Morphological and Molecular Studies of Tetracotyle-Type Metacercariae of the Genus Cotylurus Szidat, 1928 (Trematoda) from the Gravel Snail Lithoglyphus naticoides (Gastropoda) and Host Sex Dependent Differences in Infection Rate
Abstract
1. Introduction
2. Material and Methods
Molecular and Phylogenetic Analyses
3. Results
3.1. Infection Parameters
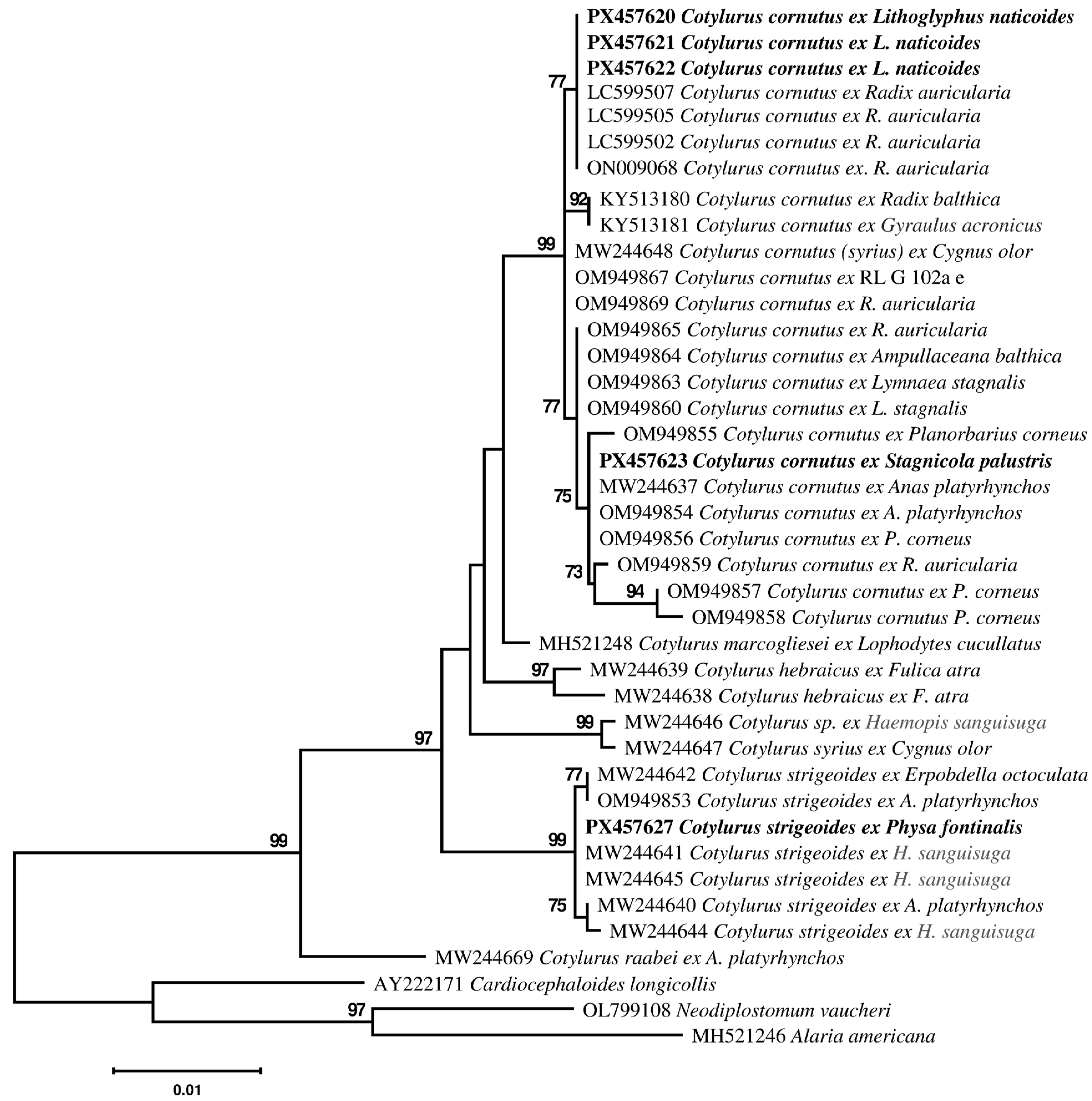
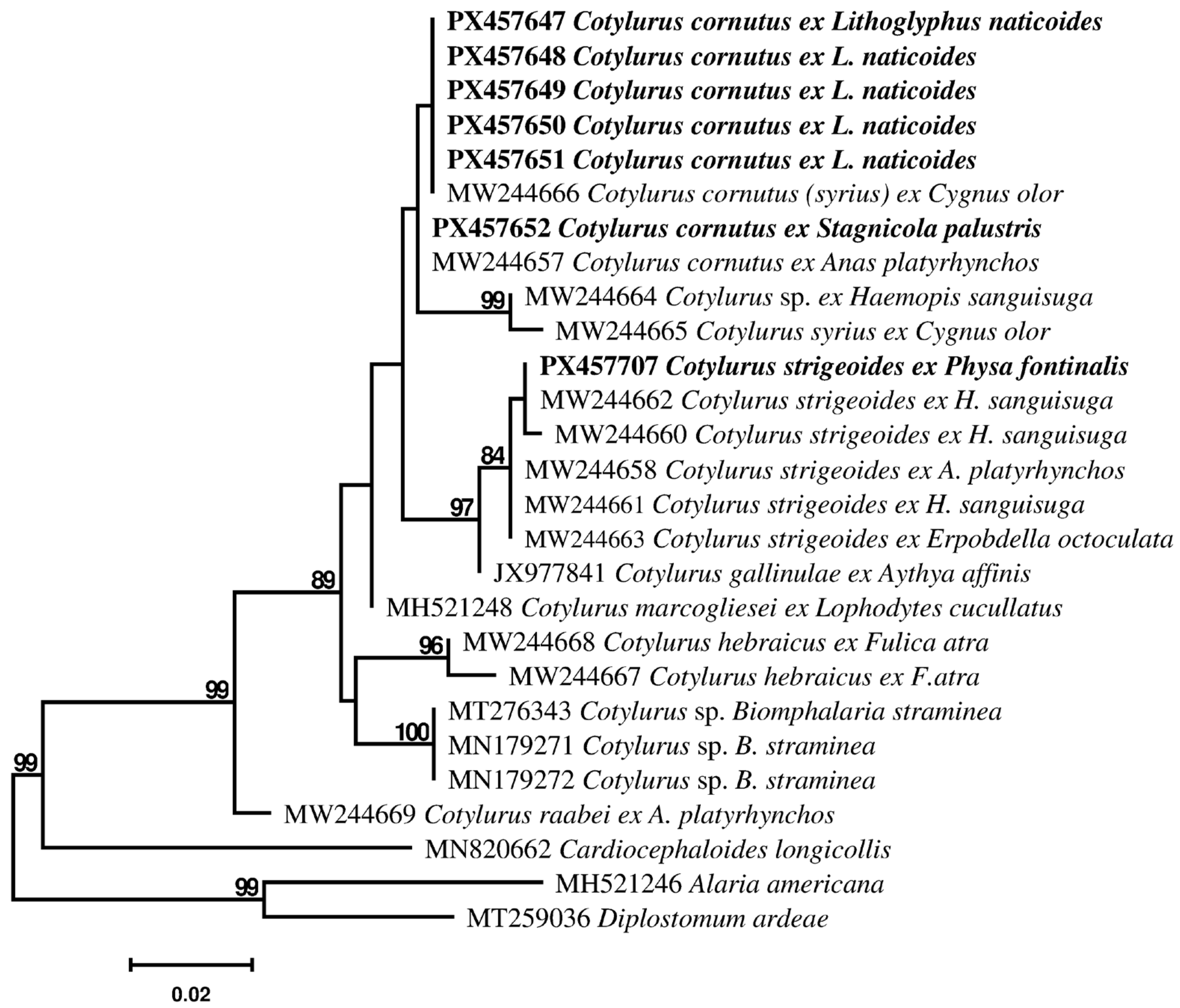
3.2. Molecular Identification and Phylogenetic Analyses
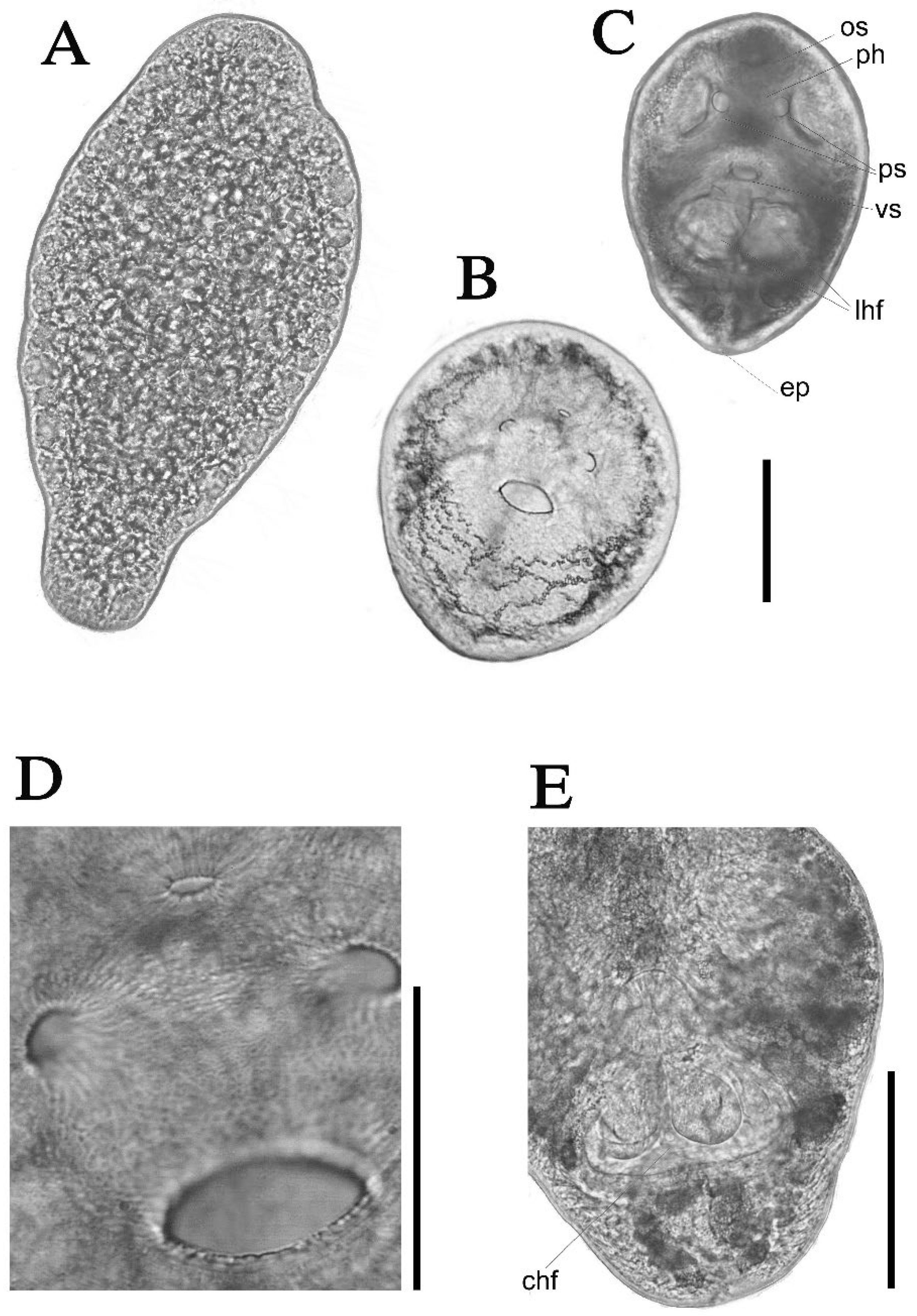
3.3. Morphological Description
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbačiauskas, K.; Višinskienė, G.; Smilgevičienė, S.; Rakauskas, V. Non-indigenous macroinvertebrate species in Lithuanian fresh waters, Part 1: Distributions, dispersal and future. Knowl. Manag. Aquat. Ecosyst. 2011, 402, 12. [Google Scholar] [CrossRef]
- Butkus, R.; Šidagytė, E.; Rakauskas, V.; Arbačiauskas, K. Distribution and current status of nonindigenous mollusc species in Lithuanian inland waters. Aquat. Invasions 2014, 9, 95–103. [Google Scholar] [CrossRef]
- Schlesch, H. Bemerkungen über die verbreitung der süsswasser- und meres-mollusken im östlichen Ostseegebiete. Tartu Ülikooli Juures Oleva Loodusuur. Seltsi Aruan. 1937, 43, 37–64. [Google Scholar]
- Schlesch, H.; Krausp, C. Zur kenntniss der land- und süsswasser-mollusken Litauens. Arch. Molluskenkd. 1938, 70, 73–125. [Google Scholar]
- Arbačiauskas, K.; Višinskienė, G.; Smilgevičienė, S. Non-indigenous macroinvertebrate species in Lithuanian fresh waters, Part 2: Macroinvertebrate assemblage deviation from naturalness in lotic systems and the consequent potential impacts on ecological quality assessment. Knowl. Manag. Aquat. Ecosyst. 2011, 402, 13. [Google Scholar] [CrossRef]
- Zdun, V. Larvae of Trematodes in Freshwater Molluscs of Ukraine; Ukrainian Academy of Sciences Press: Kiev, Ukraine, 1961; p. 141. (In Ukrainian) [Google Scholar]
- Odening, K. Der trematodenbefall von Lithoglyphus naticoides (Gastropoda) im Berliner Müggelsee 1968. Parasitol. Schrift. 1971, 21, 169–178. [Google Scholar]
