Testing a Sustainable Strategy Against Poultry Helminth Stages Developing in the Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasitophagous Fungi
2.2. Collection and Analysis of Fecal Samples
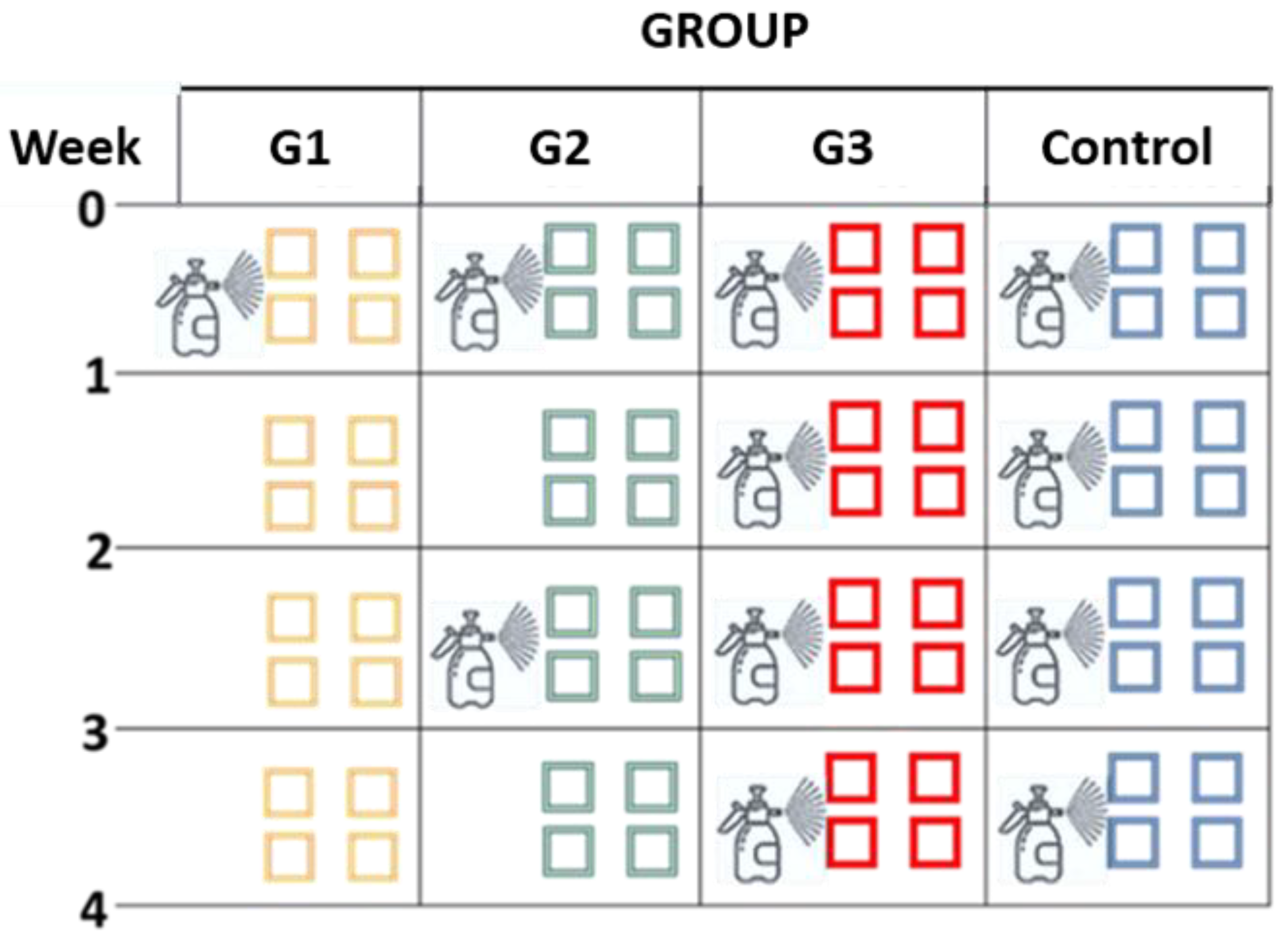
2.3. Design of the Study
2.4. Evaluation of Parasiticidal Activity in Soil
2.5. Morphological Characterization of Helminth Eggs During the Trial
2.6. Statistical Analysis
