Discrimination of Clinical and Food-Derived Candida Strains Using Biotyping and Molecular Typing Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Yeast Biotyping
2.3. Sequence Analysis of ITS Regions
2.4. Multiplex PCR Analysis
2.5. Yeast Karyotyping
2.6. Statistical Analysis
2.6.1. Agglomeration Analysis
2.6.2. Discrimination Index
3. Results
3.1. Biotyping and Yeasts Identification by API System
3.2. Genotyping and Yeast Identification Based on ITS Region Sequences
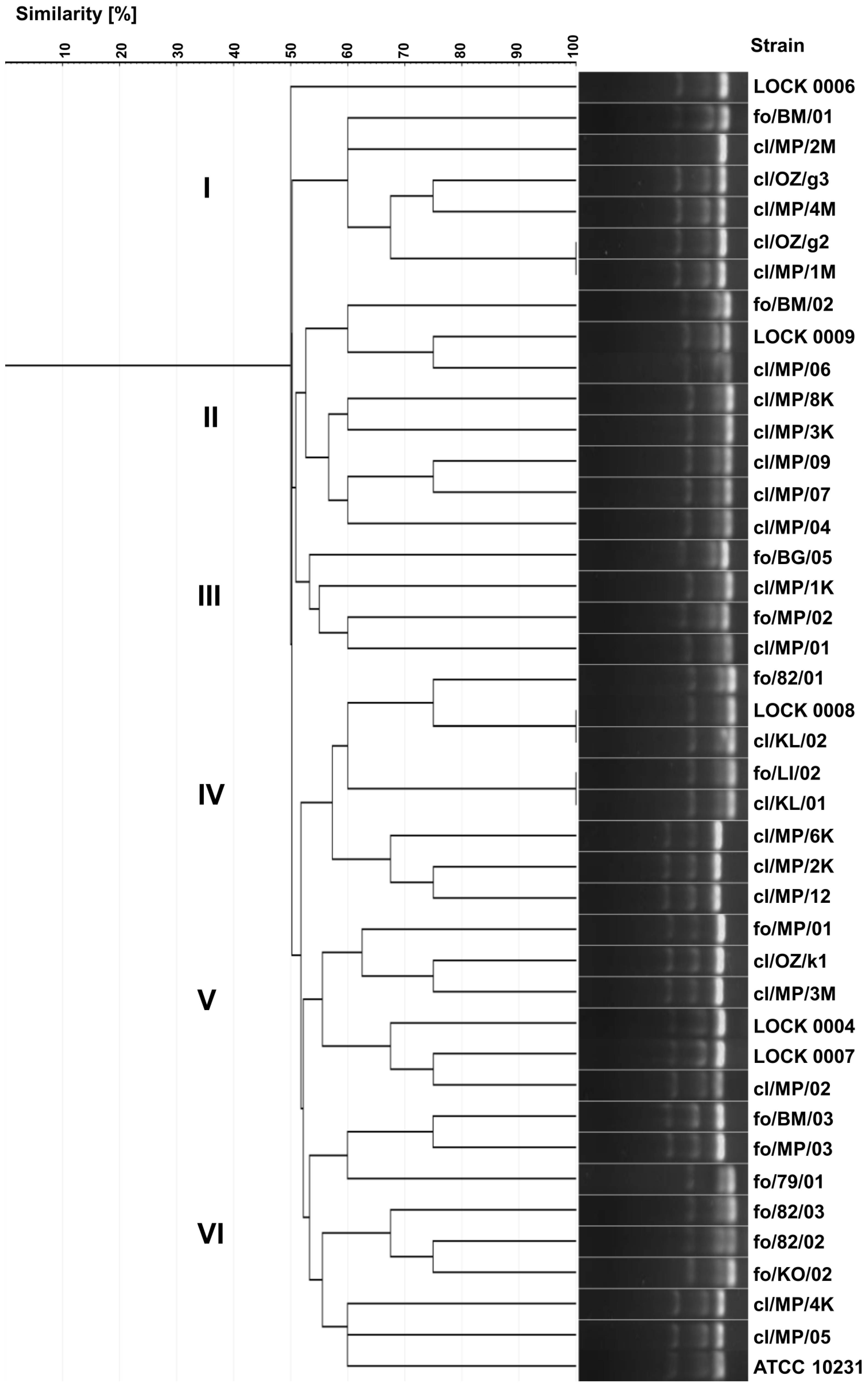
3.3. Yeasts Genotyping Based on ITS Region Polymorphism
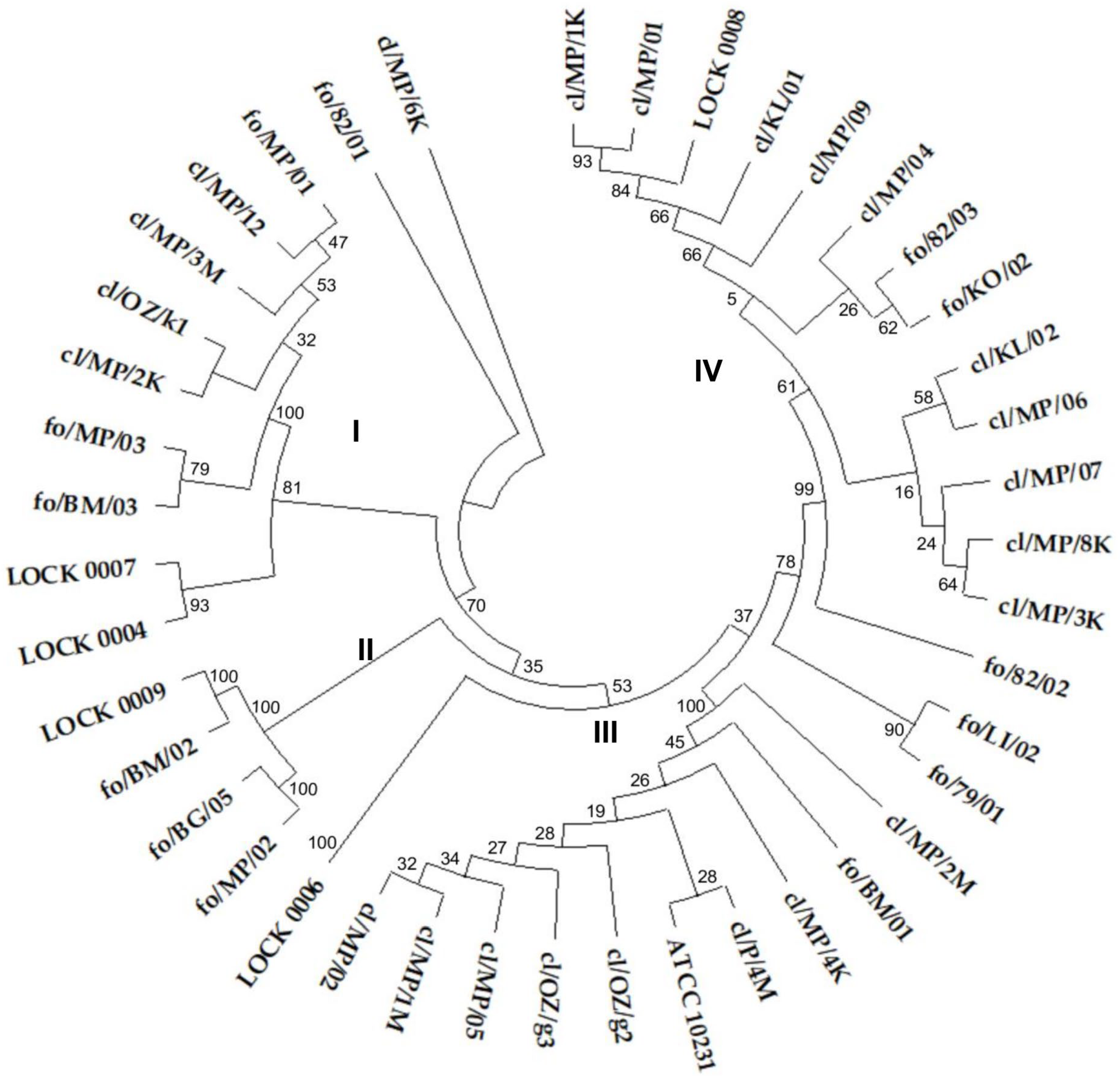
3.4. Genotyping Based on Multiplex PCR
3.5. Yeasts Karyotyping
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Substrate 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cl/MP/02 | + | − | + | − | + | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/4K | + | − | + | − | + | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/1M | + | + | + | − | + | + | + | + | + | + | + | + | − | − | + | + | + | − | − |
| cl/MP/2M | + | − | + | − | − | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/4M | + | − | + | − | + | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/OZ/g2 | + | − | + | − | + | − | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/OZ/g3 | + | − | + | − | + | + | + | + | − | + | + | + | − | − | + | + | − | − | − |
| cl/MP/05 | + | − | + | − | + | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| ATCC 10231 | + | − | + | − | + | + | + | + | + | + | + | + | − | − | + | + | + | − | − |
| cl/MP/01 | + | + | + | + | + | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/1K | + | + | + | − | + | − | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/3K | + | − | + | − | − | + | + | + | + | + | + | + | − | − | + | + | + | − | − |
| cl/MP/8K | + | − | + | − | − | + | + | + | + | + | − | + | − | − | + | + | + | − | − |
| cl/MP/04 | + | − | + | − | + | − | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/06 | + | − | + | − | − | + | + | + | − | + | − | + | − | − | + | + | + | − | − |
| cl/MP/07 | + | − | + | − | + | − | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/09 | + | − | + | − | + | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/KL/02 | + | + | + | − | + | + | + | + | − | + | − | − | + | − | + | + | + | + | − |
| cl/KL/01 | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − |
| cl/MP/12 | + | − | + | − | + | − | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/3M | + | − | + | − | + | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/OZ/k1 | + | + | + | − | + | − | + | + | − | + | + | + | − | − | + | + | − | − | − |
| cl/MP/2K | + | − | + | − | + | − | + | + | − | + | + | + | − | − | + | + | + | − | − |
| cl/MP/6K | + | + | + | − | + | + | + | + | − | + | + | + | − | − | + | + | + | − | − |
