Impact of Empirical and Definitive Antibiotics on Pediatric Febrile Urinary Tract Infection Caused by ESBL-Producing Enterobacterales
Abstract
1. Introduction
2. Materials and Methods
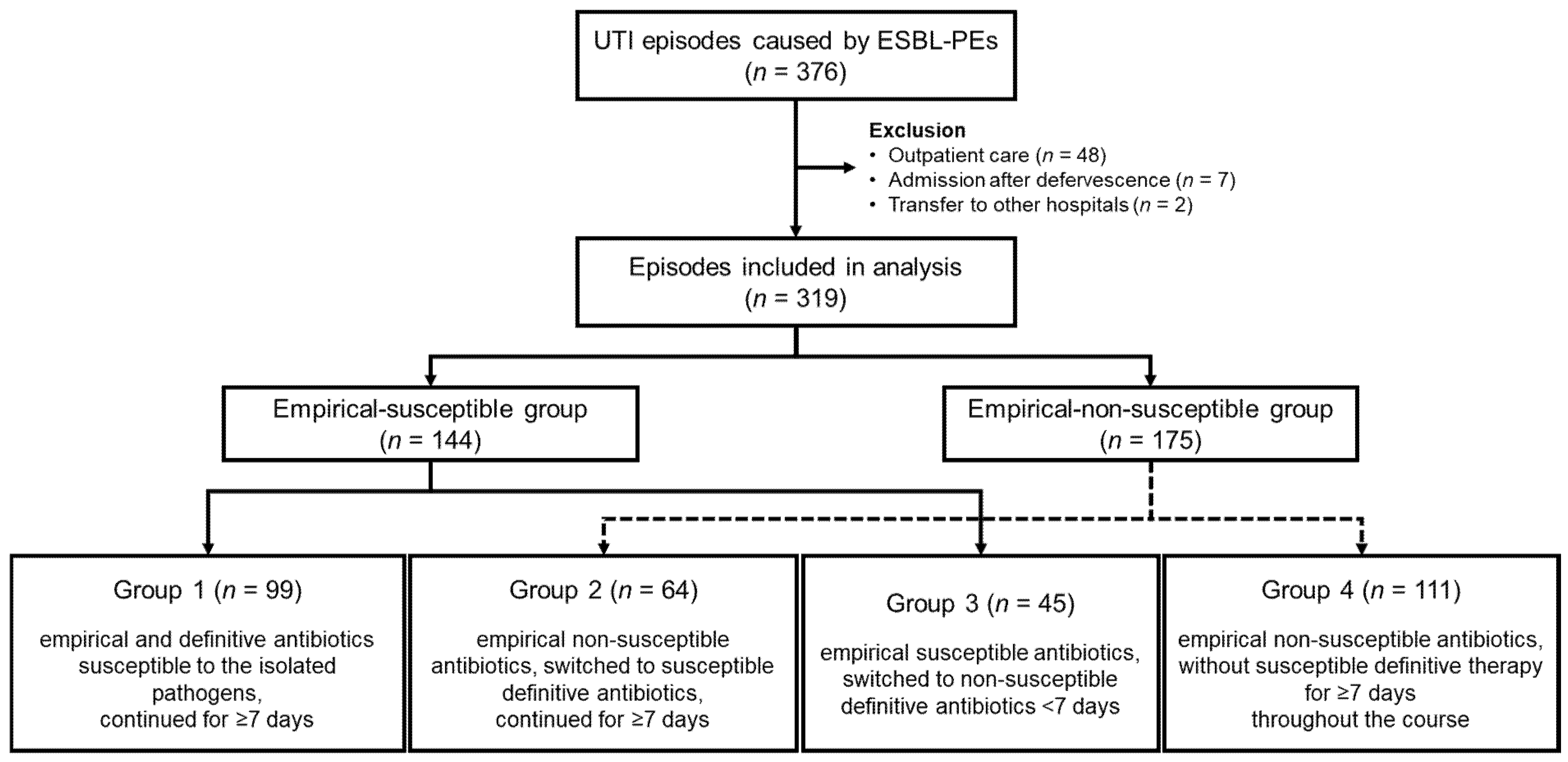
2.1. Patients and Study Design
- Group 1: Empirical and definitive therapy with antibiotics to which the isolated pathogens were susceptible, continued for ≥7 days.