3. Results
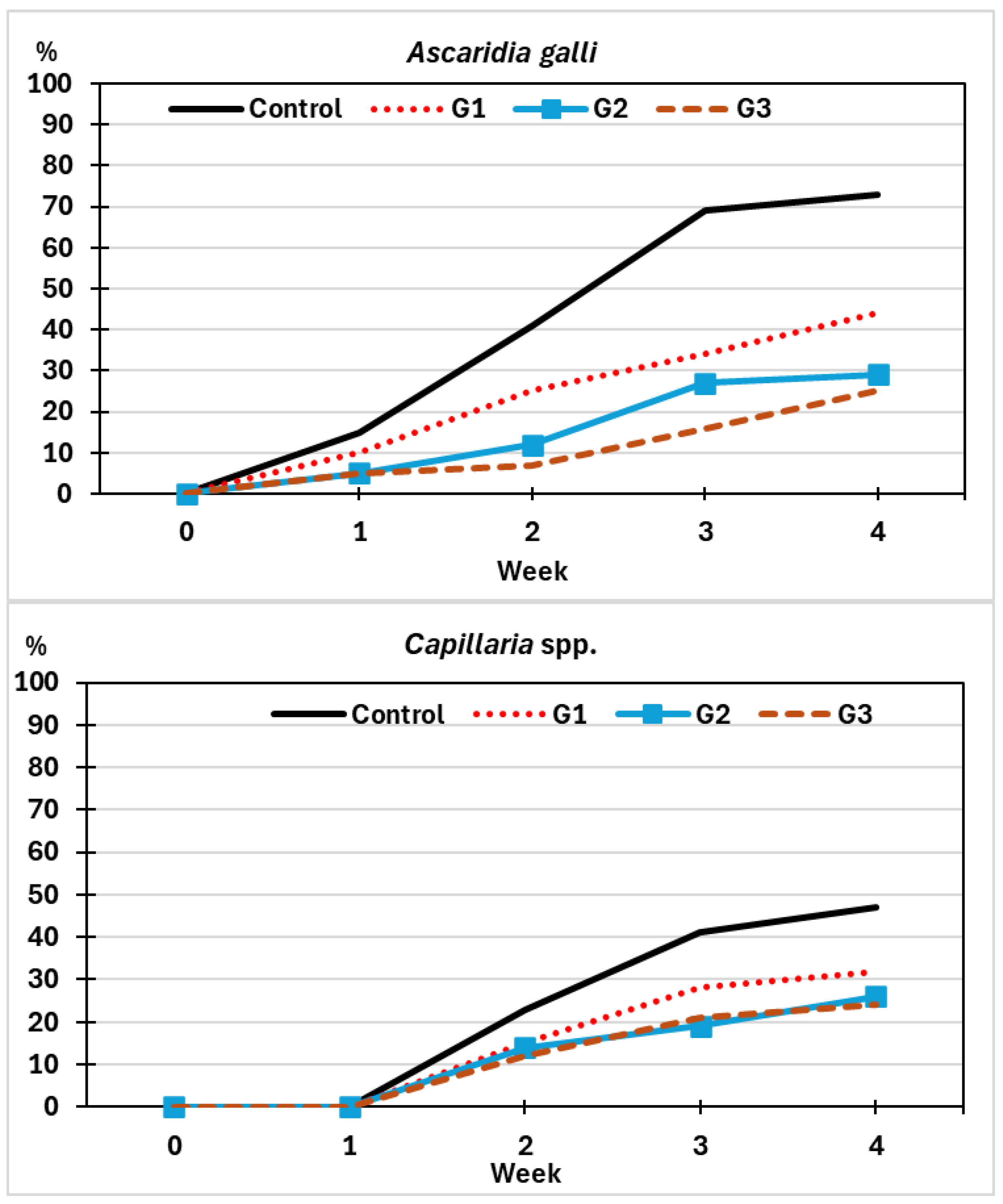
3.1. Parasitological Findings
3.2. Antagonistic Effect by Spraying Spores of M. circinelloides
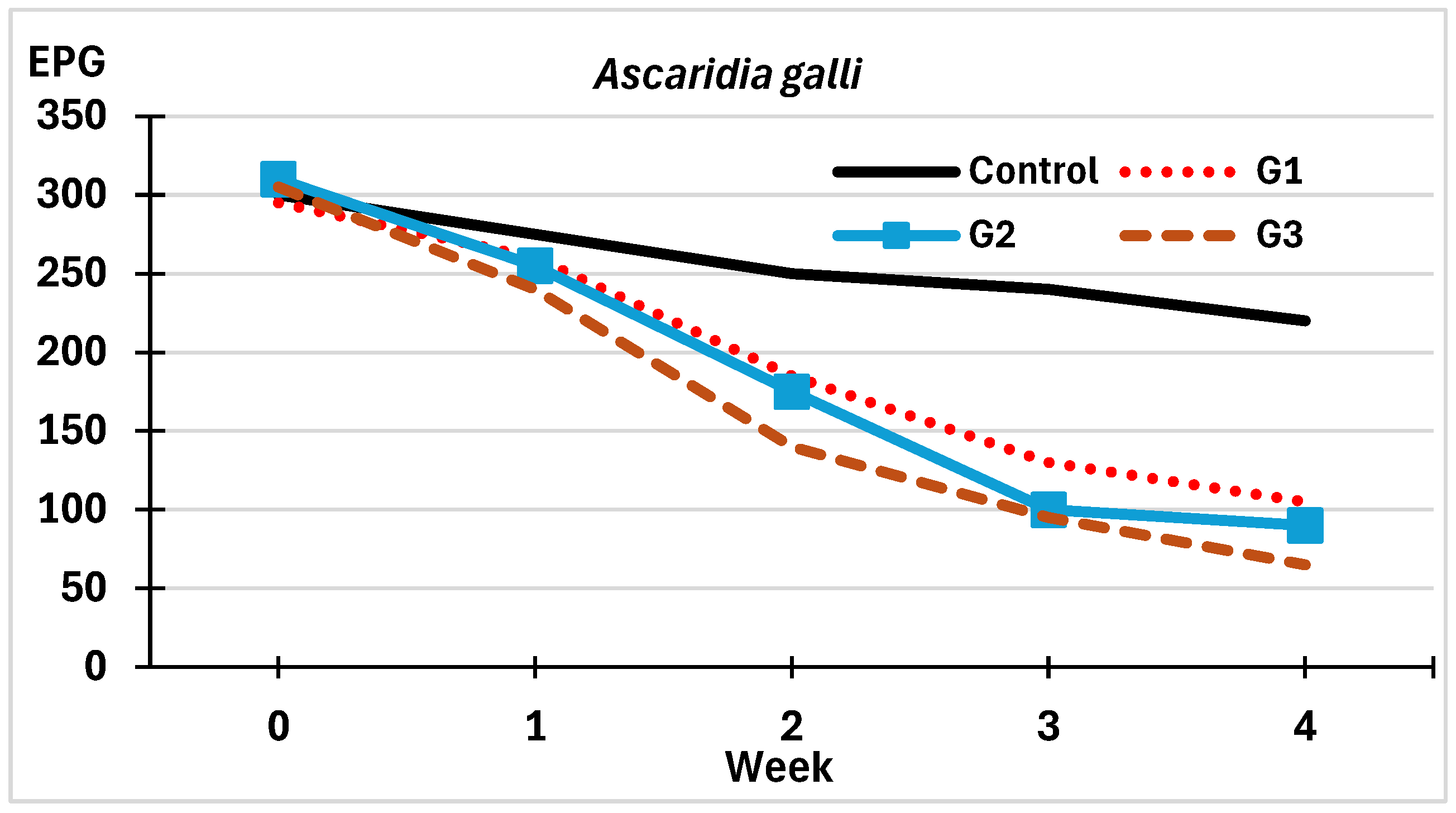
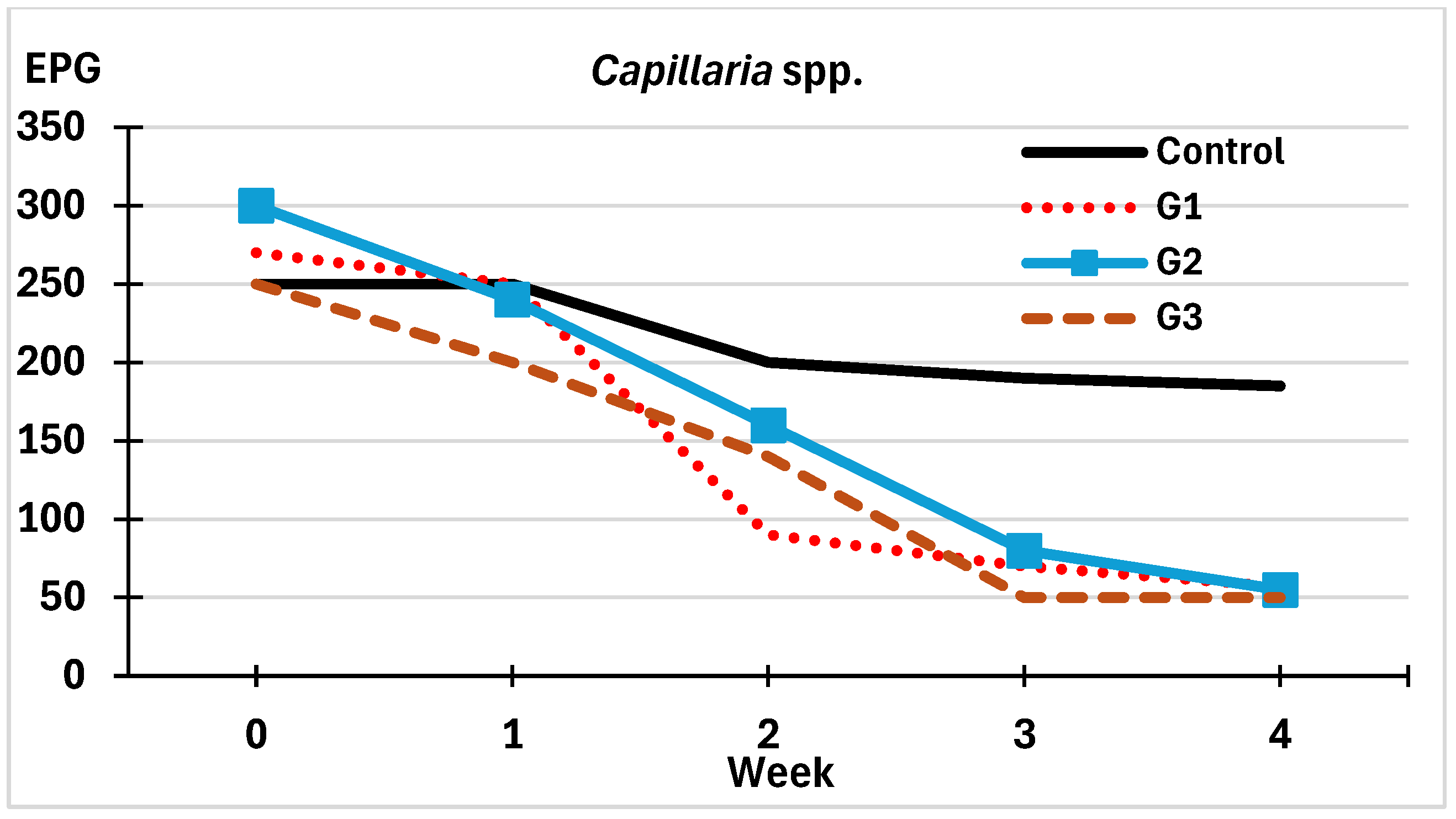
3.2.1. On the Viability of Eggs of Helminths
3.2.2. On the Development of Eggs of Helminths
3.2.3. On the Infection Risk by Helminths
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alig, B.N.; Anderson, K.E.; Malheiros, D.M.; Harding, K.L.; Malheiros, R.D. Assessment of the Effects of Stocking Density on Laying Hens Raised in Colony Cages: Part II—Egg Production, Egg Quality, and Welfare Parameters. Poultry 2025, 4, 28. [Google Scholar] [CrossRef]
- Hahn, D.L. The Effects of Feed Additives, Housing Systems, and Stress on Salmonella Shedding in Single Comb White and Brown Laying Hens. Ph.D. Dissertation, University of Nebraska-Lincoln, Lincoln, NE, USA, 2014. Available online: http://digitalcommons.unl.edu/animalscidiss/84 (accessed on 4 August 2025).
- United Egg Producers. Animal Husbandry Guidelines for U.S. Egg-Laying Flocks. 2017. Available online: https://uepcertified.com/wp-content/uploads/2019/09/CF-UEP-Guidelines_17-3.pdf (accessed on 29 August 2025).
