Alveolar Echinococcosis in 11-Month-Old Dog—Clinical Case
Abstract
1. Introduction
2. Results
2.1. Anamnesis
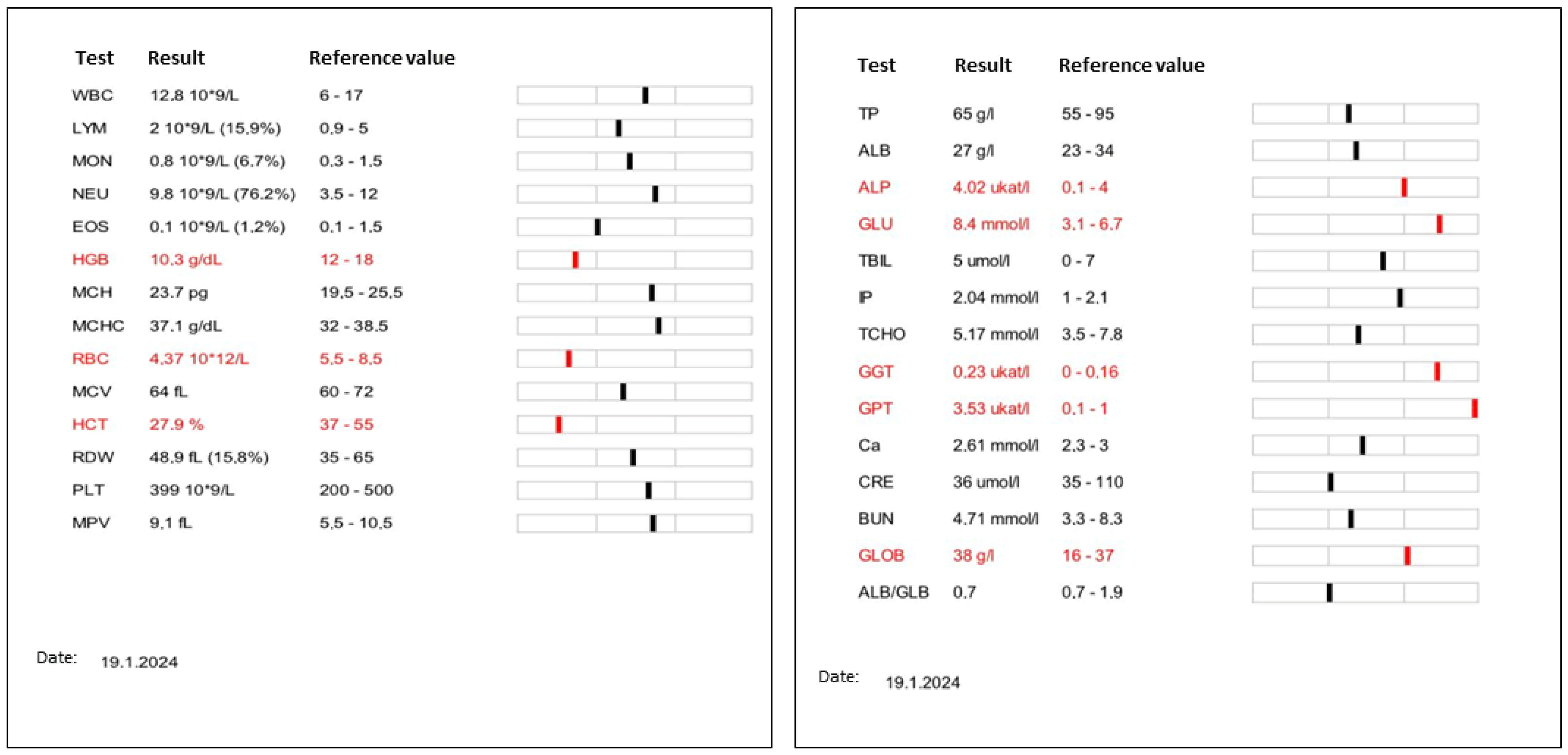
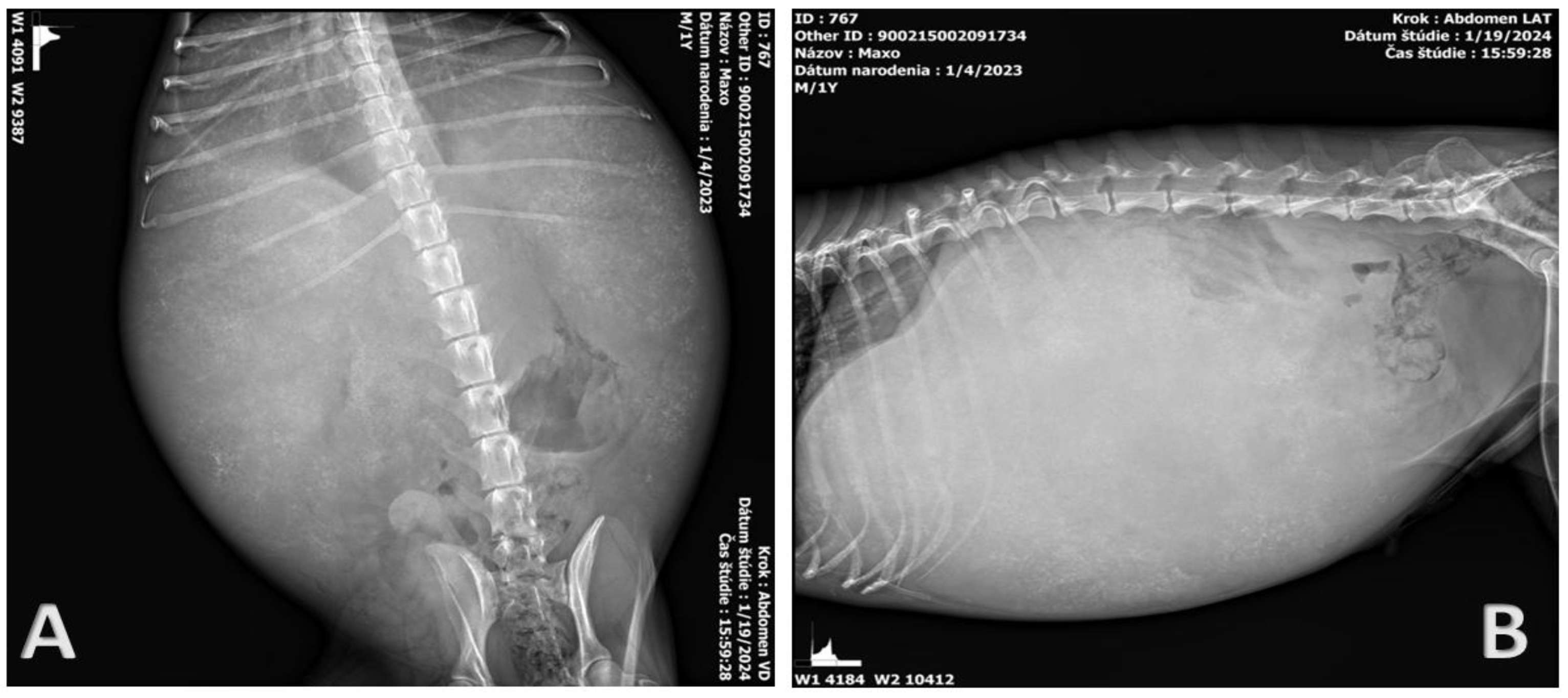
2.2. Clinical Examination
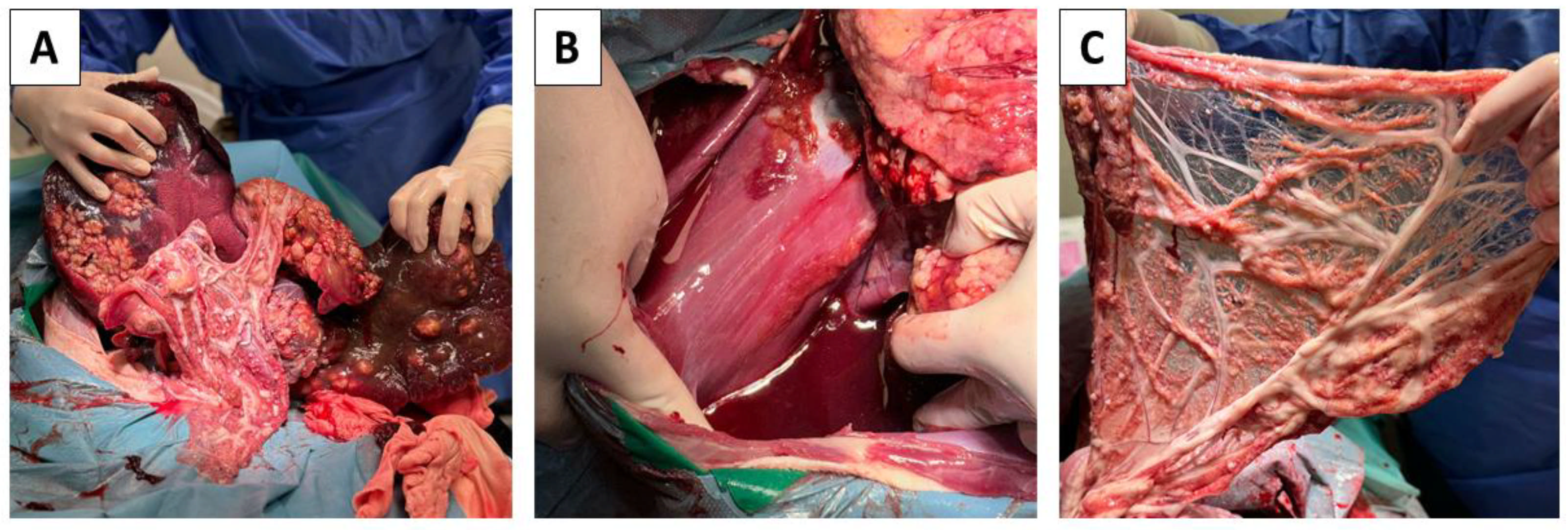
2.3. Probatory Laparotomy
2.4. Post-Mortem Findings
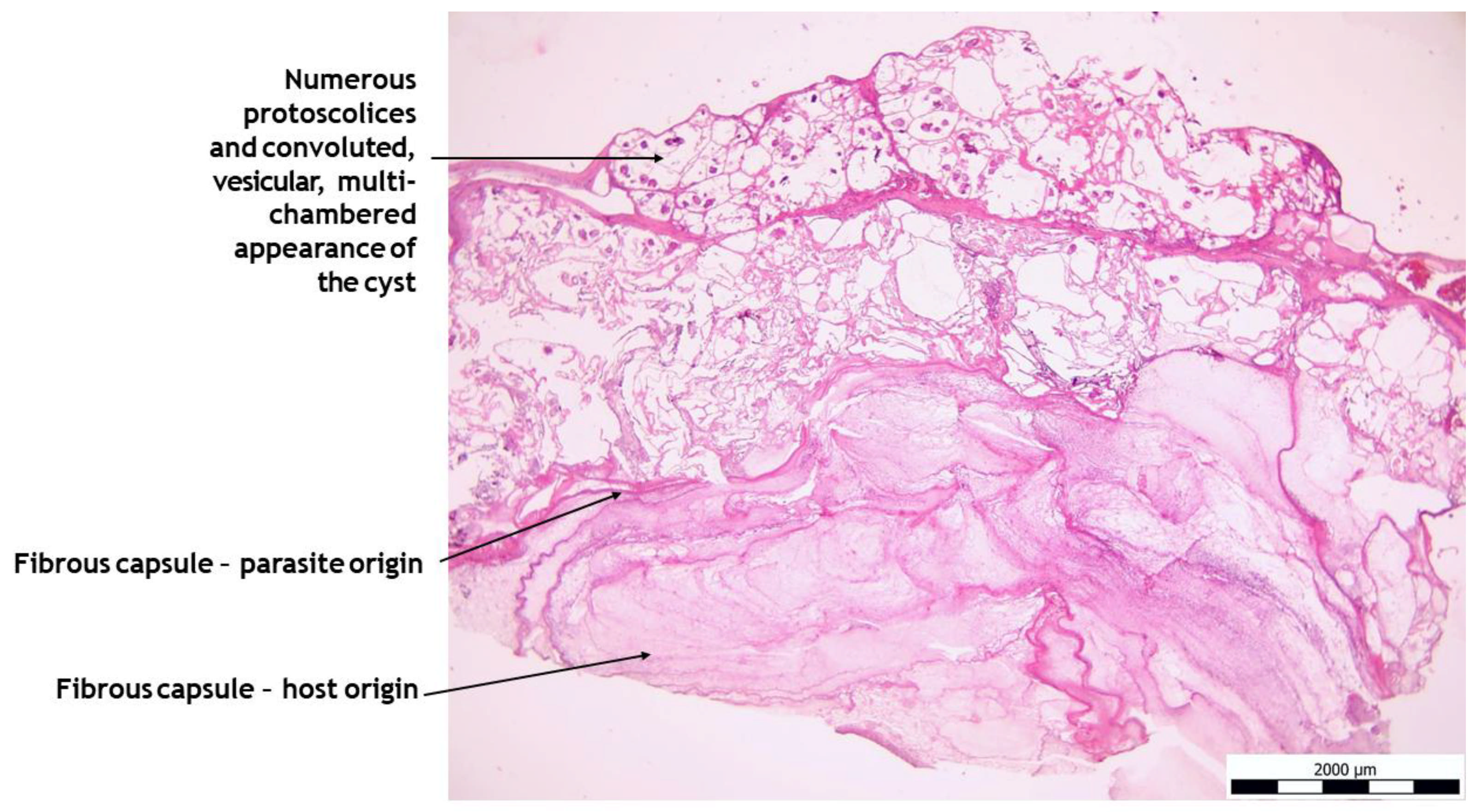