- Group 2: Empirical therapy started with non-susceptible antibiotics, but switched to susceptible definitive antibiotics, administered for ≥7 days.
- Group 3: Empirical therapy started with susceptible antibiotics, but switched to non-susceptible definitive antibiotics within 7 days.
- Group 4: Empirical therapy started with non-susceptible antibiotics, and susceptible antibiotics were not administered for ≥7 days during the entire treatment course, regardless of the definitive regimen.
2.2. Microbiological Testing
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Children with UTI Caused by ESBL-Producing Enterobacterales
3.2. Antibiotic Effects According to Empirical and Definitive Antibiotics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UTI | Urinary tract infection |
| AAP | American Academy of Pediatrics |
| ESBL | Extended-spectrum β-lactamase |
| ESBL-PE | ESBL-producing Enterobacterales |
| VUR | Vesicoureteral reflux |
| TMP-SMX | Trimethoprim-sulfamethoxazole |
| IDSA | Infectious Diseases Society of America |
| ESCMID | European Society of Clinical Microbiology and Infectious Diseases |
References
- Shaikh, N.; Morone, N.E.; Bost, J.E.; Farrell, M.H. Prevalence of urinary tract infection in childhood: A meta-analysis. Pediatr. Infect. Dis. J. 2008, 27, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Subcommittee on Urinary Tract Infection; Steering Committee on Quality Improvement and Management; Roberts, K.B. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011, 128, 595–610. [Google Scholar] [CrossRef]
- Flokas, M.E.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Prevalence of ESBL-producing Enterobacteriaceae in paediatric urinary tract infections: A systematic review and meta-analysis. J. Infect. 2016, 73, 547–557. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 guidance on the treatment of antimicrobial resistant Gram-negative infections. Clin. Infect. Dis. 2023, ciad428. [Google Scholar] [CrossRef]
- Gutierrez-Gutierrez, B.; Rodriguez-Bano, J. Current options for the treatment of infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae in different groups of patients. Clin. Microbiol. Infect. 2019, 25, 932–942. [Google Scholar] [CrossRef]
- van Loon, K.; Voor In’t Holt, A.F.; Vos, M.C. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e01730-17. [Google Scholar] [CrossRef]
