3390/pathogens14070651
APA StyleYaacob, M. A., Radin Hisam, R. S., Parina Ismail, N., Md Yusuf, N. A., Rubio Muñoz, J. M., Hashim, S., & Zhueng, T. J. (2025). Molecular and Microscopic Challenges in Detecting Plasmodium cynomolgi Co-Infections with Plasmodium vivax: A Case Report. Pathogens, 14(7), 651. https://doi.org/10.3390/pathogens14070651






