Wild Mammals as Sentinels for West Nile Virus Circulation: Evidence from Serbia
Abstract
1. Introduction
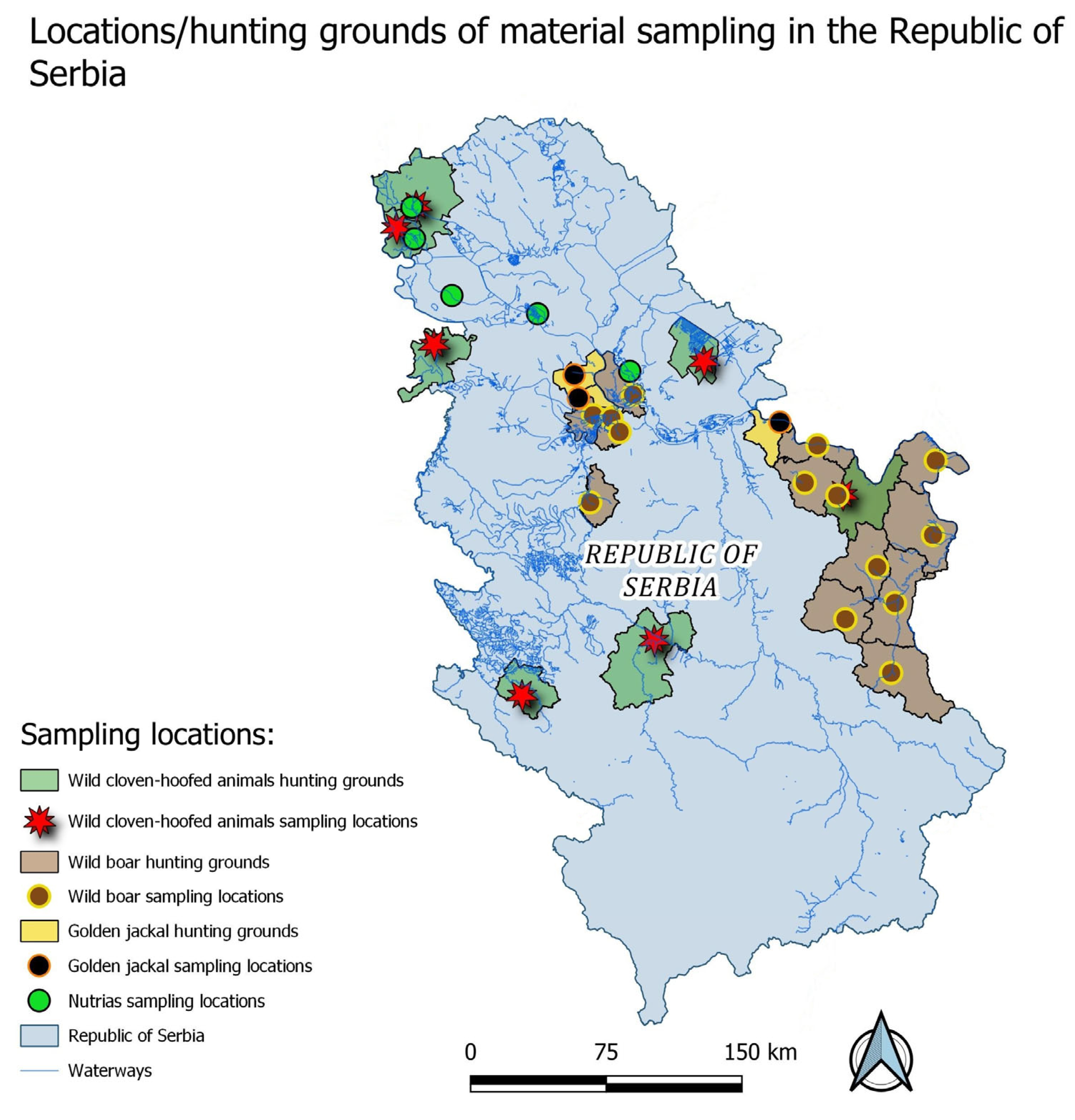
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WNV | West Nile Virus |
| ELISA | Enzyme-linked immunosorbent Assay |
| CL | Confidence Limits |
| Se | Diagnostic sensitivity |
| Sp | Diagnostic specificity |
| TP | True Prevalence |
References
- Radojicic, S.; Zivulj, A.; Petrovic, A.; Nisavic, J.; Milicevic, V.; Sipetic-Grujicic, S.; Misic, J.; Korzeniowska, M.; Stanojevic, S. Spatiotemporal Analysis of West Nile Virus Epidemic in South Banat District, Serbia, 2017–2019. Animals 2021, 11, 2951. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guggemos, H.D.; Fendt, M.; Hleike, C.; Heyde, V.; Mfune, J.K.E.; Borgemeister, C.; Junglen, S. Simultaneous circulation of two WestNile virus lineage 2 clades and Bagazavirus in the Zambezi region, Namibia. PLoS Negl. Trop. Dis. 2021, 15, e0009311. [Google Scholar] [CrossRef]
- Marini, G.; Drakulovic, B.; Jovanovic, V.; Dagostin, F.; Wint, W.; Tagliapietra, V.; Vasic, M.; Rizzoli, A. Drivers and epidemiological patterns of West Nile virus in Serbia. Front. Public Health 2024, 12, 1429583. [Google Scholar] [CrossRef]
- Nemeth, N.; Young, G.; Ndaluka, C.; Bielefeldt-Ohmann, H.; Komar, N.; Bowen, R. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Arch. Virol. 2009, 154, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Root, J.J.; Bosco-Lauth, A.M. West Nile Virus Associations in Wild Mammals: An Update. Viruses 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, L.; Roehrig, J.; Sejvar, J. Global Epidemiology of West Nile Virus. In West Nile Encephalitis Virus Infection; Emerging Infectious Diseases of the 21st Century; Springer: New York, NY, USA, 2009; pp. 1–16. [Google Scholar] [CrossRef]
- Kading, R.; Borland, E.; Cranfield, M.; Powers, A. Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater Congo basin. J. Wildl. Dis. 2013, 49, 3. [Google Scholar] [CrossRef]
- Niczyporuk, J.; Jabłonski, A. Serologic Survey for West Nile Virus in Wild Boars (Sus scrofa) in Poland. J. Wildl. Dis. 2021, 57, 168–171. [Google Scholar] [CrossRef]
- Miller, D.; Debra, L.; Radi, A.; Zaher, A.; Baldwin, C.; Dallas, I. Fatal West Nile Virus Infection in a White-tailed Deer (Odocoileus virginianus). J. Wildl. Dis. 2025, 41, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Schertler, A.; Rabitsch, W.; Moser, D.; Wessely, J.; Essl, F. The potential current distribution of the coypu (Myocastor coypus) in Europe and climate change-induced shifts in the near future. NeoBiota 2020, 58, 129–160. [Google Scholar] [CrossRef]