- Chernogorenko, M.I. Larvae of Trematodes in Molluscs in Dnepr River and Its Reservoirs (Fauna, Biology, and Peculiarities of Formation); Naukova Dumka: Kiev, Ukraine, 1983; p. 221. (In Russian) [Google Scholar]
- Stanevičiūtė, G.; Petkevičiūtė, R.; Kiselienė, V. Digenean parasites in prosobranch snail Lithoglyphus naticoides population with the morphological description of Echinochasmus sp. cercaria. Ekologija 2008, 54, 251–255. [Google Scholar] [CrossRef]
- Tyutin, A.V.; Slynko, Y.V. The first finding of the Black Sea snail Lithoglyphus naticoides (Gastropoda) and its associated species-specific Trematoda in the upper Volga basin. Russ. J. Biol. Invasions 2010, 1, 45–49. [Google Scholar] [CrossRef]
- Akimova, L.N. Trematodes associated with the alien gastropod species Lithoglyphus naticoides in Belarus. Biol. Res. 2023, 2, 29–35. [Google Scholar]
- Pyrka, E.; Kanarek, G.; Gabrysiak, J.; Jeżewski, W.; Cichy, A.; Stanicka, A.; Żbikowska, E.; Zaleśny, G.; Hildebrand, J. Life history strategies of Cotylurus spp. Szidat, 1928 (Trematoda, Strigeidae) in the molecular era—Evolutionary consequences and implications for taxonomy. Int. J. Parasitol. Parasites Wildl. 2022, 18, 201–211. [Google Scholar] [CrossRef]
- Krause, H. Untersuchungen zur anatomie und ökologie von Lithoglyphus naticoides (C. Pfeiffer). Arch. Molluskenkd. 1949, 78, 103–148. [Google Scholar]
- Zhadin, V.I. Mollusks of Freshwater and Brackish Waters of the U.S.S.R.; Academy of Sciences USSR Press: Moscow, Russia, 1952; p. 376. (In Russian) [Google Scholar]
- Stadnychenko, A.P. Pathogenic effect of trematode larvae on Viviparus viviparus (L., 1758) (Gastropoda, Prosobranchia). Parazitologiya 1972, 6, 154–160. (In Russian) [Google Scholar]
- Jeżewski, W. Occurrence of Digenea (Trematoda) in two Viviparus species from lakes, rivers and a dam reservoir. Helminthologia 2004, 41, 147–150. [Google Scholar]
- Cort, W.W.; Olivier, L.; Brackett, S. The relation of physid and planorbid snails to the life cycle of the strigeid trematode, Cotylurus flabelliformis (Faust, 1917). J. Parasitol. 1941, 27, 437–448. [Google Scholar] [CrossRef]
