A Nine-Year Review of Acinetobacter baumannii Infections Frequency and Antimicrobial Resistance in a Single-Center Study in Salerno, Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Biological Samples Collection and Processing
2.3. Bacterial Identification and Antimicrobial Profile
2.4. Data and Statistical Analysis
3. Results
3.1. Frequency of A. baumannii Strains and Patients’ Gender Distribution
3.2. Gender Distribution of Positive A.baumannii Patients Stratified by Age Group
3.3. Frequency of A. baumannii Strains by Biological Matrices and Infection Sites
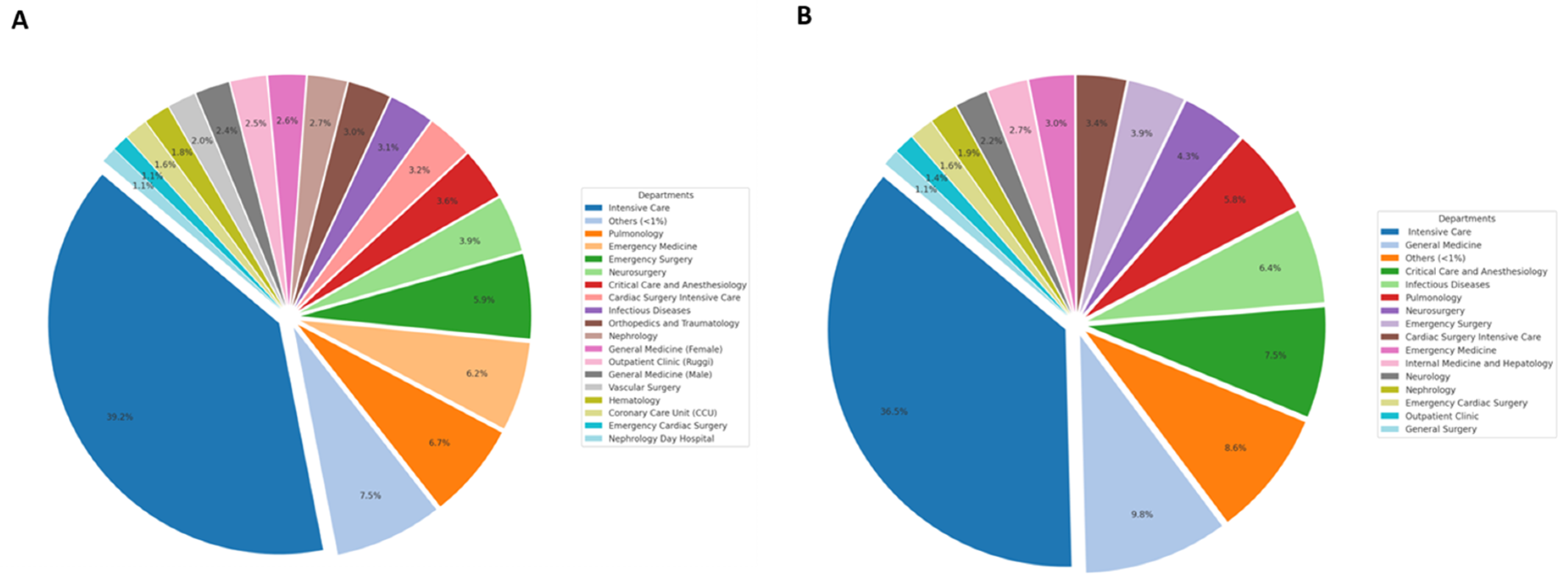
3.4. A. baumannii Frequency Across Different Hospital Units
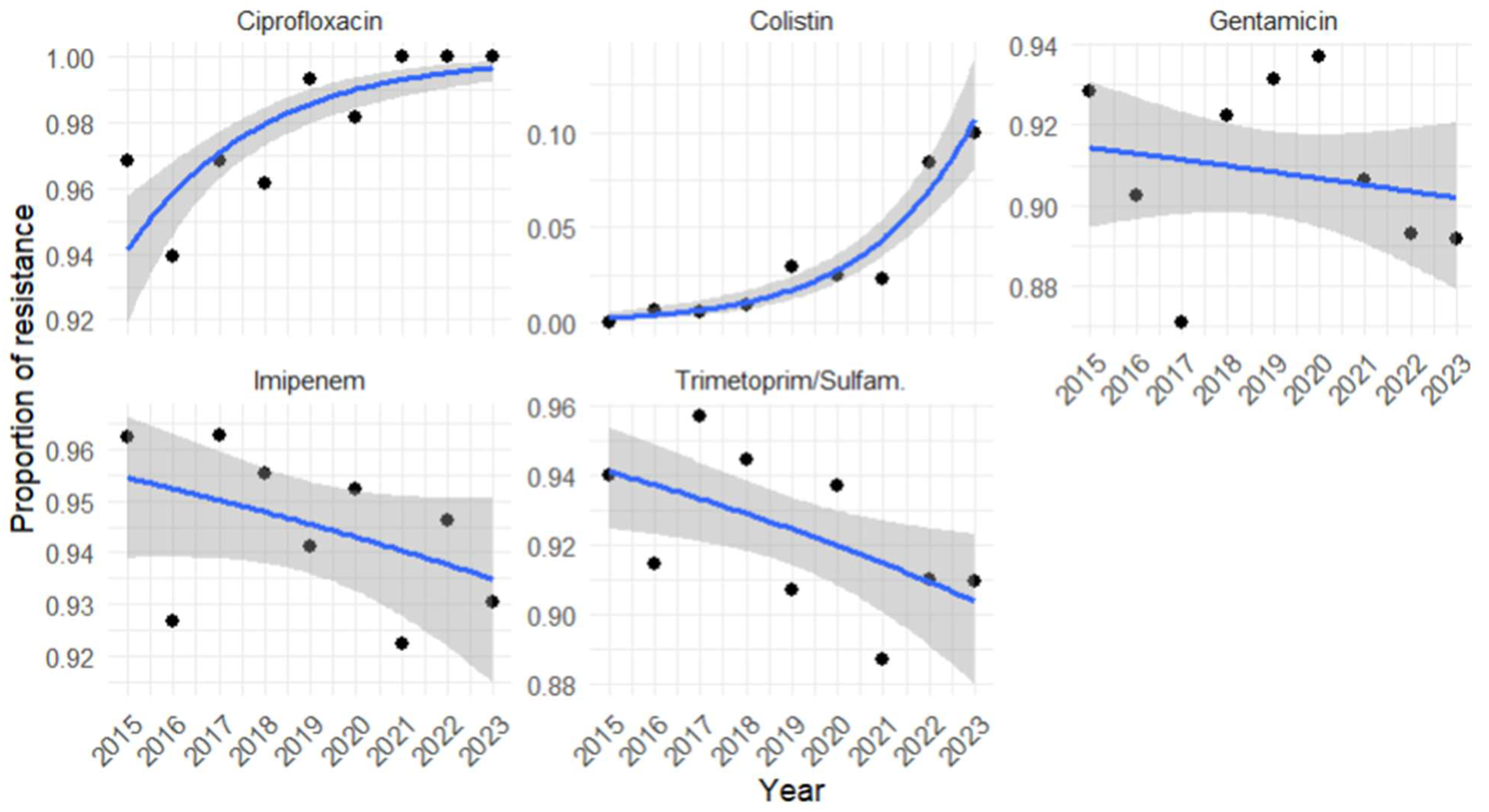
3.5. Antimicrobial Resistance Profile of A. baumannii and Its Temporal Trends
3.6. Regression Analysis of A. baumannii Antimicrobial Resistance Profile for Each Year Investigated
3.7. A. baumannii Coinfections in Respiratory Matrices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Álvarez-Ainza, M.L.; Fong-Coronado, P.A.; Ruiz-Bustos, E.; Castillón-Campaña, L.G.; Quintero-Reyes, I.E.; Duarte-Zambrano, L.A.; Bolado-Martínez, E. Antibiotic Resistance of ESKAPE Group-Microorganisms in Health Institutions from Hermosillo and Ciudad Obregón, Sonora, México. Front. Cell. Infect. Microbiol. 2024, 14, 1348093. [Google Scholar] [CrossRef]
- Bartal, C.; Rolston, K.V.I.; Nesher, L. Carbapenem-Resistant Acinetobacter baumannii: Colonization, Infection and Current Treatment Options. Infect. Dis. Ther. 2022, 11, 683–694. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2015. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2015 (accessed on 11 February 2025).
- Scoffone, V.C.; Trespidi, G.; Barbieri, G.; Arshad, A.; Israyilova, A.; Buroni, S. The Evolution of Antimicrobial Resistance in Acinetobacter baumannii and New Strategies to Fight It. Antibiotics 2025, 14, 85. [Google Scholar] [CrossRef]
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-Resistant Acinetobacter baumannii as an Emerging Concern in Hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef]
- Sun, B.; Liu, H.; Jiang, Y.; Shao, L.; Yang, S.; Chen, D. New Mutations Involved in Colistin Resistance in Acinetobacter baumannii. mSphere 2020, 5, e00895-19. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 11 February 2025).