- European Union. Council Directive 1999/74/EC of 19 July 1999 Laying Down Minimum Standards for the Protection of Laying Hens. Off. J. Eur. Communities 1999, 203, 53–57. [Google Scholar]
- Mench, J.A.; Blatchford, R.A. Determination of Space Use by Laying Hens Using Kinematic Analysis. Poult. Sci. 2014, 93, 794–798. [Google Scholar] [CrossRef]
- Riddle, E.R.; Ali, A.B.A.; Campbell, D.L.M.; Siegford, J.M. Space Use by 4 Strains of Laying Hens to Perch, Wing Flap, Dust Bathe, Stand and Lie Down. PLoS ONE 2018, 13, e0190532. [Google Scholar] [CrossRef] [PubMed]
- Knierim, U. Animal welfare aspects of outdoor runs for laying hens: A review. NJAS Wagening. J. Life Sci. 2006, 54, 133–145. [Google Scholar] [CrossRef]
- Pillay, R.; Naidoo, P.; Duma, Z.; Bhengu, K.N.; Mpaka-Mbatha, M.N.; Nembe-Mafa, N.; Mkhize-Kwitshana, Z.L. Potential Interactions Between Soil-Transmitted Helminths and Herpes Simplex Virus Type II: Implications for Sexual and Reproductive Health in Sub-Saharan African. Biology 2024, 13, 1050. [Google Scholar] [CrossRef] [PubMed]
- Schlosser-Brandenburg, J.; Midha, A.; Mugo, R.M.; Ndombi, E.M.; Gachara, G.; Njomo, D.; Rausch, S.; Hartmann, S. Infection with soil-transmitted helminths and their impact on coinfections. Front. parasitol. 2023, 2, 1197956. [Google Scholar] [CrossRef]
- Thapa, S.; Thamsborg, S.M.; Wang, R.; Meyling, N.V.; Dalgaard, T.S.; Petersen, H.H.; Mejer, H. Effect of the nematophagous fungus Pochonia chlamydosporia on soil content of ascarid eggs and infection levels in exposed hens. Parasites Vectors 2018, 11, 319. [Google Scholar] [CrossRef]
- Tarbiat, B.; Jansson, D.S.; Tydén, E.; Höglund, J. Comparison between anthelmintic treatment strategies against Ascaridia galli in commercial laying hens. Vet. Parasitol. 2016, 226, 109–115. [Google Scholar] [CrossRef]
- Tarbiat, B.; Jansson, D.S.; Höglund, J. Implementation of a targeted treatment strategy for the sustainable control of Ascaridia galli infections in laying hens. Vet. Rec. Open 2022, 9, e37. [Google Scholar] [CrossRef]
- Sulik, M.; Antoszczak, M.; Huczyński, A.; Steverding, D. Antiparasitic activity of ivermectin: Four decades of research into a “wonder drug”. Eur. J. Med. Chem. 2023, 261, 115838. [Google Scholar] [CrossRef]
- Jackson, F.; Miller, J. Alternative approaches to control--quo vadit? Vet. Parasitol. 2006, 139, 371–384. [Google Scholar] [CrossRef]
- Thamsborg, S.M.; Roepstorff, A.; Larsen, M. Integrated and biological control of parasites in organic and conventional production systems. Vet. Parasitol. 1999, 84, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Thamsborg, S.M.; Meyling, N.V.; Dhakal, S.; Mejer, H. Survival and development of chicken ascarid eggs in temperate pastures. Parasitology 2017, 144, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Valadão, M.C.; Millena de Carvalho, L.; Vieira, Í.S.; Neves, P.H.; Ferreira, V.M.; Campos, A.K.; Elias de Freitas Soares, F.; Ferraz, C.M.; Ribeiro Vilela, V.L.; Braga, F.R.; et al. Germination capacity of the Pochonia chlamydosporia fungus after its passage through the gastrointestinal tract of domestic chickens (Gallus gallus domesticus). Exp. Parasitol. 2020, 216, 107936. [Google Scholar] [CrossRef]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Baldo, D.; Ratti, C.; Piva, A.; Grilli, E. Investigating the effects of essential oils and pure botanical compounds against Eimeria tenella in vitro. Poult. Sci. 2023, 102, 102898. [Google Scholar] [CrossRef]
