Distribution of Rotavirus alphagastroenteritidis Strains in Blantyre, Malawi, During and After the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Rotavirus Alphagastroenteritidis Detection and Genotyping
2.2. Data Analysis
2.3. Ethical Approval
3. Results
3.1. Demographic Characteristics of Study Participants
Detection of R. alphagastroenteritidis in Different Age Groups During and After the COVID-19 Period
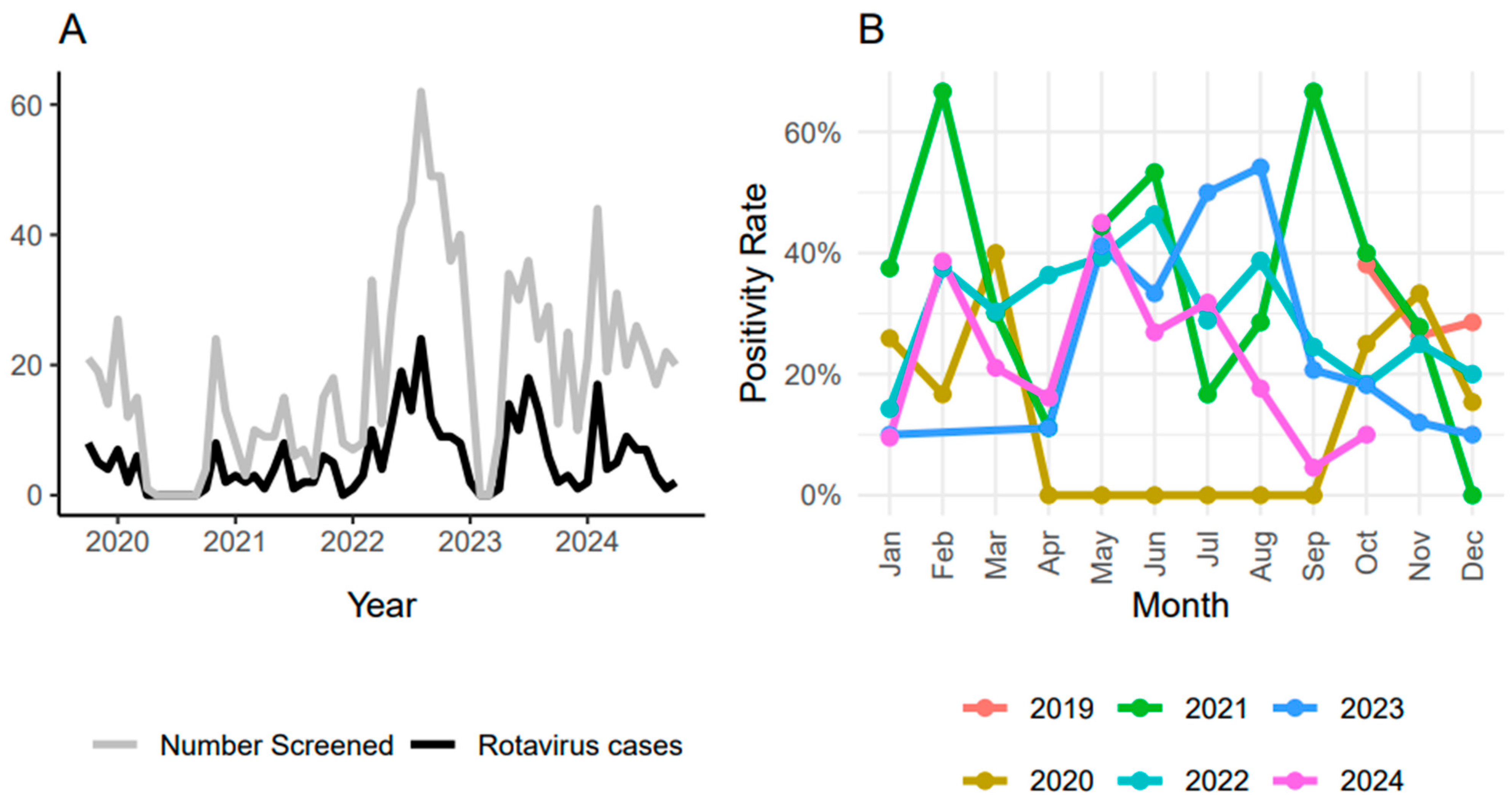
3.2. Seasonality of R. alphagastroenteritidis Infection
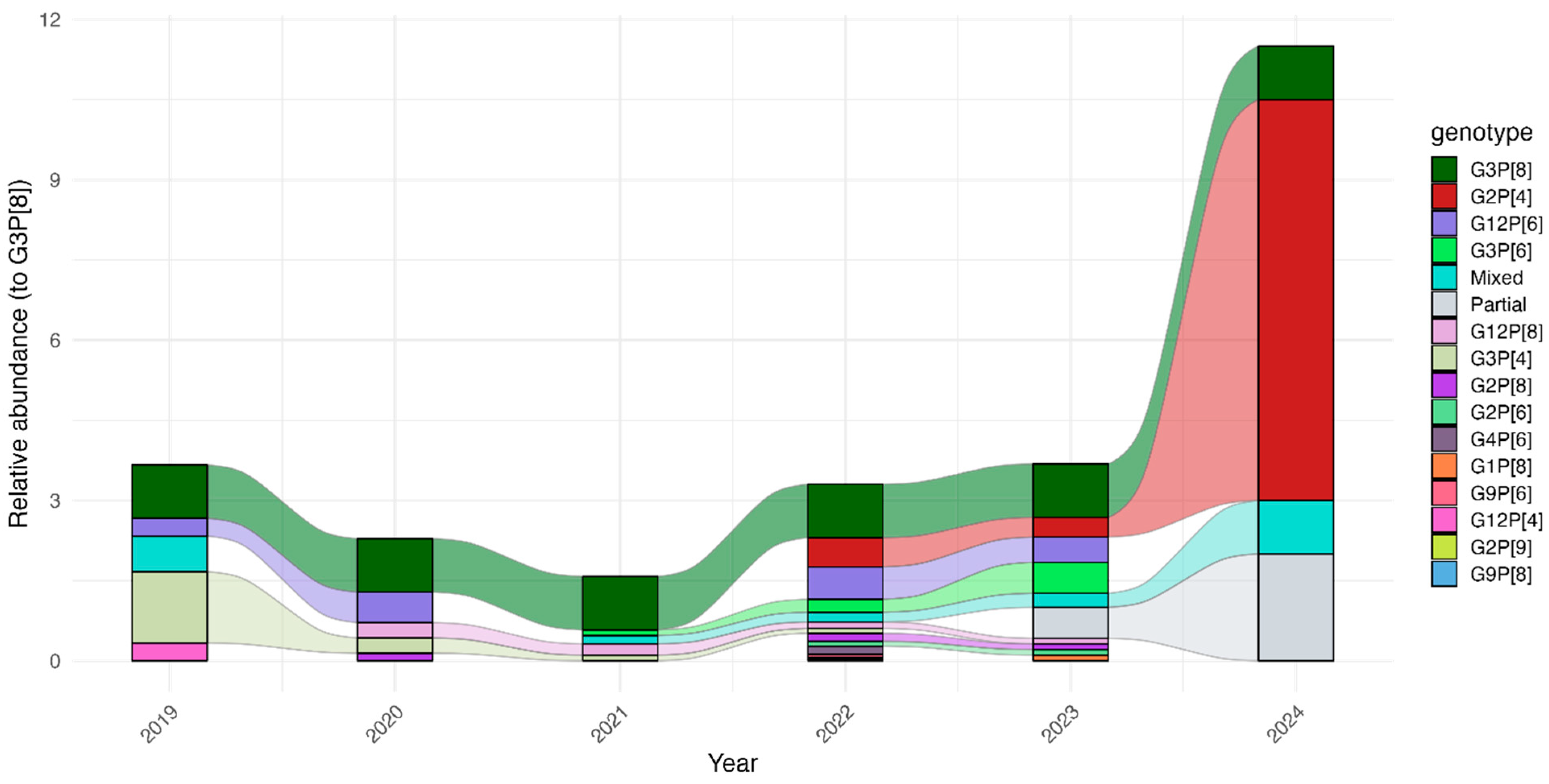
3.3. Temporal Shifts in R. alphagastroenteritidis Genotypes During and After COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus vaccination and the Global Burden of Rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef]
- Varghese, T.; Kang, G.; Steele, A.D. Understanding Rotavirus vaccine efficacy and effectiveness in countries with high child mortality. Vaccines 2022, 10, 346. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Steele, A.D. Rotavirus vaccine will have an impact in Asia. PLoS Med. 2017, 14, e1002298. [Google Scholar] [CrossRef]
- Leshem, E.; Lopman, B.; Glass, R.; Gentsch, J.; Bányai, K.; Parashar, U.; Patel, M. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 847–856. [Google Scholar] [CrossRef]