- Auerswald, H.; Ruget, A.-S.; Ladreyt, H.; In, S.; Mao, S.; Sorn, S.; Tum, S.; Duong, V.; Dussart, P.; Cappelle, J.; et al. Serological Evidence for Japanese Encephalitis and West Nile Virus Infections in Domestic Birds in Cambodia. Front. Vet. Sci. 2020, 7, 15. [Google Scholar] [CrossRef]
- Allen, S.; Jardine, C.; Hooper-McGrevy, K.; Ambagala, A.; Bosco-Lauth, A.M.; Kunkel, M.R.; Mead, D.; Nituch, L.; Ruder, K.; Nemeth, N. Serologic Evidence of Arthropod-Borne Virus Infections in Wild and Captive Ruminants in Ontario, Canada. Am. J. Trop. Med. Hyg. 2020, 103, 2100–2107. [Google Scholar] [CrossRef]
- Kvapil, P.; Racnik, J.; Kastelic, M.; Bártová, E.; Korva, M.; Jelovšek, M.; Avšic-Županc, T. Sentinel Serological Study in Selected Zoo Animals to Assess Early Detection of West Nile and Usutu Virus Circulation in Slovenia. Viruses 2021, 13, 626. [Google Scholar] [CrossRef]
- Assaid, N.; Arich, S.; Ezzikouri, S.; Benjelloun, S.; Dia, M.; Faye, O.; Akarid, K.; Beck CLecollinet, S.; Failloux, A.B.; Sarih, M. Serological evidence of West Nile virus infection in human populations and domestic birds in the Northwest of Morocco. Infect. Dis. 2021, 76, 101646. [Google Scholar] [CrossRef]
- Magallanes, S.; Llorente, F.; Ruiz-López, M.J.; Martínez-de la Puente, J.; Soriguer, R.; Calderon, J.; Jímenez-Clavero, M.Á.; Aguilera-Sepúlveda, P.; Figuerola, J. Long-term serological surveillance for West Nile and Usutu virus in horses in south-west Spain. One Health 2023, 17, 100578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vilibic-Cavlek, T.; Bogdanic, M.; Savic, V.; Hruskar, Z.; Barbic, L.; Stevanovic, V.; Antolasic, L.; Milasincic, L.; Sabadi, D.; Miletic, G.; et al. Diagnosis of West Nile virus infections: Evaluation of different laboratory methods. World J. Virol. 2024, 13, 95986. [Google Scholar] [CrossRef] [PubMed]
- Šolaja, S.; Goletić, Š.; Veljović, L.J.; Glišić, D. Complex patterns of WNV evolution: A focus on the Western Balkans and Central Europe. Front. Vet. Sci. 2024, 11, 1494746. [Google Scholar] [CrossRef]
- INgezim WEST NILE Compac R.10.WNV.K3. Available online: https://www.goldstandarddiagnostics.com/pub/media/productattachments/files/10WNVK3_Technical_sheet_westnile.pdf (accessed on 20 October 2025).
- Epitools. Available online: https://epitools.ausvet.com.au/ (accessed on 20 October 2025).
