Determination of Phylogroups, Pathotypes and Antibiotic Resistance Profiles of E. coli Isolates from Freshwater and Wastewater in the City of Panama
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of E. coli
2.3. Characterization of E. coli Isolates
2.4. DNA Extraction
2.5. Phylogenetic Group Determination of the E. coli Isolates
2.6. Determination of Pathotypes of E. coli Isolates
2.7. Detection of Quinolone-Resistance Mechanisms
3. Results
3.1. Identification of Phylogroups and Pathotypes
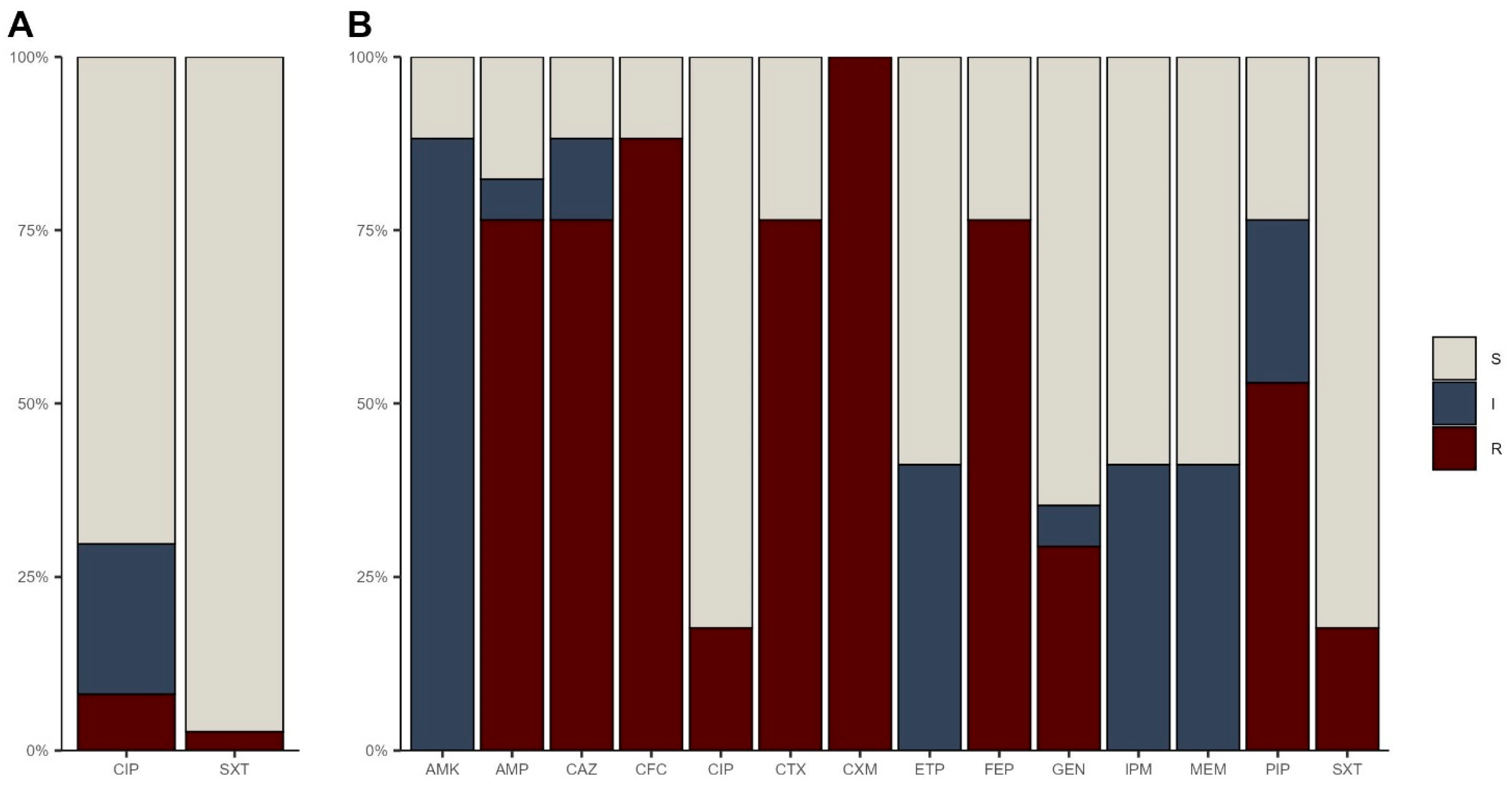
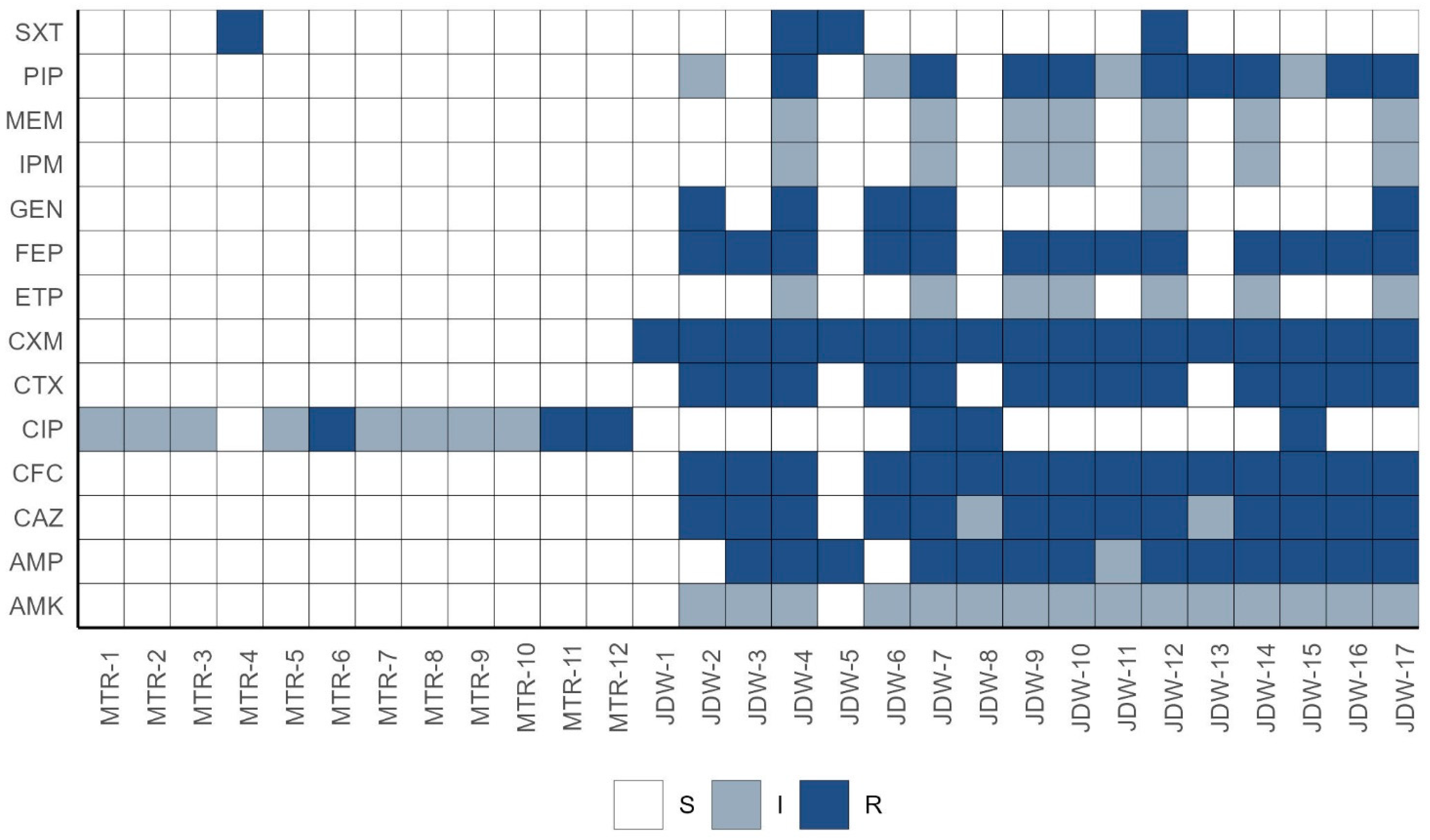
3.2. Antibiotic Resistance Profiles
3.2.1. Multidrug Resistance Profiles
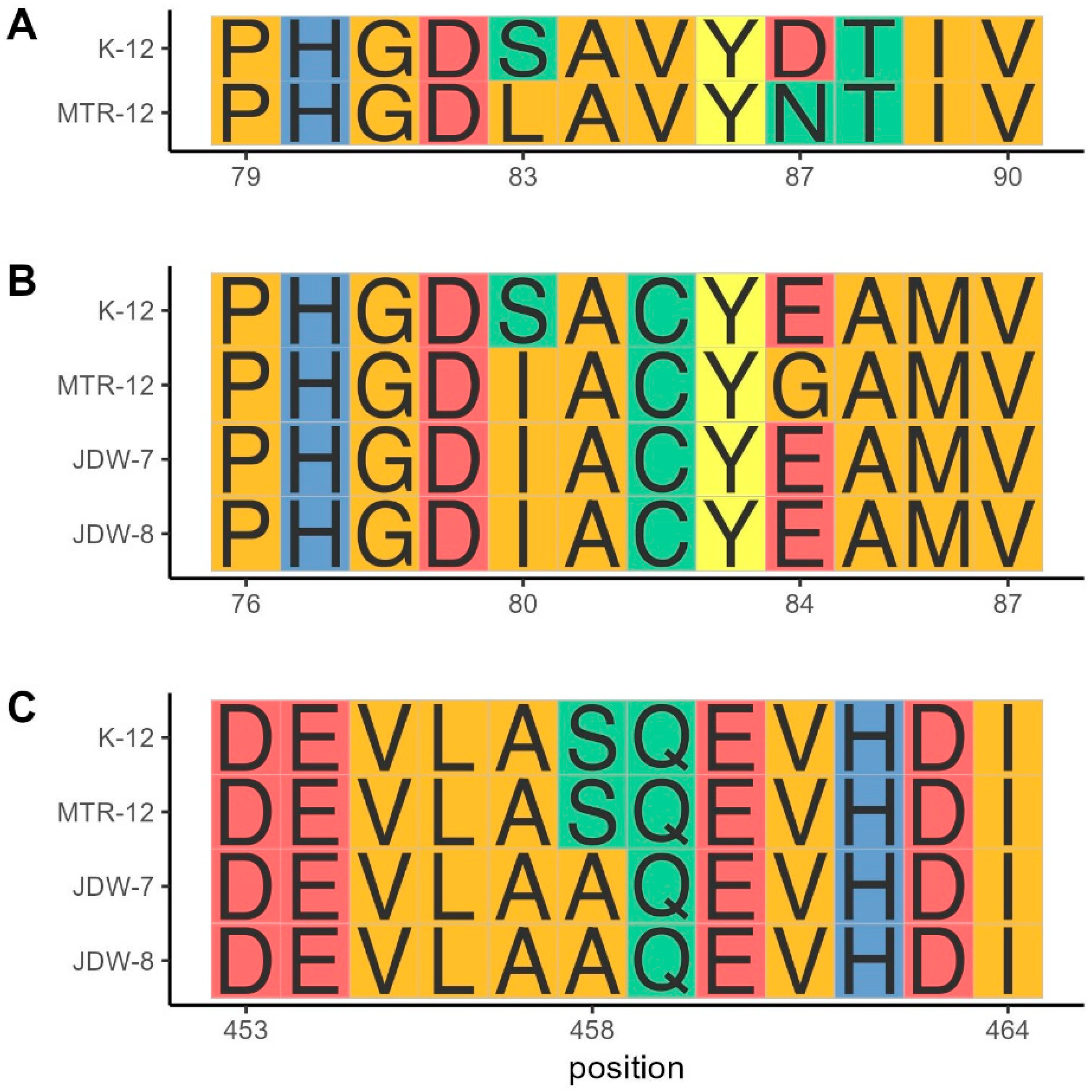
3.2.2. Quinolone Resistance Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.M.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoea in 195 Countries: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Jiwok, J.C.; Adebowale, A.S.; Wilson, I.; Kancherla, V.; Umeokonkwo, C.D. Patterns of Diarrhoeal Disease among Under-Five Children in Plateau State, Nigeria, 2013–2017. BMC Public Health 2021, 21, 2086. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.; Boisson, S.; Johnston, R.; Trouba, D.J.; Cumming, O. Unsafe Water, Sanitation and Hygiene: A Persistent Health Burden. Bull. World Health Organ. 2023, 101, 551-551A. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Ariyama, H.A.; García-Heredia, A.; Heredia, N.; García, S.; León, J.; Jaykus, L.; Solís-Soto, L. Phylogroups, Pathotypes, Biofilm Formation and Antimicrobial Resistance of Escherichia Coli Isolates in Farms and Packing Facilities of Tomato, Jalapeño Pepper and Cantaloupe from Northern Mexico. Int. J. Food Microbiol. 2019, 290, 96–104. [Google Scholar] [CrossRef]
- Halaji, M.; Fayyazi, A.; Rajabnia, M.; Zare, D.; Pournajaf, A.; Ranjbar, R. Phylogenetic Group Distribution of Uropathogenic Escherichia Coli and Related Antimicrobial Resistance Pattern: A Meta-Analysis and Systematic Review. Front. Cell. Infect. Microbiol. 2022, 12, 790184. [Google Scholar] [CrossRef]
- Morel-Journel, T.; Lehtinen, S.; Cotto, O.; Amia, R.; Clermont, O.; Dion, S.; Figueroa, C.; Martinson, J.; Ralaimazava, P.; Duval, X.; et al. Residence-Colonization Trade-off and Niche Differentiation Enable the Coexistence of Escherichia Coli Phylogroups in Healthy Humans. bioRxiv 2024. [Google Scholar] [CrossRef]