- Romandini, A.; Pani, A.; Schenardi, P.A.; Pattarino, G.A.C.; De Giacomo, C.; Scaglione, F. Antibiotic resistance in pediatric infections: Global emerging threats, predicting the near future. Antibiotics 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.S.; Kim, J.H.; Cho, M.H.; Park, E.; Ha, I.S.; Cheong, H.I.; Kang, H.G. Low relapse rate of urinary tract infections from extended-spectrum beta-lactamase-producing bacteria in young children. Pediatr. Nephrol. 2019, 34, 2399–2407. [Google Scholar] [CrossRef]
- Topaloglu, R.; Er, I.; Dogan, B.G.; Bilginer, Y.; Ozaltin, F.; Besbas, N.; Ozen, S.; Bakkaloglu, A.; Gur, D. Risk factors in community-acquired urinary tract infections caused by ESBL-producing bacteria in children. Pediatr. Nephrol. 2010, 25, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Vazouras, K.; Hisa, Y.; Folgori, L.; Bielicki, J.; Aguadisch, E.; Bamford, A.; Brett, A.; Caseris, M.; Cerkauskiene, R.; De Luca, M.; et al. Treatment and outcomes of children with febrile urinary tract infection due to extended spectrum beta-lactamase-producing bacteria in Europe: TOO CUTE Study. Pediatr. Infect. Dis. J. 2020, 39, 1081–1087. [Google Scholar] [CrossRef]
- Greenhouse, I.; Babushkin, F.; Finn, T.; Shimoni, Z.; Aliman, M.; Ben-Ami, R.; Cohen, R. Long-term outcomes of inappropriate antibiotic therapy for upper urinary tract infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: A retrospective cohort study. Diagn. Microbiol. Infect. Dis. 2017, 89, 222–229. [Google Scholar] [CrossRef]
- Chan, E.Y. Community-acquired urinary tract infections caused by ESBL-producing Enterobacteriaceae in infants less than 2 years of age. Pediatr. Nephrol. 2022, 37, 1167–1168. [Google Scholar] [CrossRef]
- Jo, K.J.; Yoo, S.; Song, J.Y.; Kim, S.H.; Park, S.E. Non-carbapenem antimicrobial therapy in young infant with urinary tract infections caused by community-acquired extended-spectrum beta-lactamase-producing Escherichia coli. Pediatr. Neonatol. 2021, 62, 271–277. [Google Scholar] [CrossRef]
- Madhi, F.; Jung, C.; Timsit, S.; Levy, C.; Biscardi, S.; Lorrot, M.; Grimprel, E.; Hees, L.; Craiu, I.; Galerne, A.; et al. Febrile urinary-tract infection due to extended-spectrum beta-lactamase-producing Enterobacteriaceae in children: A French prospective multicenter study. PLoS ONE 2018, 13, e0190910. [Google Scholar] [CrossRef] [PubMed]
- Tratselas, A.; Iosifidis, E.; Ioannidou, M.; Saoulidis, S.; Kollios, K.; Antachopoulos, C.; Sofianou, D.; Roilides, E.J. Outcome of urinary tract infections caused by extended spectrum beta-lactamase-producing Enterobacteriaceae in children. Pediatr. Infect. Dis. J. 2011, 30, 707–710. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standard for Antimicrobial Susceptibility Testing, 20th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Wong, S.W.; Tullus, K.; Chan, Y.H.E. Controversies in treating febrile infantile urinary tract infection caused by extended-spectrum beta-lactamase producing Enterobacteriaceae: An international multi-centre survey. Pediatr. Nephrol. 2025, 40, 2253–2266. [Google Scholar] [CrossRef]