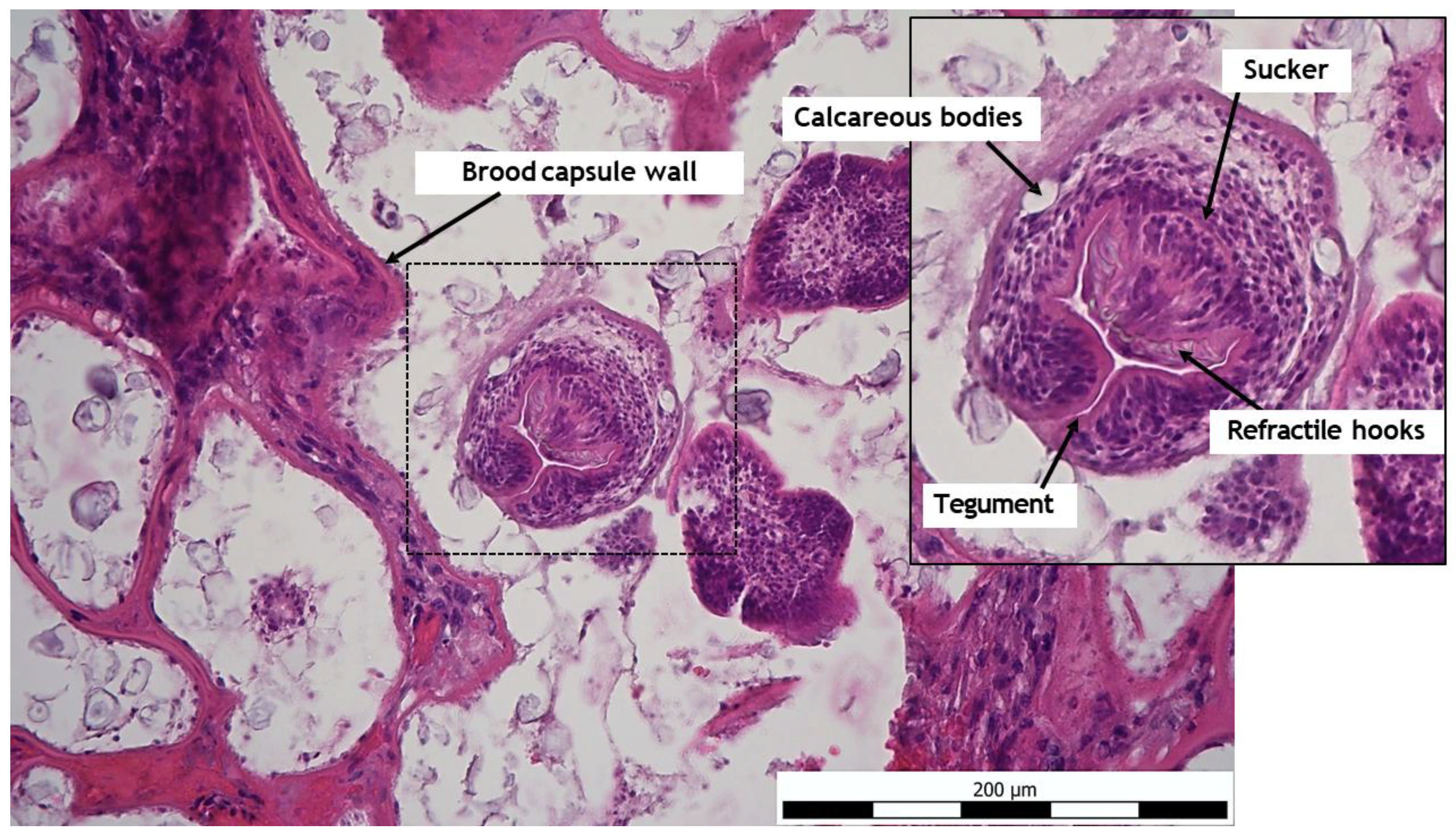
2.5. Histo-Pathological Study
2.6. Microbiology
2.7. Parasitology
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hogea, M.-O.; Ciomaga, B.-F.; Muntean, M.-M.; Muntean, A.-A.; Popa, M.I.; Popa, G.L. Cystic Echinococcosis in the Early 2020s: A Review. Trop. Med. Infect. Dis. 2024, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.M.; Dias, M.C.; Cardo, M.; Aguiar, P.; de Carvalho, L.M. The Evolution of Cystic Echinococcosis in Humans and Ruminants in Portugal—A One Health Approach. Vet. Sci. 2023, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Toews, E.; Musiani, M.; Checkley, S.; Visscher, D.; Massolo, A. A Global Assessment of Echinococcus multilocularis Infections in Domestic Dogs: Proposing a Framework to Overcome Past Methodological Heterogeneity. Int. J. Parasitol. 2021, 51, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, V.; Pantchev, N.; Gawlowska, S.; Vrhovec, M.G.; Bauer, C. Echinococcus multilocularis Infections in Domestic Dogs and Cats from Germany and Other European Countries. Vet. Parasitol. 2008, 157, 244–253. [Google Scholar] [CrossRef]
- Echinococcus Infections in Dogs: Prevalence, Symptoms and Diagnosis. LABOKLIN Europe. 2024. Available online: https://laboklin.com/ch-it/echinococcus-infections-in-dogs-prevalence-symptoms-and-diagnosis/ (accessed on 27 March 2024).