- Machado, M.; Panzenhagen, P.; Aburjaile, F.F.; Brenig, B.; da Costa, M.M.; Azevedo, V.A.d.C.; de Souza Figueiredo, E.E.; Conte-Junior, C.A. Evolution of Pathogenic Escherichia Coli Harboring the Transmissible Locus of Stress Tolerance: From Food Sources to Clinical Environments. Sci. Rep. 2025, 15, 5014. [Google Scholar] [CrossRef]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia Coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef]
- Saka, H.K.; Dabo, N.T.; Muhammad, B.; García-Soto, S.; Ugarte-Ruiz, M.; Alvarez, J. Diarrheagenic Escherichia Coli Pathotypes From Children Younger Than 5 Years in Kano State, Nigeria. Front. Public Health 2019, 7, 348. [Google Scholar] [CrossRef]
- Robert, B.; Richard, J.; Shane, K.; Attila, H.; Tom, S. Monitoring Drinking Water Quality in Nationally Representative Household Surveys in Low- and Middle-Income Countries: Cross-Sectional Analysis of 27 Multiple Indicator Cluster Surveys 2014–2020. Environ. Health Perspect. 2025, 129, 97010. [Google Scholar] [CrossRef]
- Abdulhadi, R.; Bailey, A.; Van Noorloos, F. Access Inequalities to WASH and Housing in Slums in Low- and Middle-Income Countries (LMICs): A Scoping Review. Glob. Public Health 2024, 19, 2369099. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G. Escherichia Coli Diseases in Latin America—A ‘One Health’ Multidisciplinary Approach. Pathog. Dis. 2017, 75, ftx012. [Google Scholar] [CrossRef] [PubMed]
- Canizalez-Roman, A.; Velazquez-Roman, J.; Valdez-Flores, M.A.; Flores-Villaseñor, H.; Vidal, J.E.; Muro-Amador, S.; Guadrón-Llanos, A.M.; Gonzalez-Nuñez, E.; Medina-Serrano, J.; Tapia-Pastrana, G.; et al. Detection of Antimicrobial-Resistance Diarrheagenic Escherichia Coli Strains in Surface Water Used to Irrigate Food Products in the Northwest of Mexico. Int. J. Food Microbiol. 2019, 304, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aijuka, M.; Santiago, A.E.; Girón, J.A.; Nataro, J.P.; Buys, E.M. Enteroaggregative Escherichia Coli Is the Predominant Diarrheagenic E. Coli Pathotype among Irrigation Water and Food Sources in South Africa. Int. J. Food Microbiol. 2018, 278, 44–51. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.S.; Kim, S.; Shin, E.; Oh, K.-H.; Kim, Y.; Kim, C.H.; Hwang, M.A.; Jin, C.M.; Na, K.; et al. A Waterborne Outbreak of Multiple Diarrhoeagenic Escherichia Coli Infections Associated with Drinking Water at a School Camp. Int. J. Infect. Dis. 2018, 66, 45–50. [Google Scholar] [CrossRef]