- Rianthavorn, P.; Siripen, N.; Kunnaruk, K. Cephalosporins for first febrile urinary tract infection from Enterobacteriaceae with antimicrobial resistance in young children. Acta Paediatr. 2021, 110, 2230–2232. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ahn, Y.H.; Lee, J.W.; Park, E. Clinical outcomes of non-carbapenem treatment for urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Child. Kidney Dis. 2021, 25, 22–28. [Google Scholar] [CrossRef]
- Toubiana, J.; Timsit, S.; Ferroni, A.; Grasseau, M.; Nassif, X.; Lortholary, O.; Zahar, J.R.; Chalumeau, M. Community-onset extended-spectrum beta-lactamase-producing Enterobacteriaceae invasive infections in children in a university hospital in France. Medicine 2016, 95, e3163. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, S.M.; Chang, Y.K.; Lee, D.G.; Cho, S.Y.; Lee, H.J.; Choi, J.H.; Yoo, J.H. The efficacy of non-carbapenem antibiotics for the treatment of community-onset acute pyelonephritis due to extended-spectrum beta-lactamase-producing Escherichia coli. J. Antimicrob. Chemother. 2014, 69, 2848–2856. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tangden, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Ziv-On, E.; Friger, M.D.; Saidel-Odes, L.; Borer, A.; Shimoni, O.; Nikonov, A.; Nesher, L. Impact of an antibiotic stewardship program on the incidence of resistant Escherichia coli: A quasi-experimental study. Antibiotics 2021, 10, 179. [Google Scholar] [CrossRef]
- Kim, Y.A.; Park, Y.S.; Youk, T.; Lee, H.; Lee, K. Trends in South Korean antimicrobial use and association with changes in Escherichia coli resistance rates: 12-year ecological study using a nationwide surveillance and antimicrobial prescription database. PLoS ONE 2018, 13, e0209580. [Google Scholar] [CrossRef]
- Al-Tamimi, M.; Abu-Raideh, J.; Albalawi, H.; Shalabi, M.; Saleh, S. Effective oral combination treatment for extended-spectrum beta-lactamase-producing Escherichia coli. Microb. Drug Resist. 2019, 25, 1132–1141. [Google Scholar] [CrossRef]
- Ouellet-Pelletier, J.; Guimont, C.; Gauthier, M.; Gravel, J. Adverse events following diagnostic urethral catheterization in the pediatric emergency department. Can. J. Emerg. Med. 2016, 18, 437–442. [Google Scholar] [CrossRef][Green Version]