| fo/82/01 | + | + | + | − | + | − | − | − | − | + | + | + | + | − | + | + | + | + | − |
| fo/82/02 | + | + | + | − | − | + | + | + | − | + | + | + | + | + | + | + | + | + | − |
| fo/82/03 | + | + | + | + | − | + | − | + | − | + | − | + | − | − | + | + | + | + | − |
| fo/KO/02 | + | + | + | − | − | − | + | − | − | + | − | − | − | − | − | + | + | − | + |
| fo/LI/02 | + | + | + | − | − | + | + | + | − | + | + | + | + | + | + | + | − | + | + |
| LOCK 0008 | + | − | − | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| fo/79/01 | + | + | + | − | − | − | − | + | − | + | − | + | + | − | + | + | − | + | − |
| fo/BM/01 | + | − | + | − | + | + | − | + | − | + | − | + | − | − | + | − | + | − | − |
| fo/MP/01 | + | + | − | − | + | + | + | − | − | + | − | + | − | − | − | + | − | − | − |
| fo/MP/03 | + | + | − | − | + | + | + | − | − | + | − | + | − | − | − | − | − | − | − |
| fo/BM/03 | + | + | − | − | + | + | + | − | − | + | − | + | + | − | − | − | − | − | + |
| LOCK 0006 | + | + | + | − | + | + | + | + | + | + | + | + | + | − | + | + | + | + | − |
| fo/MP/02 | + | + | − | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| fo/BG/05 | + | − | − | − | + | − | − | + | − | + | − | − | − | − | − | − | − | − | − |
| fo/BM/02 | + | + | − | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| LOCK 0009 | + | + | − | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
| LOCK 0004 | + | + | + | − | + | + | + | + | + | + | + | + | + | − | + | + | + | + | − |
| LOCK 0007 | + | + | − | − | − | − | − | − | − | − | + | − | − | − | + | + | − | + | − |
References
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2021, 13, 89–121. [Google Scholar] [CrossRef] [PubMed]
- Parambath, S.; Dao, A.; Kim, H.Y.; Zawahir, S.; Izquierdo, A.A.; Tacconelli, E.; Govender, N.; Oladele, R.; Colombo, A.; Sorrell, T.; et al. Candida albicans—A systematic review to inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae045. [Google Scholar] [CrossRef] [PubMed]
- Gaffar, N.R.; Valand, N.; Venkatraman Girija, U. Candidiasis: Insights into virulence factors, complement evasion and antifungal drug resistance. Microorganisms 2025, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Castanheira, M. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int. J. Antimicrob. Agents 2017, 50, 352–358. [Google Scholar] [CrossRef]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive candidiasis: Current clinical challenges and unmet needs in adult populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef]
- Pinho, S.; Miranda, I.M.; Costa-de-Oliveira, S. Global epidemiology of invasive infections by uncommon Candida species: A systematic review. J. Fungi 2024, 10, 558. [Google Scholar] [CrossRef]