- Lim, M.-A.; Kim, J.-Y.; Acharya, D.; Bajgain, B.B.; Park, J.-H.; Yoo, S.-J.; Lee, K. A Diarrhoeagenic Enteropathogenic Escherichia Coli (EPEC) Infection Outbreak That Occurred among Elementary School Children in Gyeongsangbuk-Do Province of South Korea Was Associated with Consumption of Water-Contaminated Food Items. Int. J. Environ. Res. Public Health 2020, 17, 3149. [Google Scholar] [CrossRef]
- Zelelie, T.Z.; Eguale, T.; Yitayew, B.; Abeje, D.; Alemu, A.; Seman, A.; Jass, J.; Mihret, A.; Abebe, T. Molecular Epidemiology and Antimicrobial Susceptibility of Diarrheagenic Escherichia Coli Isolated from Children under Age Five with and without Diarrhea in Central Ethiopia. PLoS ONE 2023, 18, e0288517. [Google Scholar] [CrossRef]
- Tapia-Pastrana, G.; Rojas-Bautista, M.; Hernández-Pérez, P.; Santiago-Martínez, O.; Gómez-Rodríguez, L.C.; Terrazas-Luna, V.M.; Montes-Yedra, J.; Bautista-Avendaño, A.A.; García-López, E.S.; Leon-Sicairos, N.; et al. Virulence Genes, Antimicrobial Resistance Profile, Phylotyping and Pathotyping of Diarrheagenic Escherichia Coli Isolated from Children in Southwest Mexico. PLoS ONE 2024, 19, e0300304. [Google Scholar] [CrossRef]
- Angulo-Zamudio, U.A.; Gutiérrez-Jiménez, J.; Monroy-Higuera, L.; Flores-Villaseñor, H.; Leon-Sicairos, N.; Velazquez-Roman, J.; Vidal, J.E.; Tapia-Pastrana, G.; Canizalez-Roman, A. Non-Diarrheagenic and Diarrheagenic E. Coli Carrying Supplementary Virulence Genes (SVG) Are Associated with Diarrhea in Children from Mexico. Microb. Pathog. 2021, 157, 104994. [Google Scholar] [CrossRef]
- Han, H.; Li, W.; Liu, J.; Zhang, X.; Huo, X.; Sun, Y.; Chen, J.; Fan, R.; Zhang, J.; Chen, Y.; et al. Seven-Year Overview of Antimicrobial Resistance in Diarrheagenic Escherichia Coli from Sporadic Human Diarrhea Cases in 20 Chinese Provinces. One Health Adv. 2024, 2, 29. [Google Scholar] [CrossRef]
- Salleh, M.Z.; Nik Zuraina, N.M.N.; Hajissa, K.; Ilias, M.I.; Deris, Z.Z. Prevalence of Multidrug-Resistant Diarrheagenic Escherichia Coli in Asia: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Bhardwaj, R.; Mishra, P.; Rajput, S.K. Antimicrobials Misuse/Overuse: Adverse Effect, Mechanism, Challenges and Strategies to Combat Resistance. Open Biotechnol. J. 2020, 14, 107–112. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; American Water Works Association: Washington, DC, USA, 2023. [Google Scholar]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and Simple Determination of the Escherichia Coli Phylogenetic Group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Cheng, P.; Rondeau, G.; Porwollik, S.; McClelland, M. A Single Step Multiplex PCR for Identification of Six Diarrheagenic E. Coli Pathotypes and Salmonella. Int. J. Med. Microbiol. 2013, 303, 210–216. [Google Scholar] [CrossRef]