- Estes, M.K.; Cohen, J. Rotavirus gene structure and function. Microbiol. Rev. 1989, 53, 410–449. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, N.; Kapanda, L.; Tate, J.E.; Jere, K.C.; Iturriza-Gomara, M.; Nakagomi, O.; Mwansambo, C.; Costello, A.; Parashar, U.D.; Heyderman, R.S.; et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: An observational and case-control study. Lancet Infect. Dis. 2015, 15, 422–428. [Google Scholar] [CrossRef]
- Mandolo, J.J.; Henrion, M.Y.R.; Mhango, C.; Chinyama, E.; Wachepa, R.; Kanjerwa, O.; Malamba-Banda, C.; Shawa, I.T.; Hungerford, D.; Kamng’ona, A.W.; et al. Reduction in severity of all-cause gastroenteritis requiring hospitalisation in children vaccinated against rotavirus in Malawi. bioRxiv 2021. [Google Scholar] [CrossRef]
- Iturriza-Gómara, M.; Jere, K.C.; Hungerford, D.; Bar-Zeev, N.; Shioda, K.; Kanjerwa, O.; Houpt, E.R.; Operario, D.J.; Wachepa, R.; Pollock, L.; et al. Etiology of diarrhea among hospitalized children in Blantyre, Malawi, following Rotavirus vaccine introduction: A case-control study. J. Infect. Dis. 2019, 220, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Anscombe, C.; Lissauer, S.; Thole, H.; Rylance, J.; Dula, D.; Menyere, M.; Kutambe, B.; van der Veer, C.; Phiri, T.; Banda, N.P.; et al. A comparison of four epidemic waves of COVID-19 in Malawi; An observational cohort study. medRxiv 2022. [Google Scholar] [CrossRef]