- Watkins, R.R.; Gowen, R.; Lionakis, M.S.; Ghannoum, M. Update on the pathogenesis, virulence, and treatment of Candida auris. Pathog. Immun. 2022, 7, 46–65. [Google Scholar] [CrossRef]
- Frías-De-León, M.G.; Hernández-Castro, R.; Conde-Cuevas, E.; García-Coronel, I.H.; Vázquez-Aceituno, V.A.; Soriano-Ursúa, M.A.; Farfán-García, E.D.; Ocharán-Hernández, E.; Rodríguez-Cerdeira, C.; Arenas, R.; et al. Candida glabrata antifungal resistance and virulence factors, a perfect pathogenic combination. Pharmaceutics 2021, 13, 1529. [Google Scholar] [CrossRef]
- Kieliszek, M.; Kot, A.M.; Bzducha-Wróbel, A.; Błażejak, S.; Gientka, I.; Kurcz, A. Biotechnological use of Candida yeasts in the food industry: A review. Fungal Biol. Rev. 2017, 31, 185–198. [Google Scholar] [CrossRef]
- Johnson, E.A.; Echavarri-Erasun, C. Yeast biotechnology. In The Yeasts, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: London, UK, 2011; pp. 22–44. [Google Scholar]
- Fleet, G.H.; Balia, R. The public health and probiotic significance of yeasts in foods and beverages. In Yeasts in Food and Beverages, 1st ed.; Querol, A., Fleet, G.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 381–398. [Google Scholar]
- Carlino, N.; Blanco-Míguez, A.; Punčochář, M.; Mengoni, C.; Pinto, F.; Tatti, A.; Manghi, P.; Armanini, F.; Avagliano, M.; Barcenilla, C.; et al. Unexplored microbial diversity from 2,500 food metagenomes and links with the human microbiome. Cell 2024, 187, 5775–5795.e15. [Google Scholar] [CrossRef]
- Levine, J.; Dykoski, R.K.; Janoff, E.N. Candida-associated diarrhea: A syndrome in search of credibility. Clin. Infect. Dis. 1995, 21, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Talwar, P.; Chakrabarti, A.; Chalwa, A.; Mehta, S.; Walza, B.N.S.; Lumar, L.; Chung, K.S. Fungal diarrhoea: Association of different fungi and seasonal variation and their incidence. Mycopathologia 1990, 110, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Barclay, G.R.; McKenzie, H.; Pennington, J.; Parratt, D.; Pennington, C.R. The effect of dietary yeast on the activity of stable chronic Crohn’s disease. Scand. J. Gastroenterol. 1992, 27, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Kunicka-Styczyńska, A. Typing and virulence factors of food-borne Candida spp. isolates. Int. J. Food Microbiol. 2018, 279, 57–63. [Google Scholar] [CrossRef]
- Arafa, S.H.; Elbanna, K.; Osman, G.E.H.; Abulreesh, H.H. Candida diagnostic techniques: A review. J. Umm Al-Qura Univ. Appll. Sci. 2023, 9, 360–377. [Google Scholar] [CrossRef]
- Bonfim-Mendonça, P.S.; Fiorini, A.; Shinobu-Mesquita, C.S.; Baeza, L.C.; Fernandez, M.A.; Svidzinski, T.I.E. Molecular typing of Candida albicans isolates from hospitalized patients. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 385–391. [Google Scholar] [CrossRef]