- Le, T.A.H.; Fabre, L.; Roumagnac, P.; Grimont, P.A.; Scavizzi, M.R.; Weill, F.X. Clonal Expansion and Microevolution of Quinolone-Resistant Salmonella Enterica Serotype Typhi in Vietnam from 1996 to 2004. J. Clin. Microbiol. 2007, 45, 3485–3492. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Shang, X.; Wang, L.; Li, H.; Wang, X. Characteristics of Quinolone-Resistant Escherichia Coli Isolated from Bovine Mastitis in China. J. Dairy Sci. 2018, 101, 6244–6252. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, T.; Xu, S.; Gao, F.; Lam, T.T.; Wang, Q.; Wu, T.; Huang, H.; Zhan, L.; Li, L.; et al. Ggmsa: A Visual Exploration Tool for Multiple Sequence Alignment and Associated Data. Brief. Bioinform. 2022, 23, bbac222. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Miró, E.; Ortega, A.; Bou, G.; González-López, J.J.; Oliver, A.; Pascual, A.; Cercenado, E.; Oteo, J.; Martínez-Martínez, L.; et al. Molecular Identification of Aminoglycoside-Modifying Enzymes in Clinical Isolates of Escherichia Coli Resistant to Amoxicillin/Clavulanic Acid Isolated in Spain. Int. J. Antimicrob. Agents 2015, 46, 157–163. [Google Scholar] [CrossRef]
- Salah, F.D.; Soubeiga, S.T.; Ouattara, A.K.; Sadji, A.Y.; Metuor-Dabire, A.; Obiri-Yeboah, D.; Banla-Kere, A.; Karou, S.; Simpore, J. Distribution of Quinolone Resistance Gene (Qnr) in ESBL-Producing Escherichia Coli and Klebsiella spp. in Lomé, Togo. Antimicrob. Resist. Infect. Control 2019, 8, 104. [Google Scholar] [CrossRef]
- Aguirre-Sánchez, J.R.; Valdez-Torres, J.B.; del Campo, N.C.; Martínez-Urtaza, J.; del Campo, N.C.; Lee, B.G.; Quiñones, B.; Chaidez-Quiroz, C. Phylogenetic Group and Virulence Profile Classification in Escherichia Coli from Distinct Isolation Sources in Mexico. Infect. Genet. Evol. 2022, 106, 105380. [Google Scholar] [CrossRef]
- Redha, M.A.; Al Sweih, N.; Albert, M.J. Virulence and Phylogenetic Groups of Escherichia Coli Cultured from Raw Sewage in Kuwait. Gut Pathog. 2022, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.; Bordalo, A.A.; Machado, A. Characterization of Escherichia Coli Isolates in Recreational Waters: Implications for Public Health and One Health Approach. Water 2024, 16, 2695. [Google Scholar] [CrossRef]