- Mzumara, G.W.; Chawani, M.; Sakala, M.; Mwandira, L.; Phiri, E.; Milanzi, E.; Phiri, M.D.; Kazanga, I.; O’Byrne, T.; Zulu, E.M.; et al. The health policy response to COVID-19 in Malawi. BMJ Glob. Health 2021, 6, e006035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, J.; Ye, Q. Nonpharmaceutical interventions against the COVID-19 pandemic significantly decreased the spread of enterovirus in children. J. Med. Virol. 2022, 94, 3581–3588. [Google Scholar] [CrossRef]
- Sadiq, A.; Bostan, N.; Jadoon Khan Aziz, A. Effect of rotavirus genetic diversity on vaccine impact. Rev. Med. Virol. 2022, 32, e2259. [Google Scholar] [CrossRef]
- Mhango, C.; Banda, A.; Chinyama, E.; Mandolo, J.J.; Kumwenda, O.; Malamba-Banda, C.; Barnes, K.G.; Kumwenda, B.; Jambo, K.C.; Donato, C.M.; et al. Comparative whole genome analysis reveals re-emergence of typical human Wa-like and DS-1-like G3 rotaviruses after Rotarix vaccine introduction in Malawi. medRxiv 2022. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Ngwira, B.M.; Dove, W.; Hindwa, B.D.; Turner, A.M.; Broadhead, R.L.; Molyneux, M.E.; Hart, C.A. Epidemiology of rotavirus infection in children in Blantyre, Malawi, 1997–2007. J. Infect. Dis. 2010, 202 (Suppl. S1), 68–74. [Google Scholar] [CrossRef] [PubMed]
- Pulse Survey on Continuity of Essential Health Services During the COVID-19 Pandemic: Interim Report; World Health Organization: Geneva, Switzerland, 2020.