- Woolsey, I.D.; Miller, A.L. Echinococcus granulosus sensu lato and Echinococcus multilocularis: A Review. Res. Vet. Sci. 2021, 135, 517–522. [Google Scholar] [CrossRef]
- Moro, P.; Schantz, P.M. Echinococcosis: A Review. Int. J. Infect. Dis. 2009, 13, 125–133. [Google Scholar] [CrossRef]
- Thompson, R.C.A. The Molecular Epidemiology of Echinococcus Infections. Pathogens 2020, 9, 453. [Google Scholar] [CrossRef]
- Schmidberger, J.; Uhlenbruck, J.; Schlingeloff, P.; Maksimov, P.; Conraths, F.J.; Mayer, B.; Kratzer, W. Dog Ownership and Risk for Alveolar Echinococcosis, Germany. Emerg. Infect. Dis. 2022, 28, 1597–1605. [Google Scholar] [CrossRef]
- Weiss, A.T.A.; Bauer, C.; Köhler, K. Morphological Characteristics of Alveolar Echinococcosis in Dogs. J. Comp. Pathol. 2009, 141, 312. [Google Scholar] [CrossRef]
- Liu, C.; Fan, H.; Ge, R. A Case of Human Hepatic Alveolar Echinococcosis Accompanied by Lung and Brain Metastases. Korean J. Parasitol. 2021, 59, 291–296. [Google Scholar] [CrossRef]
- Bakinowska, E.; Kostopanagiotou, K.; Wojtyś, M.E.; Kiełbowski, K.; Ptaszyński, K.; Gajić, D.; Ruszel, N.; Wójcik, J.; Grodzki, T.; Tomos, P. Basic Operative Tactics for Pulmonary Echinococcosis in the Era of Endostaplers and Energy Devices. Medicina 2023, 59, 543. [Google Scholar] [CrossRef] [PubMed]
- Zanib, A.; Ahmed, A.A.; Milos, A.S.; Musavi, S.S. Rare Presentation of Hepatic Alveolar Echinococcosis Mimicking Hepatocellular Carcinoma. BMJ Case Rep. CP 2024, 17, e259701. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, G.; Pinzone, M.R.; Gruttadauria, S.; Celesia, B.M.; Madeddu, G.; Malaguarnera, G.; Pavone, P.; Cappellani, A.; Cacopardo, B. Hepatic Echinococcosis: Clinical and Therapeutic Aspects. World J. Gastroenterol. 2012, 18, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Can, H.; İnceboz, T.; Caner, A.; Atalay Şahar, E.; Karakavuk, M.; Döşkaya, M.; Çelebi, F.; Değirmenci Döşkaya, A.; Gülçe İz, S.; Gürüz, Y.; et al. Detection of Echinococcus granulosus and Echinococcus multilocularis in cyst samples using a novel single tube multiplex real-time polymerase chain reaction. Mikrobiyol. Bul. 2016, 50, 266–277. [Google Scholar] [CrossRef]
- The First Finding of Echinococcus multilocularis in Dogs in Slovakia: An Emerging Risk for Spreading of Infection. ResearchGate 2025.
- Antolová, D.; Šnábel, V.; Jarošová, J.; Cavallero, S.; D’Amelio, S.; Syrota, Y.; Rosoľanka, R.; Avdičová, M.; Miterpáková, M. Human Alveolar Echinococcosis in Slovakia: Epidemiology and Genetic Diversity of Echinococcus multilocularis, 2000–2023. PLoS Negl. Trop. Dis. 2024, 18, e0011876. [Google Scholar] [CrossRef]