- Ulmer, M. Notes on the development of Cotylurus flabelliformis tetracotyles in the second intermediate host (Trematoda: Strigeidae). Trans. Am. Microsc. Soc. 1957, 76, 321–327. [Google Scholar] [CrossRef]
- Stunžėnas, V.; Petkevičiūtė, R.; Stanevičiūtė, G. Phylogeny of Sphaerium solidum (Bivalvia) based on karyotype and sequences of 16S and ITS1 rDNA. Cent. Eur. J. Biol. 2011, 6, 105–117. [Google Scholar] [CrossRef]
- Petkevičiūtė, R.; Stunžėnas, V.; Stanevičiūtė, G. Differentiation of European freshwater bucephalids (Digenea: Bucephalidae) based on karyotypes and DNA sequences. Syst. Parasitol. 2014, 87, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.; De Chambrier, A.; Kuchta, R.; Littlewood, D.T.J.; Waeschenbach, A. Macrobothriotaenia ficta (Cestoda: Proteocephalidea), a parasite of sunbeam snake (Xenopeltis unicolor): Example of convergent evolution. Zootaxa 2013, 3640, 485–499. [Google Scholar] [CrossRef]
- Tkach, V.; Grabda-Kazubska, B.; Pawlowski, J.; Swiderski, Z. Molecular and morphological evidences for close phylogenetic affinities of the genera Macrodera, Leptophallus, Metaleptophallus, and Paralepoderma (Digenea, Plagiorchioidea). Acta Parasitol. 1999, 44, 170–179. [Google Scholar] [CrossRef]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Tkach, V.V.; Littlewood, D.T.J.; Olson, P.D.; Kinsella, M.J.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Morozov, 1955 (Trematoda: Digenea): A bigger data set resolves fewer genera. Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Soldanova, M.; Georgieva, S.; Roháčová, J.; Knudsen, R.; Kuhn, J.A.; Henriksen, E.H.; Siwertsson, A.; Shaw, J.C.; Kuris, A.M.; Amundsen, P.A.; et al. Molecular analyses reveal high species diversity of trematodes in a sub-Arctic lake. Int. J. Parasitol. 2017, 47, 327–345. [Google Scholar] [CrossRef]
- Pyrka, E.; Kanarek, G.; Zaleśny, G.; Hildebrand, J. Leeches as the intermediate host for strigeid trematodes: Genetic diversity and taxonomy of the genera Australapatemon Sudarikov, 1959 and Cotylurus Szidat, 1928. Parasit. Vectors 2021, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Sasaki, M. Trematode diversity in freshwater snails from a stopover point for migratory waterfowls in Hokkaido, Japan: An assessment by molecular phylogenetic and population genetic analyses. Parasitol. Int. 2021, 83, 102329. [Google Scholar] [CrossRef]