- Stoppe, N.d.C.; Silva, J.S.; Carlos, C.; Sato, M.I.Z.; Saraiva, A.M.; Ottoboni, L.M.M.; Torres, T.T. Worldwide Phylogenetic Group Patterns of Escherichia Coli from Commensal Human and Wastewater Treatment Plant Isolates. Front. Microbiol. 2017, 8, 2512. [Google Scholar] [CrossRef] [PubMed]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The Population Genetics of Pathogenic Escherichia Coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- Hemati, S.; Halimi, S.; Jabalameli, F.; Emaneini, M.; Beigverdi, R. Phylogenetic Group, Antibiotic Resistance, Virulence Gene, and Genetic Diversity of Escherichia Coli Causing Bloodstream Infections in Iran. Front. Microbiol. 2024, 15, 1426510. [Google Scholar] [CrossRef]
- Singh, N.S.; Singhal, N.; Kumar, M.; Virdi, J.S. High Prevalence of Drug Resistance and Class 1 Integrons in Escherichia Coli Isolated from River Yamuna, India: A Serious Public Health Risk. Front. Microbiol. 2021, 12, 621564. [Google Scholar] [CrossRef]
- Bong, C.W.; Low, K.Y.; Chai, L.C.; Lee, C.W. Prevalence and Diversity of Antibiotic Resistant Escherichia Coli From Anthropogenic-Impacted Larut River. Front. Public Health 2022, 10, 794513. [Google Scholar] [CrossRef]
- Tawfick, M.M.; Elshamy, A.A.; Mohamed, K.T.; El Menofy, N.G. Gut Commensal Escherichia Coli, a High-Risk Reservoir of Transferable Plasmid-Mediated Antimicrobial Resistance Traits. Infect. Drug Resist. 2022, 15, 1077–1091. [Google Scholar] [CrossRef]
- Correa-Martinez, C.L.; Leopold, S.R.; Köck, R.; Kossow, A.; Bauwens, A.; Mellmann, A. Enterohemorrhagic E. coli (EHEC): Environmental-Vehicle-Human Interface BT—Zoonoses: Infections Affecting Humans and Animals; Sing, A., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 355–372. ISBN 978-3-031-27164-9. [Google Scholar]
- Ogura, Y.; Seto, K.; Morimoto, Y.; Nakamura, K.; Sato, M.; Gotoh, Y.; Itoh, T.; Toyoda, A.; Ohnishi, M.; Hayashi, T. Genomic Characterization of β-Glucuronidase–Positive Escherichia coli O157:H7 Producing Stx2a. Emerg. Infect. Dis. J. 2018, 24, 2219. [Google Scholar] [CrossRef]
- Moreno, J.; Conte, E.; Tribaldos, M.; Morales, Y.; Zamorano, C.; Gómez, B.; Toro, J. Antibiotic Resistance Profiles in Panama: Trends from 2007 to 2013. Pharm. Pharmacol. Int. J. 2018, 6, 350–355. [Google Scholar] [CrossRef][Green Version]