- Nahorski, W.L.; Knap, J.P.; Pawłowski, Z.S.; Krawczyk, M.; Polański, J.; Stefaniak, J.; Patkowski, W.; Szostakowska, B.; Pietkiewicz, H.; Grzeszczuk, A.; et al. Human Alveolar Echinococcosis in Poland: 1990–2011. PLoS Negl. Trop. Dis. 2013, 7, e1986. [Google Scholar] [CrossRef]
- Kolářová, L.; Matějů, J.; Hrdý, J.; Kolářová, H.; Hozáková, L.; Žampachová, V.; Auer, H.; Stejskal, F. Human Alveolar Echinococcosis, Czech Republic, 2007–2014. Emerg. Infect. Dis. 2015, 21, 2263–2265. [Google Scholar] [CrossRef]
- Auer, H.; Aspöck, H. Echlnococcosls in Austria. Zentralblatt Bakteriol. 1990, 272, 498–508. [Google Scholar] [CrossRef]
- Halász, T.; Nagy, G.; Nagy, I.; Csivincsik, Á. Micro-Epidemiological Investigation of Echinococcus multilocularis in Wild Hosts from an Endemic Area of Southwestern Hungary. Parasitologia 2021, 1, 158–167. [Google Scholar] [CrossRef]
- Sréter, T.; Széll, Z.; Sréter-Lancz, Z.; Varga, I. Echinococcus multilocularis in Northern Hungary. Emerg. Infect. Dis. 2004, 10, 1344–1346. [Google Scholar] [CrossRef]
- Catalano, S.; Lejeune, M.; Liccioli, S.; Verocai, G.G.; Gesy, K.M.; Jenkins, E.J.; Kutz, S.J.; Fuentealba, C.; Duignan, P.J.; Massolo, A. Echinococcus multilocularis in Urban Coyotes, Alberta, Canada. Emerg. Infect. Dis. 2012, 18, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Massolo, A.; Liccioli, S.; Budke, C.; Klein, C. Echinococcus multilocularis in North America: The Great Unknown. Parasite 2014, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Konyaev, S.V.; Yanagida, T.; Nakao, M.; Ingovatova, G.M.; Shoykhet, Y.N.; Bondarev, A.Y.; Odnokurtsev, V.A.; Loskutova, K.S.; Lukmanova, G.I.; Dokuchaev, N.E.; et al. Genetic Diversity of Echinococcus spp. in Russia. Parasitology 2013, 140, 1637–1647. [Google Scholar] [CrossRef]
- Giraudoux, P.; Raoul, F.; Afonso, E.; Ziadinov, I.; Yang, Y.; Li, L.; Li, T.; Quéré, J.-P.; Feng, X.; Wang, Q.; et al. Transmission Ecosystems of Echinococcus multilocularis in China and Central Asia. Parasitology 2013, 140, 1655–1666. [Google Scholar] [CrossRef]
- Dubinský, P.; Malczewski, A.; Miterpáková, M.; Gawor, J.; Reiterová, K. Echinococcus multilocularis in the Red Fox Vulpes Vulpes from the East Carpathian Region of Poland and the Slovak Republic. J. Helminthol. 2006, 80, 243–247. [Google Scholar] [CrossRef]
- Jansen, F.; Claes, M.; Bakkers, E.; Aryal, A.; Madimba, K.C.; Gabriël, S.; Dermauw, V.; Van Hul, A.; Vervaeke, M.; Dorny, P. Echinococcus multilocularis in Red Foxes in North Belgium: Prevalence and Trends in Distribution. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100470. [Google Scholar] [CrossRef]
- Alveolárna Echinokokóza Pečene u Psa–Klinický Prípad. Available online: https://www.researchgate.net/publication/316797582_Alveolarna_echinokokoza_pecene_u_psa_-_klinicky_pripad (accessed on 26 March 2025).