- Tullus, K.; Shaikh, N. Urinary tract infections in children. Lancet 2020, 395, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum beta-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, J.H.; Lee, Y. Virulence factors associated with Escherichia coli bacteremia and urinary tract infection. Ann. Lab. Med. 2022, 42, 203–212. [Google Scholar] [CrossRef]
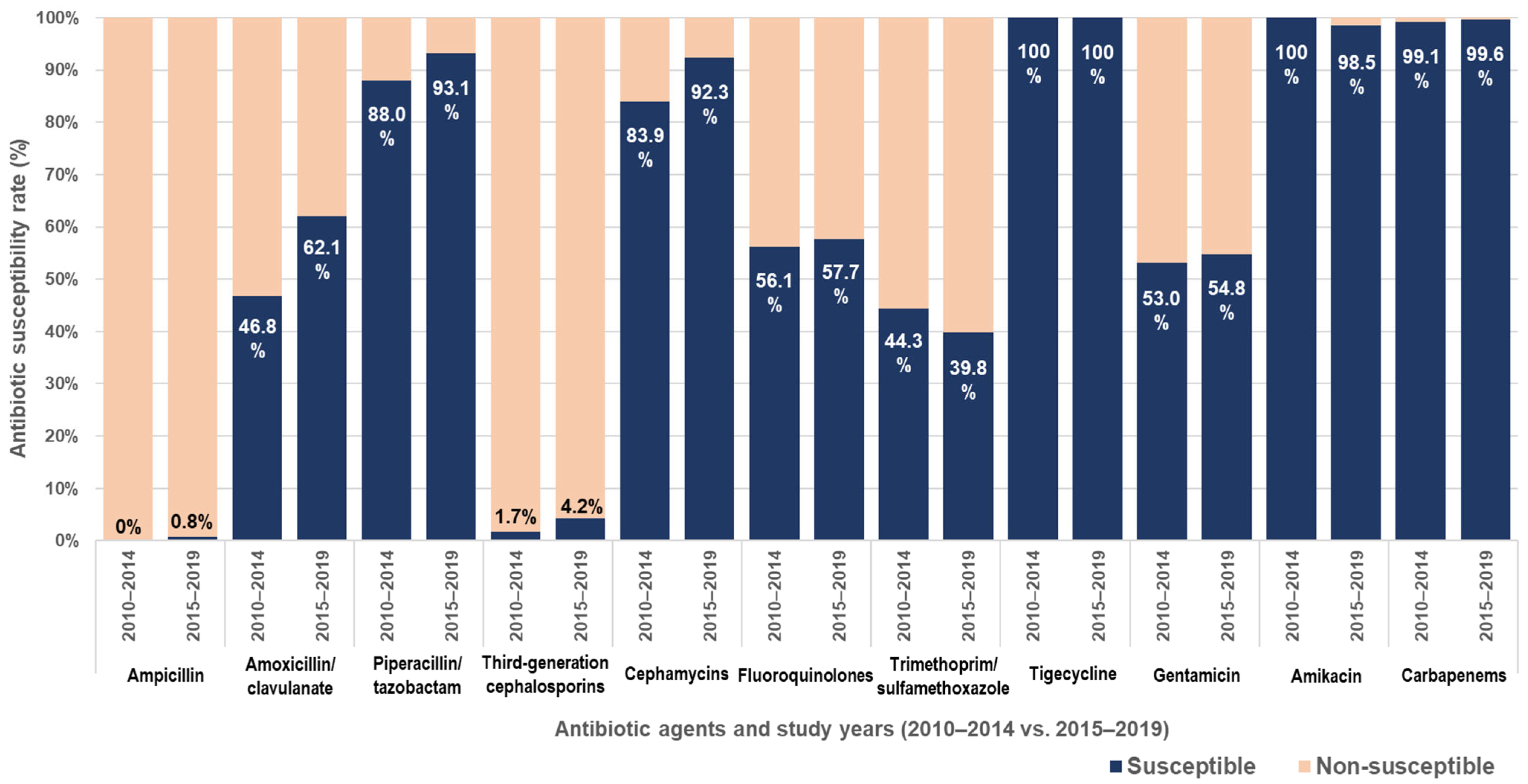
| Factor | Number (%) |
|---|---|
| Year | |
| 2010–2014 | 115 (30.6) |
| 2015–2019 | 261 (69.4) |
| Isolated pathogens | |
| Escherichia coli | 366 (97.3) |
| Klebsiella pneumoniae | 10 (2.7) |
| Sex | |
| Male | 249 (66.2) |
| Female | 127 (33.8) |
| Age, months, median (range) | 5 (0–104) |
| Age group | |
| <3 months | 91 (24.2) |
| 3–5 months | 143 (38.0) |
| 6–23 months | 101 (26.9) |
| ≥24 months | 41 (10.9) |
| Episodes of UTI | |
| First | 323 (85.9) |
| Second | 36 (9.6) |
| Third or more | 17 (4.5) |
| Treatment setting | |
| Hospitalization | 328 (87.2) |
| Outpatient care | 48 (12.8) |
| Previous hospitalization within 3 months | 63 (16.8) |
| Previous antibiotic use within 3 months | 77 (20.5) |
| Urogenital abnormality | 56 (14.9) |
| Abnormal prenatal ultrasonography | 9 (2.4) |
| Vesicoureteral reflux | 37 (9.8) |
| Hydronephrosis | 5 (1.3) |
| Multicystic dysplastic kidney | 3 (0.8) |
| Duplicated collecting system | 2 (0.5) |
| Ureterocele | 1 (0.3) |
| Bladder diverticulum | 1 (0.3) |
| Cloacal anomaly | 1 (0.3) |
| Neurogenic bladder | 4 (1.1) |
| Cryptorchidism | 1 (0.3) |
| Adrenal neuroblastoma | 1 (0.3) |
| Concurrent diagnosis | |
| Upper respiratory infection | 35 (9.3) |
| Lower respiratory infection | 8 (2.1) |
| Bacteremia | 6 (1.6) |
| Exanthem subitum | 4 (1.1) |
| Febrile seizure | 3 (0.8) |
| Cellulitis | 1 (0.3) |
| Erythema multiforme | 1 (0.3) |
| Factor | Empirical-Susceptible Group (n = 144) | Empirical-non-Susceptible Group (n = 175) | p-Value |
|---|---|---|---|
| Year | <0.001 | ||
| 2010–2014 | 72 (50.0) | 24 (13.7) | |
| 2015–2019 | 72 (50.0) | 151 (86.3) | |
| Isolated pathogens | 1.000 | ||
| Escherichia coli | 140 (97.2) | 170 (97.1) | |
| Klebsiella pneumoniae | 4 (2.8) | 5 (2.9) | |
| Sex | 0.295 | ||
| Male | 95 (66.0) | 125 (71.4) | |
| Female | 49 (34.0) | 50 (28.6) | |
| Age, months, median (range) | 5 (0–101) | 4 (0–83) | <0.001 |
| Age group | 0.138 | ||
| <3 months | 33 (22.9) | 53 (30.3) | |
| 3–5 months | 53 (36.8) | 71 (40.6) | |
| 6–23 months | 40 (27.8) | 39 (22.3) | |
| ≥24 months | 18 (12.5) | 12 (6.9) | |
| Episodes of UTI | 0.892 | ||
| First | 125 (86.8) | 154 (88.0) | |
| Second | 14 (9.7) | 16 (9.1) | |
| Third or more | 5 (3.5) | 5 (2.9) | |