- Hernández-Mena, D.I.; García-Prieto, L.; García-Varela, M. Morphological and molecular differentiation of Parastrigea (Trematoda: Strigeidae) from Mexico, with the description of a new species. Parasitol. Int. 2014, 63, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Locke, S.A.; Van Dam, A.; Caffara, M.; Pinto, H.A.; López-Hernández, D.; Blanar, C.A. Validity of the Diplostomoidea and Diplostomida (Digenea, Platyhelminthes) upheld in phylogenomic analysis. Int. J. Parasitol. 2018, 48, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, D.; Locke, S.A.; de Assis, J.C.A.; Drago, F.B.; de Melo, A.L.; Rabelo, É.M.L.; Pinto, H.A. Molecular, morphological and experimental-infection studies of cercariae of five species in the superfamily Diplostomoidea (Trematoda: Digenea) infecting Biomphalaria straminea (Mollusca: Planorbidae) in Brazil. Acta Trop. 2019, 199, 105082. [Google Scholar] [CrossRef]
- Fernández, M.V.; Hamann, M.I.; Davies, C.; Lauthier, J.J.; Davies, D. First record of Cotylurus metacercariae (Trematoda:Strigeidae) in Biomphalaria straminea (Planorbidae) from Argentina: Morphological and molecular identification. Ann. Parasitol. 2022, 68, 473–481. [Google Scholar] [CrossRef]
- Achatz, T.J.; Pulis, E.E.; González-Acuña, D.; Tkach, V.V. Phylogenetic relationships of Cardiocephaloides spp. (Digenea, Diplostomoidea) and the genetic characterization of Cardiocephaloides physalis from Magellanic penguin, Spheniscus magellanicus, in Chile. Acta Parasitol. 2020, 65, 525–534. [Google Scholar] [CrossRef]
- Locke, S.A.; Drago, F.B.; Núñez, V.; Souza, G.T.R.; Takemoto, R.M. Phylogenetic position of Diplostomum spp. from New World herons based on complete mitogenomes, rDNA operons, and DNA barcodes, including a new species with partially elucidated life cycle. Parasitol. Res. 2020, 119, 2129–2137. [Google Scholar] [CrossRef]