- Hu, H.; Da, X.; Li, Z.; Li, T.; Zhang, X.; Bian, T.; Jin, Y.; Xu, K.; Guo, Y. Determination and Ecological Risk Assessment of Quinolone Antibiotics in Drinking and Environmental Waters Using Fully Automated Disk-Based SPE Coupled with UPLC-MS/MS. Molecules 2024, 29, 4611. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Chatterjee, S. Fluoroquinolone Antibiotics: Occurrence, Mode of Action, Resistance, Environmental Detection, and Remediation—A Comprehensive Review. Environ. Pollut. 2022, 315, 120440. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.G.; Diez-Santos, I.; Sankara Krishna, P.; Clavijo, B.; Maxwell, A. Insights into Antibiotic Resistance Promoted by Quinolone Exposure. Antimicrob. Agents Chemother. 2024, 69, e00997-24. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Saha, S.R.; Islam, M.R.; Haider, S.M.A.; Hossain, M.I.; Chowdhury, A.S.M.H.K.; Rousham, E.K.; Islam, M.A. High Prevalence of Plasmid-Mediated Quinolone Resistance (PMQR) among E. Coli from Aquatic Environments in Bangladesh. PLoS ONE 2021, 16, e0261970. [Google Scholar] [CrossRef]
- Amato, M.; Dasí, D.; González, A.; Ferrús, M.A.; Castillo, M.Á. Occurrence of Antibiotic Resistant Bacteria and Resistance Genes in Agricultural Irrigation Waters from Valencia City (Spain). Agric. Water Manag. 2021, 256, 107097. [Google Scholar] [CrossRef]
- Amarasiri, M.; Takezawa, T.; Malla, B.; Furukawa, T.; Sherchand, J.B.; Haramoto, E.; Sei, K. Prevalence of Antibiotic Resistance Genes in Drinking and Environmental Water Sources of the Kathmandu Valley, Nepal. Front. Microbiol. 2022, 13, 894014. [Google Scholar] [CrossRef]
- Beshiru, A.; Isokpehi, N.A.; Igbinosa, I.H.; Akinnibosun, O.; Ogofure, A.G.; Igbinosa, E.O. Extended-Spectrum Beta-Lactamase (ESBL)- and Non-ESBL Producing Escherichia Coli Surveillance in Surface Water Sources in Edo State, Nigeria: A Public Health Concern. Sci. Rep. 2024, 14, 21658. [Google Scholar] [CrossRef]
- Naveen Kumar, V.; Saikumar, C. Prevalence of Quinolone-Resistance Uropathogenic Escherichia Coli in a Tertiary Care Hospital in South Iran. Indian J. Public Health Res. Dev. 2019, 10, 2609–2613. [Google Scholar] [CrossRef]
- Zhao, Y.-C.; Sun, Z.-H.; Xiao, M.-X.; Li, J.-K.; Liu, H.; Cai, H.-L.; Cao, W.; Feng, Y.; Zhang, B.-K.; Yan, M. Analyzing the Correlation between Quinolone-Resistant Escherichia Coli Resistance Rates and Climate Factors: A Comprehensive Analysis across 31 Chinese Provinces. Environ. Res. 2024, 245, 117995. [Google Scholar] [CrossRef]