| Previous hospitalization within 3 months | 26 (18.1) | 27 (15.4) | 0.531 |
| Previous antibiotic use within 3 months | 27 (18.8) | 32 (18.3) | 0.915 |
| Urogenital abnormality | 21 (14.6) | 24 (13.7) | 0.824 |
| Concurrent diagnosis | |||
| Upper respiratory infection | 13 (9.0) | 16 (9.1) | 0.972 |
| Lower respiratory infection | 4 (2.8) | 4 (2.3) | 1.000 |
| Bacteremia 1 | 4 (2.8) | 2 (1.2) | 0.417 |
| Hospital days, median (range) | 7 (3–35) | 7 (3–16) | <0.001 |
| Antibiotic duration, days, median (range) | |||
| Total duration | 13 (6–34) | 13 (2–25) | <0.001 |
| Intravenous antibiotics | 6 (2–34) | 6 (1–15) | <0.001 |
| Oral antibiotics | 7 (0–14) | 7 (0–15) | <0.001 |
| Fever duration | |||
| Total, days, median (range) | 3 (1–12) | 3 (1–12) | <0.001 |
| After initiation of antibiotics, hours, median (range) | 11 (0–129) | 15 (0–106) | <0.001 |
| Defervescence within 2 days of hospitalization | 109 (75.7) | 124 (70.9) | 0.333 |
| Defervescence during empirical therapy | 132 (91.7) | 156 (89.1) | 0.449 |
| 99mTc-DMSA scan abnormality 2 | 58 (81.7) | 47 (52.8) | <0.001 |
| Urological intervention | 7 (4.9) | 8 (4.6) | 0.903 |
| Recurrent UTI within 3 months | 2 (1.4) | 9 (5.1) | 0.067 |
| Factor | Group 1 (n = 99) | Group 2 (n = 64) | Group 3 (n = 45) | Group 4 (n = 111) | p-Value |
|---|---|---|---|---|---|
| Year | 0.017 | ||||
| 2010–2014 | 40 (40.4) | 7 (10.9) | 32 (71.1) | 17 (15.3) | |
| 2015–2019 | 59 (59.6) | 57 (89.1) | 13 (28.9) | 94 (84.7) | |
| Isolated pathogens | 0.201 | ||||
| Escherichia coli | 95 (96.0) | 61 (95.3) | 45 (100.0) | 109 (98.2) | |
| Klebsiella pneumoniae | 4 (4.0) | 3 (4.7) | 0 (0.0) | 2 (1.8) | |
| Sex | 0.098 | ||||
| Male | 68 (68.7) | 38 (59.4) | 27 (60.0) | 87 (78.4) | |
| Female | 31 (31.3) | 26 (40.6) | 18 (40.0) | 24 (21.6) | |
| Age, months, median (range) | 5 (0–97) | 5 (0–80) | 5 (1–101) | 4 (0–83) | 0.072 |
| Age group | 0.008 | ||||
| <3 months | 21 (21.2) | 17 (26.6) | 12 (26.7) | 36 (32.4) | |
| 3–5 months | 37 (37.4) | 23 (35.9) | 16 (35.6) | 48 (43.2) | |
| 6–23 months | 29 (29.3) | 18 (28.1) | 11 (24.4) | 21 (18.9) | |
| ≥24 months | 12 (12.1) | 6 (9.4) | 6 (13.3) | 6 (5.4) | |
| Episodes of UTI | 0.147 | ||||
| First | 84 (84.9) | 55 (85.9) | 41 (91.1) | 99 (89.2) | |
| Second | 11 (11.1) | 5 (7.8) | 3 (6.7) | 11 (9.9) | |
| Third or more | 4 (4.0) | 4 (6.3) | 1 (2.2) | 1 (0.9) | |
| Previous hospitalization within 3 months | 21 (21.2) | 10 (15.6) | 5 (11.1) | 17 (15.3) | 0.234 |
| Previous antibiotic use within 3 months | 22 (22.2) | 11 (17.2) | 5 (11.1) | 21 (18.9) | 0.485 |