- Jha, V.; Giri, R.; Koli, J.; Poojari, D.; Dhamapurkar, V.; Jhangiani, A.; Nikumb, D.; Rumani, S.; Markam, M.; Sahu, A.; et al. RAPD typing, antibiotic resistance profiling and genetic diversity of Candida isolates. Microbiol. Infect. Dis. 2022, 6, 1–8. [Google Scholar] [CrossRef]
- Bautista-Muñoz, C.; Boldo, X.M.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Identification of Candida spp. by randomly amplified polymorphic DNA analysis and differentiation between Candida albicans and Candida dubliniensis by direct PCR methods. J. Clin. Microbiol. 2003, 41, 414–420. [Google Scholar] [CrossRef]
- Małek, M.; Paluchowska, P.; Bogusz, B.; Budak, A. Molecular characterization of Candida isolates from intensive care unit patients, Krakow, Poland. Rev. Iberoam. Micol. 2017, 34, 10–16. [Google Scholar] [CrossRef]
- Makled, A.F.; Ali, S.A.M.; Labeeb, A.Z.; Salman, S.S.; Shebl, D.Z.M.; Hegazy, S.G.; Sabal, M.S. Characterization of Candida species isolated from clinical specimens: Insights into virulence traits, antifungal resistance and molecular profiles. BMC Microbiol. 2024, 24, 388. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Shinsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Fujita, S.I.; Senda, Y.; Nakaguchi, S.; Hashimoto, T. Multiplex PCR using internal transcribed spacer 1 and 2 regions for rapid detection and identification of yeast strains. J. Clin. Microbiol. 2001, 39, 3617–3622. [Google Scholar] [CrossRef] [PubMed]
- Kunicka-Styczyńska, A.; Rajkowska, K. Phenotypic and genotypic diversity of wine yeasts used for acidic musts. World J. Microbiol. Biotechnol. 2012, 28, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Heras, J.; Domínguez, C.; Mata, E.; Pascual, V. GelJ—A tool for analyzing DNA fingerprint gel images. BMC Bioinform. 2015, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. Molecular evolutionary genetics analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]
- Bartie, K.L.; Williams, D.W.; Wilson, M.J.; Potts, A.J.; Lewis, M.A. PCR fingerprinting of Candida albicans associated with chronic hyperplastic candidosis and other oral conditions. J. Clin. Microbiol. 2001, 39, 4066–4075. [Google Scholar] [CrossRef]
- Giammanco, G.M.; Lopes, M.M.; Coimbra, R.S.; Pignato, S.; Grimont, P.A.; Grimont, F.; Freitas, G.; Giammanco, G. Value of morphotyping for the characterization of Candida albicans clinical isolates. Mem. Inst. Oswaldo Cruz. 2005, 100, 483–490. [Google Scholar] [CrossRef]
- Gouliamova, D.; Dimitrov, R.; Petrova, P.; Stoyancheva, G.; Petrov, K. Genomic approaches to yeast taxonomy. Biotechnol. Biotechnol. Equip. 2009, 23 (Suppl. S1), 519–523. [Google Scholar] [CrossRef]
- Mendoza-Reyes, D.F.; Gómez-Gaviria, M.; Mora-Montes, H.M. Candida lusitaniae: Biology, pathogenicity, virulence factors, diagnosis, and treatment. Infect. Drug Resist. 2022, 15, 5121–5135. [Google Scholar] [CrossRef]