- Bilotto, F.; Fusé, L.A.; Sagües, M.F.; Iglesias, L.E.; Fernández, A.S.; Zegbi, S.; Guerrero, I.; Saumell, C.A. Predatory effect of Duddingtonia flagrans on infective larvae of gastro-intestinal parasites under sunny and shaded conditions. Exp. Parasitol. 2018, 193, 27–32. [Google Scholar] [CrossRef]
- Canhão-Dias, M.; Paz-Silva, A.; Madeira de Carvalho, L.M. The efficacy of predatory fungi on the control of gastrointestinal parasites in domestic and wild animals-A systematic review. Vet. Parasitol. 2020, 283, 109173. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P.; López-Arellano, M.E.; Olmedo-Juárez, A.; Higuera-Pierdrahita, R.I.; von Son-de Fernex, E. Recent Advances in the Control of Endoparasites in Ruminants from a Sustainable Perspective. Pathogens 2023, 12, 1121. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Cunha, E.; de Carvalho, L.M.; Paz-Silva, A.; Oliveira, M. First insights on the susceptibility of native coccidicidal fungi Mucor circinelloides and Mucor lusitanicus to different avian antiparasitic drugs. BMC Vet. Res. 2024, 20, 63. [Google Scholar] [CrossRef]
- Hernández, J.Á.; Sánchez-Andrade, R.; Cazapal-Monteiro, C.F.; Arroyo, F.L.; Sanchís, J.M.; Paz-Silva, A.; Arias, M.S. A combined effort to avoid strongyle infection in horses in an oceanic climate region: Rotational grazing and parasiticidal fungi. Parasites Vectors 2018, 11, 240. [Google Scholar] [CrossRef]
- Lozano, J.; Almeida, C.; Vicente, E.; Sebastião, D.; Palomero, A.M.; Cazapal-Monteiro, C.; Arias, M.S.; Oliveira, M.; Carvalho, L.M.; Paz-Silva, A. Assessing the efficacy of the ovicidal fungus Mucor circinelloides in reducing coccidia parasitism in peacocks. Sci. Rep. 2024, 14, 11352. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.M.; Allanson, M.; Kwa, B.; Azizan, A.; Izurieta, R. Morphological changes of Ascaris spp. eggs during their development outside the host. J. Parasitol. 2012, 98, 63–68. [Google Scholar] [CrossRef]
- Wuthijaree, K.; Lambertz, C.; Gauly, M. Prevalence of gastrointestinal helminth infections in free-range laying hens under mountain farming production conditions. Br. Poult. Sci. 2017, 58, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Hunt, P.W.; Hine, B.C.; Ruhnke, I. The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: New insights into an old problem. Poult. Sci. 2019, 98, 6517–6526. [Google Scholar] [CrossRef]
- Höglund, J.; Daş, G.; Tarbiat, B.; Geldhof, P.; Jansson, D.S.; Gauly, M. Ascaridia galli—An old problem that requires new solutions. Int. J. Parasitol. Drugs Drug Resist. 2023, 23, 1–9. [Google Scholar] [CrossRef]
- Cortiñas, F.J.; Cazapal-Monteiro, C.F.; Hernández, J.A.; Arroyo, F.L.; Miguélez, S.; Suárez, J.; López de Arellano, M.E.; Sánchez-Andrade, R.; Mendoza de Gives, P.; Paz-Silva, A.; et al. Potential Use of Mucor circinelloides for the Biological Control of Certain Helminths Affecting Livestock Reared in a Care Farm. Biocontrol Sci. Technol. 2015, 25, 1443–1452. [Google Scholar] [CrossRef]
- Hernández, J.A.; Cazapal-Monteiro, C.F.; Arroyo, F.L.; Silva, M.I.; Palomero, A.M.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias, M.S. Biological Control of Soil Transmitted Helminths (STHs) in a Zoological Park by Using Saprophytic Fungi. Biol. Control 2018, 122, 24–30. [Google Scholar] [CrossRef]
- Anane, A.; Dufailu, O.A.; Addy, F. Ascaridia galli and Heterakis gallinarum prevalence and genetic variance of A. galli in rural chicken from the Northern Region, Ghana. Vet. Parasitol. Reg. Stud. Rep. 2022, 29, 100692. [Google Scholar] [CrossRef] [PubMed]