- Jańczak, D.; Skibiński, F.; Borkowski, A.; Jerchewicz, M.; Włodarz, K.; Klimiuk, P.; Sapierzyński, R.A.; Gawor, J. First Cases of Alveolar Echinococcosis in Dogs in Poland. Ann. Agric. Environ. Med. 2023, 30, 561–565. [Google Scholar] [CrossRef]
- Zajac, A.; Fairman, D.; McGee, E.; Wells, B.; Peregrine, A.; Jenkins, E.; LeRoith, T.; St John, B. Alveolar Echinococcosis in a Dog in the Eastern United States. J. Vet. Diagn. Investig. 2020, 32, 742–746. [Google Scholar] [CrossRef]
- Kolapo, T.U.; Hay, A.; Gesy, K.M.; Frey, C.F.; Rothenburger, J.L.; Joffe, D.J.; Spotswood, T.; Huang, Y.; Massolo, A.; Peregrine, A.S.; et al. Canine Alveolar Echinococcosis: An Emerging and Costly Introduced Problem in North America. Transbound. Emerg. Dis. 2023, 2023, 5224160. [Google Scholar] [CrossRef]
- Kantarci, M.; Bayraktutan, U.; Karabulut, N.; Aydinli, B.; Ogul, H.; Yuce, I.; Calik, M.; Eren, S.; Atamanalp, S.S.; Oto, A. Alveolar Echinococcosis: Spectrum of Findings at Cross-Sectional Imaging. RadioGraphics 2012, 32, 2053–2070. [Google Scholar] [CrossRef] [PubMed]
- Menghi, C.; Gatta, C.; Arias, L. Human Cystic and Alveolar Echinococcosis. Curr. Treat. Options Infect. Dis. 2017, 9, 210–222. [Google Scholar] [CrossRef]
- Blutke, A.; Hamel, D.; Hüttner, M.; Gehlen, H.; Romig, T.; Pfister, K.; Hermanns, W. Cystic Echinococcosis Due to Echinococcus equinus in a Horse from Southern Germany. J. Vet. Diagn. Investig. 2010, 22, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Eckert, J. Veterinary Aspects of Alveolar Echinococcosis—A Zoonosis of Public Health Significance. Vet. Parasitol. 2001, 98, 65–87. [Google Scholar] [CrossRef]
- Piotrowski, I.L.; Fabian, R.; Ohlerth, S.M.; Grimm, F.; Wehrli Eser, M.E. Cystic Echinococcosis Due to Echinococcus equinus in a Swiss Donkey. Equine Vet. Educ. 2025, 37, e71–e76. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.; An, X.; Xu, D.; Cai, S.; Chu, C.; Liu, G. Advances in Novel Diagnostic Techniques for Alveolar Echinococcosis. Diagnostics 2025, 15, 585. [Google Scholar] [CrossRef]
- Guo, H.; Liu, W.; Wang, J.; Xing, Y. Extrahepatic Alveolar Echinococcus on Multi-Slice Computed Tomography and Magnetic Resonance Imaging. Sci. Rep. 2021, 11, 9409. [Google Scholar] [CrossRef]
- Oberli, A.; Furrer, L.; Skoko, L.; Müller, N.; Gottstein, B.; Bittel, P. A Novel Multiplex Real-Time Polymerase Chain Reaction for the Molecular Diagnosis of Metacestode Infections in Human Patients. Clin. Microbiol. Infect. 2023, 29, 1451.e1–1451.e5. [Google Scholar] [CrossRef]
- Maksimov, P.; Bergmann, H.; Wassermann, M.; Romig, T.; Gottstein, B.; Casulli, A.; Conraths, F.J. Species Detection within the Echinococcus granulosus sensu lato Complex by Novel Probe-Based Real-Time PCRs. Pathogens 2020, 9, 791. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šufliarska, Z.; Tóth, Š.; Gentil, M.; Humeník, F. Alveolar Echinococcosis in 11-Month-Old Dog—Clinical Case. Pathogens 2025, 14, 450. https://doi.org/10.3390/pathogens14050450
Šufliarska Z, Tóth Š, Gentil M, Humeník F. Alveolar Echinococcosis in 11-Month-Old Dog—Clinical Case. Pathogens. 2025; 14(5):450. https://doi.org/10.3390/pathogens14050450
Chicago/Turabian StyleŠufliarska, Zuzana, Štefan Tóth, Michaela Gentil, and Filip Humeník. 2025. "Alveolar Echinococcosis in 11-Month-Old Dog—Clinical Case" Pathogens 14, no. 5: 450. https://doi.org/10.3390/pathogens14050450
APA StyleŠufliarska, Z., Tóth, Š., Gentil, M., & Humeník, F. (2025). Alveolar Echinococcosis in 11-Month-Old Dog—Clinical Case. Pathogens, 14(5), 450. https://doi.org/10.3390/pathogens14050450