- Chilanga, E.; Dzimbiri, M.; Mwanjawala, P.; Keller, A.; Mbeya, R.A. Religion, politics and COVID-19 risk perception among urban residents in Malawi. BMC Public Health 2022, 22, 1430. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.; Talha, M.; Rahman, S.; Hasan, M.; Alam, S.; Hassan, Z.; Moni, S.; Khan, S.H.; Hossain, M.E.; Faruque, A.S.G.; et al. Rotavirus trends and distribution of genotypes before and during COVID-19 pandemic era: Bangladesh, 2017–2021. J. Med. Virol. 2024, 96, e29681. [Google Scholar] [CrossRef]
- Sangeda, R.Z.; James, D.; Mariki, H.; Mbwambo, M.E.; Mwenesi, M.E.; Nyaki, H.; Tinuga, F.; Manyanga, D.P. Childhood vaccination trends during 2019 to 2022 in Tanzania and the impact of the COVID-19 pandemic. Hum. Vaccin. Immunother. 2024, 20, 2356342. [Google Scholar] [CrossRef] [PubMed]
- Cassocera, M.; Bauhofer, A.F.L.; Chissaque, A.; Munlela, B.; Guimarães, E.; Isaías, T.; Conjo, C.; Maculuve, B.; Chicumbe, S.; de Deus, N. Regional difference on rotavirus vaccine coverage in children with diarrhea in Mozambique, before and during COVID-19 pandemic: A cross-sectional analysis. BMC Infect. Dis. 2025, 25, 382. [Google Scholar] [CrossRef]
- Turner, A.; Ngwira, B.; Witte, D.; Mwapasa, M.; Dove, W.; Cunliffe, N. Surveillance of rotavirus gastro-enteritis in children in Blantyre, Malawi. Paediatr. Int. Child Health 2013, 33, 42–45. [Google Scholar] [CrossRef]
- Nyasulu, J.C.Y.; Munthali, R.J.; Nyondo-Mipando, A.L.; Pandya, H.; Nyirenda, L.; Nyasulu, P.S.; Manda, S. COVID-19 pandemic in Malawi: Did public sociopolitical events gatherings contribute to its first-wave local transmission? Int. J. Infect. Dis. 2021, 106, 269–275. [Google Scholar] [CrossRef]
- Seheri, L.M.; Magagula, N.B.; Peenze, I.; Rakau, K.; Ndadza, A.; Mwenda, J.M.; Weldegebriel, G.; Steele, A.D.; Mphahlele, M.J. Rotavirus strain diversity in Eastern and Southern African countries before and after vaccine introduction. Vaccine 2018, 36, 7222–7230. [Google Scholar] [CrossRef] [PubMed]
- Mhango, C.; Mandolo, J.J.; Chinyama, E.; Wachepa, R.; Kanjerwa, O.; Malamba-Banda, C.; Matambo, P.B.; Barnes, K.G.; Chaguza, C.; Shawa, I.T.; et al. Rotavirus Genotypes in Hospitalized Children with Acute Gastroenteritis Before and After Rotavirus Vaccine Introduction in Blantyre, Malawi, 1997–2019. J. Infect. Dis. 2022, 225, 2127–2136. [Google Scholar] [CrossRef]
- Mwangi, P.N.; Potgieter, R.L.; Simwaka, J.; Mpabalwani, E.M.; Mwenda, J.M.; Mogotsi, M.T.; Magagula, N.; Esona, M.D.; Steele, A.D.; Seheri, M.L.; et al. Genomic Analysis of G2P[4] Group A Rotaviruses in Zambia Reveals Positive Selection in Amino Acid Site 7 of Viral Protein 3. Viruses 2023, 15, 501. [Google Scholar] [CrossRef]
- Bibera Lourdes, G.; Chen, J.; Pereira, P.; Benninghoff, B. Dynamics of G2P[4] Strain Evolution and Rotavirus Vaccination: A Review of Evidence for Rotarix. Vaccine 2020, 38, 5591–5600. [Google Scholar] [CrossRef]