- Wongrak, K.; Daş, G.; Moors, E.; Sohnrey, B.; Gauly, M. Establishment of gastro-intestinal helminth infections in free-range chickens: A longitudinal on farm study. Berl. Münch. Tierarztl. Wochenschr. 2014, 127, 314–321. [Google Scholar] [CrossRef]
- Bestman, M.; Verwer, C.; van Niekerk, T.; Leenstra, F.; Reuvekamp, B.; Amsler-Kepalaite, Z.; Maurer, V. Factors related to free-range use in commercial laying hens. Appl. Anim. Behav. Sci. 2019, 214, 57–63. [Google Scholar] [CrossRef]
- Bestman, M.; van Niekerk, T.; Göransson, L.; Ferrante, V.; Gunnarsson, S.; Grilli, G.; Arndt, S.S.; Bas Rodenburg, T. Free-range use and intestinal parasites in organic/free-range laying hens. J. Appl. Poult. Res. 2023, 32, 100321. [Google Scholar] [CrossRef]
- Jansson, D.S.; Nyman, A.; Vågsholm, I.; Christensson, D.; Göransson, M.; Fossum, O.; Höglund, J. Ascarid infections in laying hens kept in different housing systems. Avian Pathol. 2010, 39, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Hinrichsen, L.K.; Brenninkmeyer, C.; Gunnarsson, S.; Heerkens, J.L.; Verwer, C.; Niebuhr, K.; Willett, A.; Grilli, G.; Thamsborg, S.M.; et al. Prevalence and magnitude of helminth infections in organic laying hens (Gallus gallus domesticus) across Europe. Vet. Parasitol. 2015, 214, 118–124. [Google Scholar] [CrossRef]
- Walker, J.G.; Morgan, E.R. Generalists at the interface: Nematode transmission between wild and domestic ungulates. Int. J. Parasitol. Parasites Wildl. 2014, 3, 242–250. [Google Scholar] [CrossRef]
- Faedo, M.; Larsen, M.; Grønvold, J. Predacious activity of Duddingtonia flagrans within the cattle faecal pat. J. Helminthol. 2002, 76, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, B.; Iorio, R.; Morelli, S.; Di Teodoro, L.; De Angelis, E.; Bartolini, R.; Di Cesare, A. A Pilot Study of the in Vitro Efficacy of Different Concentrations of Duddingtonia flagrans for the Control of Gastrointestinal Nematodes of Sheep. Ann. Parasitol. 2024, 70, 113–118. [Google Scholar]
- Mendoza-de Gives, P.; López-Arellano, M.E.; Aguilar-Marcelino, L.; Olazarán-Jenkins, S.; Reyes-Guerrero, D.; Ramírez-Vargas, G.; Vega-Murillo, V.E. The nematophagous fungus Duddingtonia flagrans reduces the gastrointestinal parasitic nematode larvae population in faeces of orally treated calves maintained under tropical conditions-Dose/response assessment. Vet. Parasitol. 2018, 263, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Voinot, M.; Cazapal-Monteiro, C.; Hernández, J.Á.; Palomero, A.M.; Arroyo, F.L.; Sanchís, J.; Pedreira, J.; Sánchez-Andrade, R.; Paz-Silva, A.; Arias, M.S. Integrating the control of helminths in dairy cattle: Deworming, rotational grazing and nutritional pellets with parasiticide fungi. Vet. Parasitol. 2020, 278, 109038. [Google Scholar] [CrossRef]
- Paz-Silva, A.; Salmo, R.; Viña, C.; Miguel Palomero, A.; Ángel Hernández, J.; Sánchez-Andrade, R.; Cazapal-Monteiro, C.; Sol Arias, M. Gelatin Treats Containing Filamentous Fungi to Promote Sustainable Control of Helminths among Pets and Zoo Animals. Biol. Control 2023, 179, 105184. [Google Scholar] [CrossRef]
- Salmo, R.; Viña, C.; Zubiria, I.; Malagón, J.Á.H.; Sanchís, J.M.; Cazapal, C.; Arias, M.S.; Sánchez-Andrade, R.; Paz-Silva, A. Formulating Parasiticidal Fungi in Dried Edible Gelatins to Reduce the Risk of Infection by Trichuris sp. among Continuous Grazing Bison. Pathogens 2024, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, S.; Gauly, M.; Daş, G. Embryonation ability of Ascaridia galli eggs isolated from worm uteri or host faeces. Vet. Parasitol. 2016, 215, 29–34. [Google Scholar] [CrossRef]