- Achatz, T.J.; Pulis, E.E.; Woodyard, E.T.; Rosser, T.G.; Martens, J.R.; Weinstein, S.B.; Fecchio, A.; McAllister, C.T.; Bonilla, C.C.; Tkach, V.V. Molecular phylogenetic analysis of Neodiplostomum and Fibricola (Digenea, Diplostomidae) does not support host-based systematics. Parasitology 2022, 149, 542–554. [Google Scholar] [CrossRef]
- Sudarikov, V.E. Trematodes of the Fauna USSR. Strigeids; Nauka: Moscow, Russia, 1984; p. 169. (In Russian) [Google Scholar]
- Heneberg, P.; Sitko, J.; Těšínský, M.; Rząd, M.; Bizos, J. Central European Strigeidae Railliet, 1919 (Trematoda: Strigeidida): Molecular and comparative morphological analysis suggests the reclassification of Parastrigea robusta Szidat, 1928 into Strigea Abildgaard, 1790. Parasitol. Int. 2018, 67, 688–701. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000; p. 464. [Google Scholar]
- Bray, R.A.; Cutmore, S.C.; Cribb, T.H. A paradigm for the recognition of cryptic trematode species in tropical Indo-west Pacific fishes: The problematic genus Preptetos (Trematoda: Lepocreadiidae). Int. J. Parasitol. 2022, 52, 169–203. [Google Scholar] [CrossRef]
- Duneau, D.; Ebert, D. Host sexual dimorphism and parasite adaptation. PLoS Biol. 2012, 10, e1001271. [Google Scholar] [CrossRef]
- Matozzo, V.; Marin, M.G. First cytochemical study of haemocytes from the crab Carcinus aestuarii (Crustacea, Decapoda). Eur. J. Histochem. 2010, 54, e9. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shi, Y.; Yao, T.; Bai, C.; Jiang, J.; Ye, L. Gender differences in hemocyte immune parameters of Hong Kong oyster Crassostrea hongkongensis during immune stress. Front. Immunol. 2021, 12, 659469. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, M.B.; Fournier, M.; Auffret, M. Seasonal variations of immune parameters in diploid and triploid Pacific oysters, Crassostrea gigas (Thunberg). Aquaculture 2007, 264, 73–81. [Google Scholar] [CrossRef]
- Campbell, R.A. Host-finding behavior of Cotylurus flabelliformis (Trematoda: Strigeidae) cercariae for snail hosts. Folia Parasitol. 1997, 44, 199–204. [Google Scholar]
| Parasite Species | Host Species | Locality | GenBank ID * [Reference] | |
|---|---|---|---|---|
| 28S | 5.8S-ITS2-28S | |||