- Adah, M.I.; Rohwedder, A.; Olaleyle, O.D.; Werchau, H. Nigerian rotavirus serotype G8 could not be typedby PCR due to nucleotide mutation at the 3ʹ endof the primer binding site. Arch. Virol. 1997, 142, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, N.; Jere, K.C.; Bennett, A.; Pollock, L.; Tate, J.E.; Nakagomi, O.; Iturriza-Gomara, M.; Costello, A.; Mwansambo, C.; Parashar, U.D.; et al. Population impact and effectiveness of monovalent Rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: Ecological and case-control analyses. Clin. Infect. Dis. 2016, 62 (Suppl. S2), 13–19. [Google Scholar] [CrossRef] [PubMed]
| Age Group | Sample Size (%) | Positive (%) | % Positive Within Age Group | % of All R. alphagastroenteritidis-Positive Samples |
|---|---|---|---|---|
| <6 months | 110 (9.7) | 36 | 32.7% | 10.9% |
| 6–11 months | 426 (37.5%) | 137 | 32.2% | 41.5% |
| >11–23 months | 412 (36.3%) | 124 | 30.1% | 37.6% |
| >23 months | 184 (16.2%) | 32 | 17.4% | 9.7% |
| NA | 3 (0.3%) | 1 | 33.3% | 0.3% |
| P[4] | [6] | P[8] | P[9] | P-Mix | Untypable | Total | |
|---|---|---|---|---|---|---|---|
| G1 | - | - | 2 (0.6%) | - | - | - | 2 (0.6%) |
| G2 | 59 (17.9%) | 8 (2.4%) | 8 (2.4%) | 1 (0.3%) | 3 (0.9%) | 7 (2.1%) | 86 (26.1%) |
| G3 | 11 (3.3%) | 21 (6.4%) | 83 (25.2%) | - | 2 (0.6%) | 14 (4.2%) | 131 (39.7%) |
| G4 | - | 5 (1.5%) | - | - | - | - | 5 (1.5%) |
| G9 | - | 2 (0.6%) | 1 (0.3%) | - | - | - | 3 (0.9%) |
| G10 | - | - | - | - | 1 (0.3%) | - | 1 (0.3%) |
| G12 | 1 (0.3%) | 34 (10.3%) | 12 (3.6%) | - | 2 (0.6%) | 9 (2.7%) | 58 (17.6%) |
| G-mix | 3 (0.9%) | 3 (0.9%) | 4 (1.2%) | - | - | 0 (0%) | 10 (3.0%) |
| Untypable | 1 (0.3%) | 5 (1.5%) | 6 (1.8%) | 1 (0.3%) | - | 21 (6.4%) | 34 (10.3%) |
| Total | 75 (22.7%) | 78 (23.6%) | 116 (35.2%) | 2 (0.6%) | 8 (2.4%) | 51 (15.5%) | 330 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinyama, E.; Mhango, C.; Taia, R.; Gauti, L.; Mandolo, J.; Kawonga, F.; Matambo, E.; Matambo, P.; Chibwe, I.; Wachepa, R.; et al. Distribution of Rotavirus alphagastroenteritidis Strains in Blantyre, Malawi, During and After the COVID-19 Pandemic. Pathogens 2025, 14, 1169. https://doi.org/10.3390/pathogens14111169
Chinyama E, Mhango C, Taia R, Gauti L, Mandolo J, Kawonga F, Matambo E, Matambo P, Chibwe I, Wachepa R, et al. Distribution of Rotavirus alphagastroenteritidis Strains in Blantyre, Malawi, During and After the COVID-19 Pandemic. Pathogens. 2025; 14(11):1169. https://doi.org/10.3390/pathogens14111169
Chicago/Turabian StyleChinyama, End, Chimwemwe Mhango, Rothwell Taia, Landilani Gauti, Jonathan Mandolo, Flywell Kawonga, Ernest Matambo, Prisca Matambo, Innocent Chibwe, Richard Wachepa, and et al. 2025. "Distribution of Rotavirus alphagastroenteritidis Strains in Blantyre, Malawi, During and After the COVID-19 Pandemic" Pathogens 14, no. 11: 1169. https://doi.org/10.3390/pathogens14111169
APA StyleChinyama, E., Mhango, C., Taia, R., Gauti, L., Mandolo, J., Kawonga, F., Matambo, E., Matambo, P., Chibwe, I., Wachepa, R., Cunliffe, N. A., Msefula, C. L., & Jere, K. C. (2025). Distribution of Rotavirus alphagastroenteritidis Strains in Blantyre, Malawi, During and After the COVID-19 Pandemic. Pathogens, 14(11), 1169. https://doi.org/10.3390/pathogens14111169