- Yanat, B.; Rodríguez-Martínez, J.-M.; Touati, A. Plasmid-Mediated Quinolone Resistance in Enterobacteriaceae: A Systematic Review with a Focus on Mediterranean Countries. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 421–435. [Google Scholar] [CrossRef]
- Pallecchi, L.; Riccobono, E.; Mantella, A.; Bartalesi, F.; Sennati, S.; Gamboa, H.; Gotuzzo, E.; Bartoloni, A.; Rossolini, G.M. High Prevalence of Qnr Genes in Commensal Enterobacteria from Healthy Children in Peru and Bolivia. Antimicrob. Agents Chemother. 2009, 53, 2632–2635. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Delgado Suárez, E.J.; Bonelli, R.R.; Oliveira, C.J.B.; Moreno-Switt, A.I.; Adell, A.D.; Reyes-Jara, A.; Grim, C.J.; Allard, M.W.; Tallent, S.M.; et al. Exploring the Genomic and Antimicrobial Resistance Tapestry: Comparative Insights into Salmonella Enterica Serotypes Agona, Braenderup, Muenchen, and Panama in Latin American Surface Waters. Microbiol. Spectr. 2024, 13, e01706-24. [Google Scholar] [CrossRef] [PubMed]
- Chiluisa Guacho, C.; Gómez Martínez, N.; Vilema Vizuete, E.G.; Dutra Asensi, M. The first report of the qnrB19 and aac(6’)-Ib-cr in isolates uropathogenic Escherichia coli ciprofloxacin-resistant in Ecuador. Interam. J. Health Sci. 2024, 4, 179. [Google Scholar] [CrossRef]
- Furmanek-Blaszk, B.; Sektas, M.; Rybak, B. High Prevalence of Plasmid-Mediated Quinolone Resistance among ESBL/AmpC-Producing Enterobacterales from Free-Living Birds in Poland. Int. J. Mol. Sci. 2023, 24, 12804. [Google Scholar] [CrossRef]
- Tyson, G.H.; Li, C.; Hsu, C.-H.; Bodeis-Jones, S.; McDermott, P.F. Diverse Fluoroquinolone Resistance Plasmids From Retail Meat E. Coli in the United States. Front. Microbiol. 2019, 10, 2826. [Google Scholar] [CrossRef]
- Laurent, P.; Jean-Yves, M.; Agnese, L.; Anne-Kathrin, S.; Nicolas, K.; Patrice, N.; Stefan, S. Antimicrobial Resistance in Escherichia Coli. Microbiol. Spectr. 2018, 6, 10-1128. [Google Scholar] [CrossRef]
- Moreno-Switt, A.I.; Pezoa, D.; Sepúlveda, V.; González, I.; Rivera, D.; Retamal, P.; Navarrete, P.; Reyes-Jara, A.; Toro, M. Transduction as a Potential Dissemination Mechanism of a Clonal QnrB19-Carrying Plasmid Isolated from Salmonella of Multiple Serotypes and Isolation Sources. Front. Microbiol. 2019, 10, 2503. [Google Scholar] [CrossRef]
- Thanh, H.H.T.; Mayumi, Y.; Manuel, C.; Carlos, B.-C.; Yoshimasa, Y. Chromosomal QnrB19-Carrying Escherichia Coli Isolated from the Stool Sample of a Community Resident in Ecuador. Microbiol. Resour. Announc. 2024, 13, e00046-24. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, J.; Bae, K.-S.; Lee, H.; Kim, J.; Park, E.-R.; Yoon, J.-K.; Lee, S.-H. Evaluation and Characterization of Quinolone-Resistant Escherichia Coli in Wastewater Treatment Plant Effluents. Water 2023, 15, 4040. [Google Scholar] [CrossRef]