| Cotylurus cornutus (mt) | Lithoglyphus naticoides | Kaunas water Reservoir, Lithuania | PX457620, PX457621, PX457622 | PX457647, PX457648, PX457649, PX457650, PX457651 |
| Cotylurus cornutus (mt) | Galba truncatula | Gintaras Bay, Juodkrantė, Lithuania | PX457623 | PX457652 |
| Cotylurus cornutus (mt) | Radix balthica | Lake Takvatn, Norway | KY513180 [28] | |
| Cotylurus cornutus (mt) | Gyraulus acronicus | Lake Takvatn, Norway | KY513181 [28] | |
| Cotylurus cornutus | Anas platyrhynchos | Gdańsk Pomerania, Poland | MW244637 [29] | MW244657 [29] |
| Cotylurus cornutus | Anas platyrhynchos | Lower Silesia, Poland | OM949854 [12] | |
| Cotylurus cornutus (mt) | Planorbarius corneus | Lower Silesia, Poland | OM949855 [12] | |
| Cotylurus cornutus (mt) | Planorbarius corneus | Lower Silesia, Poland | OM949857 [12] | |
| Cotylurus cornutus (mt) | Planorbarius corneus | Lower Silesia, Poland | OM949858 [12] | |
| Cotylurus cornutus (mt) | Radix auricularia | Lower Silesia, Poland | OM949859 [12] | |
| Cotylurus cornutus (mt) | Lymnaea stagnalis | Lower Silesia, Poland | OM949863 [12] | |
| Cotylurus cornutus (mt) | Ampullaceana balthica | Lower Silesia, Poland | OM949864 [12] | |
| Cotylurus cornutus (mt) | Radix auricularia | Lower Silesia, Poland | OM949865 [12] | |
| Cotylurus cornutus (mt) | Radix auricularia | Shihezi, Xinjiang, China | ON009068 (unpubl) | |
| Cotylurus cornutus (mt, sporocyst) | Radix auricularia | Hokkaido, Asahikawa, Japan | LC599502, LC599505, LC599507 [30] | |
| Cotylurus cornutus (mt) | Radix auricularia | Lower Silesia, Poland | OM949869 [12] | |
| Cotylurus cornutus (mt) | Peregriana labiata (=Radix labiata) | Lower Silesia, Poland | OM949867 [12] | |
| Cotylurus cornutus (mt) | Lymnaea stagnalis | Lower Silesia, Poland | OM949860 [12] | |
| Cotylurus cornutus (mt) | Planorbarius corneus | Lower Silesia, Poland | OM949856 [12] | |
| Cotylurus cornutus (syrius) | Cygnus olor | Lower Silesia, Poland | MW244648 [29] | MW244666 [29] |
| Cotylurus gallinulae | Aythya affinis | Mexico | JX977841 [31] | |
| Cotylurus hebraicus | Fulica atra | Gdańsk Pomerania, Poland | MW244638, MW244639 [29] | MW244667, MW244668 [29] |