- Linton, C.J.; Borman, A.M.; Cheung, G.; Holmes, A.D.; Szekely, A.; Palmer, M.D.; Bridge, P.D.; Campbell, C.K.; Johnson, E.M. Molecular identification of unusual pathogenic yeast isolates by large ribosomal subunit gene sequencing: 2 years of experience at the United Kingdom mycology reference laboratory. J. Clin. Microbiol. 2007, 45, 1152–1158. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Kofteridis, D.P. Fungemia by Wickerhamomyces anomalus—A narrative review. Pathogens 2024, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Douglass, A.P.; Offei, B.; Braun-Galleani, S.; Coughlan, A.Y.; Martos, A.A.R.; Ortiz-Merino, R.A.; Byrne, K.P.; Wolfe, K.H. Population genomics shows no distinction between pathogenic Candida krusei and environmental Pichia kudriavzevii: One species, four names. PLoS Pathog. 2018, 14, e1007138. [Google Scholar] [CrossRef] [PubMed]
- Maroszyńska, M.; Kunicka-Styczyńska, A.; Rajkowska, K.; Maroszyńska, I. Antibiotics sensitivity of Candida clinical and food-borne isolates. Acta Biochim. Pol. 2013, 60, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Jeggo, M. The One Health approach-why is it so important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef]
- Metcalf, R.; Akinbobola, A.; Woodford, L.; Quilliam, R.S. Thermotolerance, virulence, and drug resistance of human pathogenic Candida species colonising plastic pollution in aquatic ecosystems. Environ. Sci. Pollut. Res. 2025. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Tyler, A.C.; Hoffman, M.J.; Savka, M.A.; Hudson, A.O. Is plastic pollution in aquatic and terrestrial environments a driver for the transmission of pathogens and the evolution of antibiotic resistance? Environ. Sci. Technol. 2019, 53, 1744–1745. [Google Scholar] [CrossRef]
- Gkoutselis, G.; Rohrbach, S.; Harjes, J.; Obst, M.; Brachmann, A.; Horn, M.A.; Rambold, G. Microplastics accumulate fungal pathogens in terrestrial ecosystems. Sci. Rep. 2021, 11, 13214. [Google Scholar] [CrossRef]
- Akinbobola, A.B.; Kean, R.; Hanifi, S.M.A.; Quilliam, R.S. Environmental reservoirs of the drug-resistant pathogenic yeast Candida auris. PLoS Pathog. 2023, 19, e1011268. [Google Scholar] [CrossRef]
- Biedunkiewicz, A.; Ozimek, T. Qualitative and quantitative changes of potentially pathogenic fungi in a hydrophyte wastewater treatment plant. Pol. J. Environ. Stud. 2009, 18, 161–166. [Google Scholar]


| Strain | Source of Isolation |
|---|---|
| clinical strains | |
| cl/MP/01, cl/MP/02, cl/MP/12, cl/MP/1K, cl/MP/3K, cl/MP/4K, cl/MP/8K, cl/MP/1M, cl/MP/2M, cl/MP/3M, cl/MP/4M, cl/OZ/g2, cl/OZ/k1, cl/KL/02 | faces |
| cl/MP/04, cl/OZ/g3 | throat |
| cl/MP/05, cl/MP/2K | stomach |