| Urogenital abnormality | 16 (16.2) | 14 (21.9) | 5 (11.1) | 10 (9.0) | 0.059 |
| Concurrent diagnosis | |||||
| Upper respiratory infection | 9 (9.1) | 5 (7.8) | 4 (8.9) | 11 (9.9) | 0.788 |
| Lower respiratory infection | 4 (4.0) | 1 (1.6) | 0 (0.0) | 3 (2.7) | 0.527 |
| Bacteremia 1 | 4 (4.0) | 2 (3.2) | 0 (0.0) | 0 (0.0) | 0.019 |
| Hospital days, median (range) | 8 (4–35) | 8 (4–16) | 6 (3–7) | 6 (3–13) | <0.001 |
| Antibiotic duration, days, median (range) | |||||
| Total duration | 13 (7–34) | 14 (9–25) | 13 (6–18) | 12 (2–18) | <0.001 |
| Intravenous antibiotics | 7 (3–34) | 7 (3–15) | 5 (2–6) | 5 (1–12) | <0.001 |
| Oral antibiotics | 5 (0–14) | 7 (0–15) | 8 (0–13) | 7 (0–12) | <0.001 |
| Fever duration | |||||
| Total, days, median (range) | 3 (1–12) | 3 (1–10) | 3 (1–8) | 3 (1–12) | 0.336 |
| After initiation of antibiotics, hours, median (range) | 12 (0–129) | 29 (0–106) | 7 (0–85) | 7 (0–97) | <0.001 |
| Defervescence within 2 days of hospitalization | 73 (73.7) | 36 (56.3) | 36 (80.0) | 88 (79.3) | 0.101 |
| Defervescence during empirical therapy | 89 (89.9) | 49 (71.9) | 43 (95.6) | 107 (96.4) | 0.014 |
| 99mTc-DMSA scan abnormality 2 | 46 (90.2) | 21 (65.6) | 12 (60.0) | 26 (45.6) | <0.001 |
| Urological intervention | 6 (6.1) | 4 (6.3) | 1 (2.2) | 4 (3.6) | 0.301 |
| Recurrent UTI within 3 months | 1 (1.0) | 2 (3.1) | 1 (2.2) | 7 (6.3) | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, Y.; Kim, Y.J.; Han, S.B.; Suh, J.-S.; Lee, S.Y.; Kim, J.-H. Impact of Empirical and Definitive Antibiotics on Pediatric Febrile Urinary Tract Infection Caused by ESBL-Producing Enterobacterales. Pathogens 2025, 14, 1103. https://doi.org/10.3390/pathogens14111103
Lee J, Kim Y, Kim YJ, Han SB, Suh J-S, Lee SY, Kim J-H. Impact of Empirical and Definitive Antibiotics on Pediatric Febrile Urinary Tract Infection Caused by ESBL-Producing Enterobacterales. Pathogens. 2025; 14(11):1103. https://doi.org/10.3390/pathogens14111103
Chicago/Turabian StyleLee, Jin, Yejin Kim, Ye Ji Kim, Seung Beom Han, Jin-Soon Suh, Soo Young Lee, and Jong-Hyun Kim. 2025. "Impact of Empirical and Definitive Antibiotics on Pediatric Febrile Urinary Tract Infection Caused by ESBL-Producing Enterobacterales" Pathogens 14, no. 11: 1103. https://doi.org/10.3390/pathogens14111103
APA StyleLee, J., Kim, Y., Kim, Y. J., Han, S. B., Suh, J.-S., Lee, S. Y., & Kim, J.-H. (2025). Impact of Empirical and Definitive Antibiotics on Pediatric Febrile Urinary Tract Infection Caused by ESBL-Producing Enterobacterales. Pathogens, 14(11), 1103. https://doi.org/10.3390/pathogens14111103