| Cotylurus marcogliesei | Lophodytes cucullatus | Hudson, Montreal area, QC, Canada | MH521248 [32] | MH521248 [32] |
| Cotylurus raabei (=Cotylurostrigea raabei) | Anas platyrhynchos | Gdańsk Pomerania | MW244649 [29] | MW244669 [29] |
| Cotylurus strigeoides (sporocyst) | Physa fontinalis | Gintaras Bay, Juodkrantė, Lithuania | PX457627 | PX457707 |
| Cotylurus strigeoides (mt) | Haemopis sanguisuga | Lower Silesia, Poland | MW244641, MW244645 [29] | MW244662 [29] |
| Cotylurus strigeoides (mt) | Haemopis sanguisuga | Gdańsk Pomerania, Poland | MW244660, MW244661, [29] | |
| Cotylurus strigeoides (mt) | Haemopis sanguisuga | Gdańsk Pomerania, Poland | MW244644 [29] | |
| Cotylurus strigeoides | Anas platyrhynchos | Gdańsk Pomerania, Poland | MW244640 [29] | MW244658 [29] |
| Cotylurus strigeoides (mt) | Erpobdella octoculata | Lower Silesia, Poland | MW244642 [29] | MW244663 [29] |
| Cotylurus strigeoides | Anas platyrhynchos | Poland | OM949853 [12] | |
| Cotylurus syrius | Cygnus olor | Gdańsk Pomerania, Poland | MW244647 [29] | MW244665 [29] |
| Cotylurus sp. (syrius) (mt) | Haemopis sanguisuga | Gdańsk Pomerania, Poland | MW244646 [29] | MW244664 [29] |
| Cotylurus sp. (cercaria, mt) | Biomphalaria straminea | Brazil: Minas Gerais | MN179271 MN179272 [33] | |
| Cotylurus sp. (mt) | Biomphalaria straminea | Corrientes province, Argentina | MT276343 [34] | |
| Outgroup | ||||
| Alaria americana | Vulpes vulpes | Canada | MH521246 [32] | MH521246 [32] |
| Cardiocephaloides longicollis | Chroicocephalus ridibundus | Ukraine | AY222171 [23] | MN820662 [35] |
| Diplostomum ardeae | Nycticorax nycticorax | Puerto Rico | MT259036 [36] | |
| Neodiplostomum vaucheri | Trachops cirrhosus | USA | OL799108 [37] | |
| 2010 | 2011 | 2018 | 2019 | |||||
|---|---|---|---|---|---|---|---|---|
| Host sex | Dissected/infected | % | Dissected/infected | % | Dissected/infected | % | Dissected/infected | % |
| ♂ | 61/26 | 42.6 | 120/54 | 45 | 104/32 | 30.8 | 15/11 | 73.3 |
| ♀ | 61/4 | 6.6 | 120/3 | 2.5 | 103/8 | 7.8 | 27/0 | - |
| Not identified | 169/16 | 9.5 | ||||||
| Total number of snails | 291 | 240 | 207 | 42 | ||||