- López-Rojas, R.; Jiménez-Mejías, M.E.; Lepe, J.A.; Pachón, J. Acinetobacter baumannii Resistant to Colistin Alters Its Antibiotic Resistance Profile: A Case Report from Spain. J. Infect. Dis. 2011, 204, 1147–1148. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.-M.; Roch, A.; Castanier, M.; Papazian, L.; Raoult, D. Acinetobacter baumannii Resistant to Colistin with Impaired Virulence: A Case Report from France. J. Infect. Dis. 2011, 204, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Ahuatzin-Flores, O.E.; Torres, E.; Chávez-Bravo, E. Acinetobacter baumannii, a Multidrug-Resistant Opportunistic Pathogen in New Habitats: A Systematic Review. Microorganisms 2024, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, K.N.; Bonnesen, B.; Hansen, E.F.; Jensen, J.-U.S.; Lapperre, T.S.; Weinreich, U.M.; Hilberg, O. Guideline for the Management of COVID-19 Patients during Hospital Admission in a Non-Intensive Care Setting. Eur. Clin. Respir. J. 2020, 7, 1761677. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2023—2021 Data. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2023-2021-data (accessed on 11 February 2025).
- Saeed, N.K.; Almusawi, S.K.; Albalooshi, N.A.; Al-Beltagi, M. Unveiling the Impact: COVID-19’s Influence on Bacterial Resistance in the Kingdom of Bahrain. World J. Virol. 2025, 14, 100501. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The Impact of Immune System Aging on Infectious Diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Crimmins, E.M. Mortality and Morbidity in Ageing Men: Biology, Lifestyle and Environment. Rev. Endocr. Metab. Disord. 2022, 23, 1285–1304. [Google Scholar] [CrossRef] [PubMed]
- Isigi, S.S.; Parsa, A.D.; Alasqah, I.; Mahmud, I.; Kabir, R. Predisposing Factors of Nosocomial Infections in Hospitalized Patients in the United Kingdom: Systematic Review. JMIR Public Health Surveill. 2023, 9, e43743. [Google Scholar] [CrossRef] [PubMed]
- Tadese, B.K.; DeSantis, S.M.; Mgbere, O.; Fujimoto, K.; Darkoh, C. Clinical Outcomes Associated with Co-Infection of Carbapenem-Resistant Enterobacterales and Other Multidrug-Resistant Organisms. Infect. Prev. Pract. 2022, 4, 100255. [Google Scholar] [CrossRef]
- Pogue, J.M.; Zhou, Y.; Kanakamedala, H.; Cai, B. Burden of Illness in Carbapenem-Resistant Acinetobacter baumannii Infections in US Hospitals between 2014 and 2019. BMC Infect. Dis. 2022, 22, 36. [Google Scholar] [CrossRef]
- Mishra, A.; Aggarwal, A.; Khan, F. Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies. Antibiotics 2024, 13, 623. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Narimisa, N.; Mirshekar, M.; Dadgar-Zankbar, L.; Taki, E.; Navidifar, T.; Darban-Sarokhalil, D. Prevalence of Colistin Resistance in Clinical Isolates of Acinetobacter baumannii: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control 2024, 13, 24. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzaq, N.M.; AlRand, H.; Everett, D.B.; Senok, A.; Menezes, G.A.; Ayoub Moubareck, C. Epidemiology and Antimicrobial Resistance Trends of Acinetobacter Species in the United Arab Emirates: A Retrospective Analysis of 12 Years of National AMR Surveillance Data. Front. Public Health 2023, 11, 1245131. [Google Scholar] [CrossRef]
- Regione Campania. Report on Antibiotic Resistance and Antibiotic Use 2019. Available online: https://www.regione.campania.it/assets/documents/report-2019-sull-antibotico-resistenza-8rjc6720un7pwyo7.pdf (accessed on 13 February 2025).
- Regione Campania. Report on Antibiotic Resistance 2022. Available online: https://www.regione.campania.it/assets/documents/rapporto-sirear-2022-con-isbn.pdf (accessed on 13 February 2025).
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net) Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/TQ-AC-20-001-EN-N_0.pdf (accessed on 13 February 2025).
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Rapporto AR-ISS—I Dati 2023. Available online: https://www.epicentro.iss.it/antibiotico-resistenza/ar-iss-rapporto-acinetobacter-species (accessed on 13 February 2025).