| cl/MP/06, cl/MP/07, cl/MP/6K | vagina |
| cl/MP/09 | blood |
| cl/KL/01 | urinary tract |
| C. albicans ATCC 10231 | lungs |
| food-borne strains | |
| fo/79/01, fo/82/01, fo/82/02, fo/82/03, fo/KO/02 | fruit yogurt |
| fo/LI/02 | herring salad |
| fo/MP/01, fo/MP/02, fo/MP/03, fo/BM/01, fo/BM/02, fo/BM/03 | pickled cucumbers |
| fo/BG/05 | sauerkraut |
| LOCK 0004, LOCK 0006, LOCK 0007 | feed yeast |
| LOCK 0008, LOCK 0009 | baker’s yeast |
| Primer | Primer Sequence | Reference |
|---|---|---|
| ITS1 | 5′–TCCGTAGGTGAACCTGCGG–3′ | [23] |
| ITS3 | 5′–GCATCGATGAAGAACGCAGC–3′ | [24] |
| ITS4 | 5′–TCCTCCGCTTATTGATATGC–3′ | [23,24] |
| Strain | Source of Isolation | Identification Based on Assimilation Profiles | Identification Based on ITS Sequence | Accession Number 1 |
|---|---|---|---|---|
| clinical strains | ||||
| cl/MP/02 | Faces | Candida albicans | Candida albicans | PV670445 |
| cl/MP/4K | Faces | Candida albicans | Candida albicans | PV670447 |
| cl/MP/1M | Faces | Candida albicans | Candida albicans | PV670444 |
| cl/MP/2M | Faces | Candida albicans | Candida albicans | PV670446 |
| cl/MP/4M | Faces | Candida albicans | Candida albicans | PV670448 |
| cl/OZ/g2 | Faces | Candida albicans | Candida albicans | PV670450 |
| cl/OZ/g3 | Throat | Candida albicans | Candida albicans | PV670451 |
| cl/MP/05 | stomach | Candida albicans | Candida albicans | PV670449 |
| ATCC 10231 | Lungs | Candida albicans | Candida albicans | PV785352 |
| cl/MP/01 | Faces | Candida albicans | Clavispora lusitaniae | PV670459 |
| cl/MP/1K | Faces | Candida albicans | Clavispora lusitaniae | PV670460 |
| cl/MP/3K | Faces | Candida albicans | Clavispora lusitaniae | PV670461 |
| cl/MP/8K | Faces | Candida albicans | Clavispora lusitaniae | PV670465 |
| cl/MP/04 | Throat | Candida albicans | Clavispora lusitaniae | PV670462 |
| cl/MP/06 | Vagina | Candida albicans | Clavispora lusitaniae | PV670463 |
| cl/MP/07 | Vagina | Candida albicans | Clavispora lusitaniae | PV670464 |
| cl/MP/09 | Blood | Candida albicans | Clavispora lusitaniae | PV670466 |
| cl/KL/02 | Faces | Candida lusitaniae (Clavispora lusitaniae) | Clavispora lusitaniae | PV670458 |
| cl/KL/01 | urinary tract | Candida glabrata (Nakaseomyces glabratus) | Clavispora lusitaniae | PV670457 |
| cl/MP/12 | Faces | Candida albicans | Candida boidinii | PV670455 |
| cl/MP/3M | Faces | Candida albicans | Candida boidinii | PV670453 |
| cl/OZ/k1 | Faces | Candida albicans | Candida boidinii | PV670456 |
| cl/MP/2K | stomach | Candida albicans | Candida boidinii | PV670452 |
| cl/MP/6K | Vagina | Candida albicans | Candida boidinii | PV670454 |
| food-borne strains | ||||
| fo/82/01 | fruit yogurt | Candida lusitaniae (Clavispora lusitaniae) | Clavispora lusitaniae | PV686768 |