| Year, Host Sex | Intensity of Infection (Number of Metacercariae per Specimen) | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 13 | 15 | |||
| Number of specimens infected | 2010, sex not identified | 4 | 4 | 3 | 2 | 1 | 1 | 1 | 16 | |||||
| 2010, ♂ | 12 | 4 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 26 | ||||
| 2010, ♀ | 2 | 1 | 1 | 4 | ||||||||||
| 2011, ♂ | 12 | 15 | 8 | 8 | 3 | 3 | 1 | 3 | 1 | 54 | ||||
| 2011, ♀ | 2 | 1 | 3 | |||||||||||
| 2018, ♂ | 18 | 6 | 1 | 4 | 1 | 1 | 1 | 32 | ||||||
| 2018, ♀ | 5 | 2 | 1 | 8 | ||||||||||
| 2019, ♂ | 2 | 5 | 1 | 2 | 1 | 11 | ||||||||
| 2019, ♀ | 0 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanevičiūtė, G.; Stunžėnas, V.; Petkevičiūtė, R. Morphological and Molecular Studies of Tetracotyle-Type Metacercariae of the Genus Cotylurus Szidat, 1928 (Trematoda) from the Gravel Snail Lithoglyphus naticoides (Gastropoda) and Host Sex Dependent Differences in Infection Rate. Pathogens 2025, 14, 1063. https://doi.org/10.3390/pathogens14101063
Stanevičiūtė G, Stunžėnas V, Petkevičiūtė R. Morphological and Molecular Studies of Tetracotyle-Type Metacercariae of the Genus Cotylurus Szidat, 1928 (Trematoda) from the Gravel Snail Lithoglyphus naticoides (Gastropoda) and Host Sex Dependent Differences in Infection Rate. Pathogens. 2025; 14(10):1063. https://doi.org/10.3390/pathogens14101063
Chicago/Turabian StyleStanevičiūtė, Gražina, Virmantas Stunžėnas, and Romualda Petkevičiūtė. 2025. "Morphological and Molecular Studies of Tetracotyle-Type Metacercariae of the Genus Cotylurus Szidat, 1928 (Trematoda) from the Gravel Snail Lithoglyphus naticoides (Gastropoda) and Host Sex Dependent Differences in Infection Rate" Pathogens 14, no. 10: 1063. https://doi.org/10.3390/pathogens14101063
APA StyleStanevičiūtė, G., Stunžėnas, V., & Petkevičiūtė, R. (2025). Morphological and Molecular Studies of Tetracotyle-Type Metacercariae of the Genus Cotylurus Szidat, 1928 (Trematoda) from the Gravel Snail Lithoglyphus naticoides (Gastropoda) and Host Sex Dependent Differences in Infection Rate. Pathogens, 14(10), 1063. https://doi.org/10.3390/pathogens14101063