- Al-Mustapha, A.I.; Tiwari, A.; Laukkanen-Ninios, R.; Lehto, K.-M.; Oikarinen, S.; Lipponen, A.; Pitkänen, T.; Heikinheimo, A.; Heljanko, V.; Johansson, V.; et al. Wastewater Based Genomic Surveillance Key to Population Level Monitoring of AmpC/ESBL Producing Escherichia Coli. Sci. Rep. 2025, 15, 7400. [Google Scholar] [CrossRef]
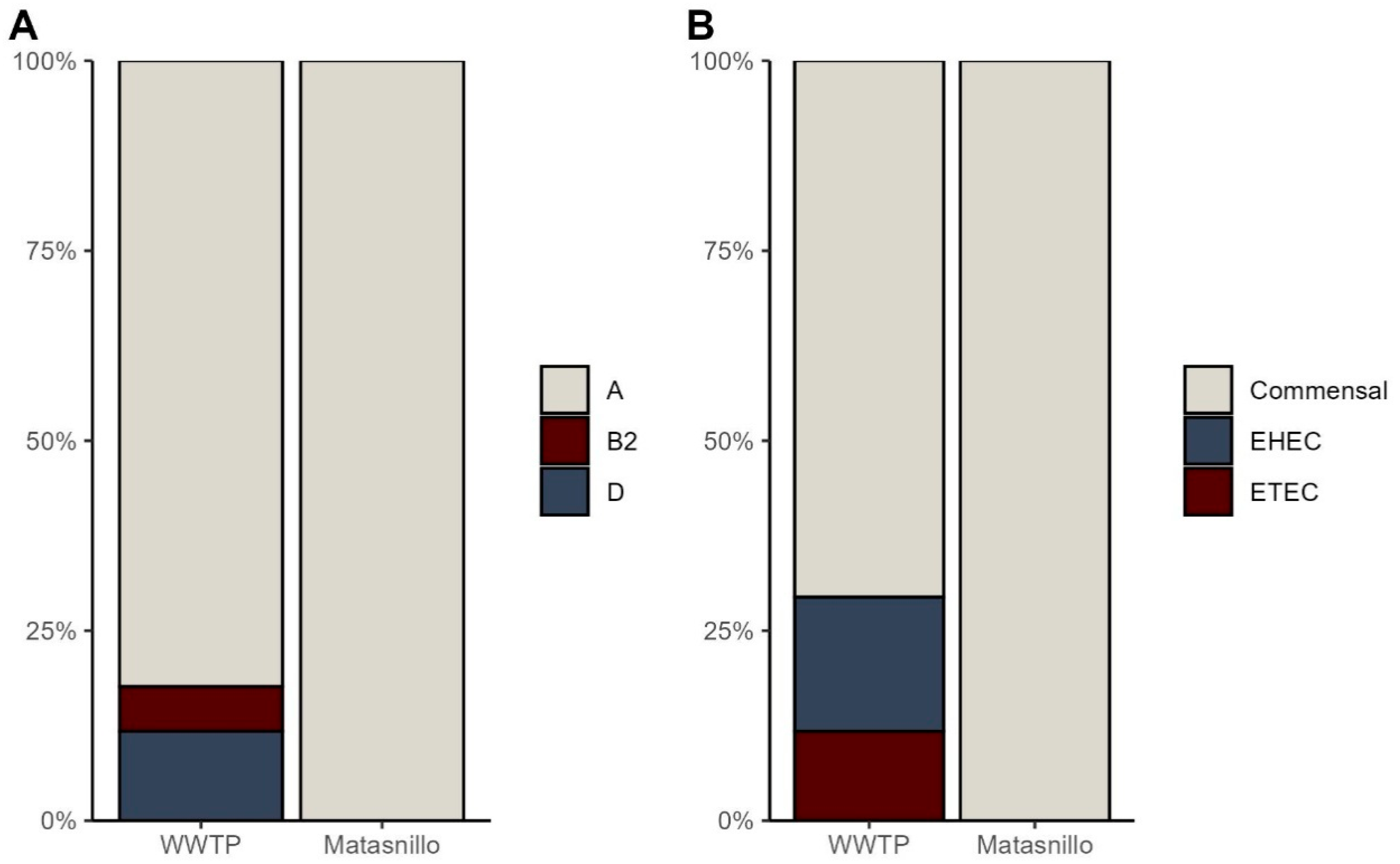
| Pathotype | Gene Target | Multiplex PCR 1 | Multiplex PCR 2 |
|---|---|---|---|
| EPEC | eae, bfpB | eae, bfpB | |
| EHEC | eae, stx1, stx2 | stx2 | stx1 |
| EIEC | virF, ipaH | virF | ipaH |
| DAEC | daaE | daaE | |
| ETEC | estB1 | estB1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Guevara, G.A.; Michelangelli, E.; Medina-Sánchez, J.R.; Mejía-Meléndez, F.; Espino, C.I.; Moreno P., J.E.; Martínez Torres, A.O.; Querol-Audí, J. Determination of Phylogroups, Pathotypes and Antibiotic Resistance Profiles of E. coli Isolates from Freshwater and Wastewater in the City of Panama. Pathogens 2025, 14, 617. https://doi.org/10.3390/pathogens14070617
Rodríguez Guevara GA, Michelangelli E, Medina-Sánchez JR, Mejía-Meléndez F, Espino CI, Moreno P. JE, Martínez Torres AO, Querol-Audí J. Determination of Phylogroups, Pathotypes and Antibiotic Resistance Profiles of E. coli Isolates from Freshwater and Wastewater in the City of Panama. Pathogens. 2025; 14(7):617. https://doi.org/10.3390/pathogens14070617
Chicago/Turabian StyleRodríguez Guevara, Gabriela A., Emmanuel Michelangelli, Juan R. Medina-Sánchez, Fermín Mejía-Meléndez, Carmen Indira Espino, José E. Moreno P., Alex O. Martínez Torres, and Jordi Querol-Audí. 2025. "Determination of Phylogroups, Pathotypes and Antibiotic Resistance Profiles of E. coli Isolates from Freshwater and Wastewater in the City of Panama" Pathogens 14, no. 7: 617. https://doi.org/10.3390/pathogens14070617
APA StyleRodríguez Guevara, G. A., Michelangelli, E., Medina-Sánchez, J. R., Mejía-Meléndez, F., Espino, C. I., Moreno P., J. E., Martínez Torres, A. O., & Querol-Audí, J. (2025). Determination of Phylogroups, Pathotypes and Antibiotic Resistance Profiles of E. coli Isolates from Freshwater and Wastewater in the City of Panama. Pathogens, 14(7), 617. https://doi.org/10.3390/pathogens14070617