| fo/82/02 | fruit yogurt | Candida famata (Debaryomyces hansenii) | Clavispora lusitaniae | PV686769 |
| fo/82/03 | fruit yogurt | Candida parapsilosis | Clavispora lusitaniae | PV686770 |
| fo/KO/02 | fruit yogurt | Candida colliculosa | Clavispora lusitaniae | PV686771 |
| fo/LI/02 | herring salad | Candida famata (Debaryomyces hansenii) | Clavispora lusitaniae | PV686772 |
| LOCK 0008 | baker’s yeast | Candida krusei (Issatchenkia orientalis)/C. inconspicua (Pichia inconspicua) | Clavispora lusitaniae | PV686773 |
| fo/79/01 | fruit yogurt | Candida lusitaniae (Clavispora lusitaniae) | Candida albicans | PV686762 |
| fo/BM/01 | pickled cucumbers | Candida tropicalis | Candida albicans | PV686763 |
| fo/MP/01 | pickled cucumbers | Candida boidinii | Candida boidinii | PV686765 |
| fo/MP/03 | pickled cucumbers | Candida boidinii | Candida boidinii | PV686766 |
| fo/BM/03 | pickled cucumbers | Candida boidinii | Candida boidinii | PV686764 |
| LOCK 0006 | feed yeast | Candida lusitaniae (Clavispora lusitaniae) | Candida tropicalis | PV686767 |
| fo/MP/02 | pickled cucumbers | Candida krusei (Issatchenkia orientalis)/C. inconspicua (Pichia inconspicua) | Pichia membranifaciens | PV686778 |
| fo/BG/05 | sauerkraut | Candida rugosa (Diutina rugosa) | Pichia membranifaciens | PV686777 |
| fo/BM/02 | pickled cucumbers | Candida krusei (Issatchenkia orientalis)/C. inconspicua (Pichia inconspicua) | Pichia fermentans | PV686775 |
| LOCK 0009 | baker’s yeast | Candida krusei (Issatchenkia orientalis)/C. inconspicua (Pichia inconspicua) | Pichia fermentans | PV686776 |
| LOCK 0004 | feed yeast | Candida lusitaniae (Clavispora lusitaniae) | Meyerozyma guilliermondii | PV686774 |
| LOCK 0007 | feed yeast | Candida pelliculosa (Wickerhamomyces anomalus) | Wickerhamomyces anomalus | PV686779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkowska, K.; Otlewska, A.; Simińska, D. Discrimination of Clinical and Food-Derived Candida Strains Using Biotyping and Molecular Typing Approaches. Pathogens 2025, 14, 614. https://doi.org/10.3390/pathogens14070614
Rajkowska K, Otlewska A, Simińska D. Discrimination of Clinical and Food-Derived Candida Strains Using Biotyping and Molecular Typing Approaches. Pathogens. 2025; 14(7):614. https://doi.org/10.3390/pathogens14070614
Chicago/Turabian StyleRajkowska, Katarzyna, Anna Otlewska, and Dorota Simińska. 2025. "Discrimination of Clinical and Food-Derived Candida Strains Using Biotyping and Molecular Typing Approaches" Pathogens 14, no. 7: 614. https://doi.org/10.3390/pathogens14070614
APA StyleRajkowska, K., Otlewska, A., & Simińska, D. (2025). Discrimination of Clinical and Food-Derived Candida Strains Using Biotyping and Molecular Typing Approaches. Pathogens, 14(7), 614. https://doi.org/10.3390/pathogens14070614