- Soares, P.R.; Pato, R.L.; Dias, S.; Santos, D. Effects of Grazing Indigenous Laying Hens on Soil Properties: Benefits and Challenges to Achieving Soil Fertility. Sustainability 2022, 14, 3407. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Cullere, M.; Pellattiero, E.; Sartori, A.; Marangon, A.; Bondesan, V. Is the farming method (cage, barn, organic) a relevant factor for marketed egg quality traits? Livest. Sci. 2021, 246, 104453. [Google Scholar] [CrossRef]
- Tarbiat, B.; Enweji, N.; Jansson, D.S.; Wallström, E.; Osterman-Lind, E.; Höglund, J. A follow-up on the Swedish roundworm control program: Strengths and weaknesses. JAPR 2023, 32, 100356. [Google Scholar] [CrossRef]
- Feyera, T.; Sharpe, B.; Elliott, T.; Shifaw, A.Y.; Ruhnke, I.; Walkden-Brown, S.W. Anthelmintic efficacy evaluation against different developmental stages of Ascaridia galli following individual or group administration in artificially trickle-infected chickens. Vet. Parasitol. 2022, 301, 109636. [Google Scholar] [CrossRef]
- Zirintunda, G.; Biryomumaisho, S.; Kasozi, K.I.; Batiha, G.E.; Kateregga, J.; Vudriko, P.; Nalule, S.; Olila, D.; Kajoba, M.; Matama, K.; et al. Emerging Anthelmintic Resistance in Poultry: Can Ethnopharmacological Approaches Offer a Solution? Front. pharmacol. 2022, 12, 774896. [Google Scholar] [CrossRef]
- Tarbiat, B.; Jansson, D.S.; Höglund, J. Environmental tolerance of free-living stages of the poultry roundworm Ascaridia galli. Vet. Parasitol. 2015, 209, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Jansson, D.S. Infection dynamics of Ascaridia galli in non-caged laying hens. Vet. Parasitol. 2011, 180, 267–273. [Google Scholar] [CrossRef] [PubMed]


| Group | Week | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Control | 8.3 | 16.7 | 20.0 | 26.7 |
| G1 | 11.9 | 37.3 | 55.9 | 64.4 |
| G2 | 17.7 | 43.5 | 67.7 | 71.0 |
| G3 | 21.3 | 54.1 | 68.9 | 78.7 |
| Group | Week | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Control | 0.0 | 20.0 | 24.0 | 26.0 |
| G1 | 7.4 | 66.7 | 74.1 | 78.9 |
| G2 | 20.0 | 46.7 | 73.3 | 81.7 |
| G3 | 20.0 | 44.0 | 80.0 | 80.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León, J.A.; Pérez-Anzúrez, G.; Ramos, I.A.; Magos Amado, C.E.; Boso Dafonte, D.; Lozano, J.; Hernández Malagón, J.Á.; Cazapal-Monteiro, C.; Bonilla, R.; Sanchís, J.; et al. Testing a Sustainable Strategy Against Poultry Helminth Stages Developing in the Soil. Pathogens 2025, 14, 1168. https://doi.org/10.3390/pathogens14111168
León JA, Pérez-Anzúrez G, Ramos IA, Magos Amado CE, Boso Dafonte D, Lozano J, Hernández Malagón JÁ, Cazapal-Monteiro C, Bonilla R, Sanchís J, et al. Testing a Sustainable Strategy Against Poultry Helminth Stages Developing in the Soil. Pathogens. 2025; 14(11):1168. https://doi.org/10.3390/pathogens14111168
Chicago/Turabian StyleLeón, Jorge Alexander, Gustavo Pérez-Anzúrez, Inês Abreu Ramos, Carlos Emiliano Magos Amado, David Boso Dafonte, João Lozano, José Ángel Hernández Malagón, Cristiana Cazapal-Monteiro, Rodrigo Bonilla, Jaime Sanchís, and et al. 2025. "Testing a Sustainable Strategy Against Poultry Helminth Stages Developing in the Soil" Pathogens 14, no. 11: 1168. https://doi.org/10.3390/pathogens14111168
APA StyleLeón, J. A., Pérez-Anzúrez, G., Ramos, I. A., Magos Amado, C. E., Boso Dafonte, D., Lozano, J., Hernández Malagón, J. Á., Cazapal-Monteiro, C., Bonilla, R., Sanchís, J., Paz-Silva, A., Sánchez-Andrade, R., Madeira de Carvalho, L. M., & Arias, M. S. (2025). Testing a Sustainable Strategy Against Poultry Helminth Stages Developing in the Soil. Pathogens, 14(11), 1168. https://doi.org/10.3390/pathogens14111168