- Veljović, L.J.; Maksimović Zorić, J.; Radosavljević, V.; Stanojević, S.; Žutić, J.; Kureljušić, B.; Pavlović, I.; Jezdimirović, N.; Milićević, V. Seroprevalence of West Nile fever virus in horses in the Belgrade epizootiological area. Vet. Glas. 2020, 74, 194–201. [Google Scholar] [CrossRef]
- Kramer, L.; Styer, L.; Ebel, G. A Global Perspective on the Epidemiology of West Nile Virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef]
- Boadella, M.; Delgado, I.; Gutiérrez-Guzmán, A.V.; Höfle, U.; Gortázar, C. Do Wild Ungulates Allow Improved Monitoring of Flavivirus Circulation in Spain? Vector-Borne Zoonotic Dis. 2012, 12, 6. [Google Scholar] [CrossRef]
- Niedrig, M.; Mantke, O.; Altmann, D.; Zeller, H. First international diagnostic accuracy study for the serological detection of West Nile virus infection. BMC Infect. Dis. 2007, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Casades-Martí, L.; Cuadrado-Matías, R.; Peralbo-Moreno, A.; Baz-Flores, S.; Fierro, Y.; Ruiz-Fons, F. Insights into the spatiotemporal dynamics of West Nile virus transmission in emerging scenarios. One Health 2023, 16, 100557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hubálek, Z.; Juricová, Z.; Straková, P.; Blazejová, H.; Betásová, L.; Rudolf, I. Serological Survey for West Nile Virus in Wild Artiodactyls, Southern Moravia (Czech Republic). Vector Borne Zoonotic Dis. 2017, 17, 9. [Google Scholar] [CrossRef]
- Halouzka, J.; Juricova, Z.; Jankova, J.; Hubalek, Z. Serologic survey of wild boars for mosquito-borne viruses in South Moravia (Czech Republic). Vet. Med. 2008, 53, 266–271. [Google Scholar] [CrossRef]
- Milićević, V.; Sapundžić, Z.Z.; Glišić, D.; Kureljušić, B.; Vasković, N.; Đorđević, M.; Mirčeta, J. Cross-sectional serosurvey of selected infectious diseases in wild ruminants in Serbia. Res. Vet. Sci. 2024, 170, 105183. [Google Scholar] [CrossRef] [PubMed]
| Species | Number of Samples | Adults | Juvenile |
|---|---|---|---|
| Wild boar | 100 | 53 | 47 |
| Nutria | 101 | 87 | 14 |
| Golden jackal | 68 | 57 | 11 |
| Red deer | 172 | 137 | 35 |
| Roe deer | 81 | 69 | 12 |
| Total No of samples | 522 | 403 | 119 |
| Species | Total Tested | Total Positive (%) | TP (%) | 95% CI (Lower–Upper) | Adults Tested | Adults Positive (%) | Juveniles Tested | Juveniles Positive (%) |
|---|---|---|---|---|---|---|---|---|
| Wild boar | 100 | 37 (37.0) | 36.0 | 0.28–0.47 | 53 | 23 (43.4) | 47 | 14 (29.8) |
| Nutria | 101 | 12 (11.9) | 10.5 | 0.07–0.20 | 87 | 10 (11.5) | 14 | 2 (14.3) |
| Golden jackal | 68 | 22 (32.4) | 31.3 | 0.22–0.44 | 57 | 17 (29.8) | 11 | 5 (45.5) |
| Red deer | 172 | 87 (50.6) | 48.8 | 0.42–0.58 | 137 | 81 (59.1) | 35 | 6 (17.1) |
| Roe deer | 81 | 7 (8.6) | 7.2 | 0.03–0.15 | 69 | 5 (7.2) | 12 | 2 (16.7) |
| Total | 522 | 165 (31.6) | 30.5 | 0.27–0.35 | 403 | 136 (33.7) | 119 | 29 (24.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veljović, L.; Paunović, M.; Glišić, D.; Šolaja, S.; Zurovac Sapundžić, Z.; Maletić, J.; Milovanović, B.; Milićević, V. Wild Mammals as Sentinels for West Nile Virus Circulation: Evidence from Serbia. Pathogens 2025, 14, 1167. https://doi.org/10.3390/pathogens14111167
Veljović L, Paunović M, Glišić D, Šolaja S, Zurovac Sapundžić Z, Maletić J, Milovanović B, Milićević V. Wild Mammals as Sentinels for West Nile Virus Circulation: Evidence from Serbia. Pathogens. 2025; 14(11):1167. https://doi.org/10.3390/pathogens14111167
Chicago/Turabian StyleVeljović, Ljubiša, Milan Paunović, Dimitrije Glišić, Sofija Šolaja, Zorana Zurovac Sapundžić, Jelena Maletić, Bojan Milovanović, and Vesna Milićević. 2025. "Wild Mammals as Sentinels for West Nile Virus Circulation: Evidence from Serbia" Pathogens 14, no. 11: 1167. https://doi.org/10.3390/pathogens14111167
APA StyleVeljović, L., Paunović, M., Glišić, D., Šolaja, S., Zurovac Sapundžić, Z., Maletić, J., Milovanović, B., & Milićević, V. (2025). Wild Mammals as Sentinels for West Nile Virus Circulation: Evidence from Serbia. Pathogens, 14(11), 1167. https://doi.org/10.3390/pathogens14111167