- Emons, M.; Blanquart, F.; Lehtinen, S. The Evolution of Antibiotic Resistance in Europe, 1998–2019. PLoS Pathog. 2025, 21, e1012945. [Google Scholar] [CrossRef]
- Rangel, K.; De-Simone, S.G. Treatment and Management of Acinetobacter Pneumonia: Lessons Learned from Recent World Events. Infect. Drug Resist. 2024, 17, 507–529. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Ioannou, P.; Kritsotakis, E.I. Co-Isolates of Acinetobacter baumannii Complex in Polymicrobial Infections: A Meta-Analysis. Access Microbiol. 2022, 4, acmi000348. [Google Scholar] [CrossRef] [PubMed]
- Semenec, L.; Cain, A.K.; Dawson, C.J.; Liu, Q.; Dinh, H.; Lott, H.; Penesyan, A.; Maharjan, R.; Short, F.L.; Hassan, K.A.; et al. Cross-Protection and Cross-Feeding between Klebsiella pneumoniae and Acinetobacter baumannii Promotes Their Co-Existence. Nat. Commun. 2023, 14, 702. [Google Scholar] [CrossRef]
- Timme, S.; Wendler, S.; Klassert, T.E.; Saraiva, J.P.; da Rocha, U.N.; Wittchen, M.; Schramm, S.; Ehricht, R.; Monecke, S.; Edel, B.; et al. Competitive Inhibition and Mutualistic Growth in Co-Infections: Deciphering Staphylococcus aureus–Acinetobacter baumannii Interaction Dynamics. ISME Commun. 2024, 4, ycae077. [Google Scholar] [CrossRef] [PubMed]
- Sophonsri, A.; Kelsom, C.; Lou, M.; Nieberg, P.; Wong-Beringer, A. Risk Factors and Outcome Associated with Coinfection with Carbapenem-Resistant Klebsiella pneumoniae and Carbapenem-Resistant Pseudomonas aeruginosa or Acinetobacter baumannii: A Descriptive Analysis. Front. Cell. Infect. Microbiol. 2023, 13, 1231740. [Google Scholar] [CrossRef]
- Rangel, K.; Betzler, D.; Gomes, C.; Genteluci, G.L.; De Souza, M.J.; Bôas, M.H.S.V. Coinfection with Acinetobacter baumannii Carbapenem-Resistant and Carbapenem-Susceptible Strains. Clin. Microbiol. Newsl. 2018, 40, 6–7. [Google Scholar] [CrossRef]
- Yamin, D.; Uskoković, V.; Wakil, A.M.; Goni, M.D.; Shamsuddin, S.H.; Mustafa, F.H.; Alfouzan, W.A.; Alissa, M.; Alshengeti, A.; Almaghrabi, R.H.; et al. Current and Future Technologies for the Detection of Antibiotic-Resistant Bacteria. Diagnostics 2023, 13, 3246. [Google Scholar] [CrossRef]
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Uzo, V.C.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Mukhopadhyay, H.; Bairagi, A.; Mukherjee, A.; Prasad, A.K.; Roy, A.D.; Nayak, A. Multidrug Resistant Acinetobacter baumannii: A Study on Its Pathogenesis and Therapeutics. Curr. Res. Microb. Sci. 2025, 8, 100331. [Google Scholar] [CrossRef]

| Years Gender | 2015 | 2016 | 2017 | 2018 | 2019 | 2015–2019 | 2020 | 2021 | 2022 | 2023 | 2020–2023 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male n. (%) | 229 (65.6) | 223 (67.6) | 200 (57.5) | 248 (68.7) | 197 (67.7) | 1097 (65.3) | 198 (73.1) | 186 (72.4) | 206 (58.2) | 174 (57.2) | 764 (64.4) |
| Female n. (%) | 120 (34.4) | 107 (32.4) | 148 (42.5) | 113 (31.3) | 94 (32.3) | 582 (34.7) | 73 (26.9) | 71 (58,2) | 148 (41.8) | 130 (42.8) | 422 (35.6) |
| Total | 349 | 330 | 348 | 361 | 291 | 1679 | 271 | 257 | 354 | 304 | 1186 |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2015–2019 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | M (%) | F (%) | M (%) | F (%) | M (%) | F (%) | M (%) | F (%) | M (%) | F (%) | M (%) | F (%) |
| 0–10 | 0 | 0.8 | 0 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 |
| 11–20 | 6.1 | 4.2 | 3.1 | 0 | 1.5 | 4.1 | 2.8 | 0.9 | 11.2 | 0 | 4.8 | 2.1 |
| 21–30 | 5.2 | 1.7 | 5.4 | 4.7 | 5.0 | 2.7 | 4.4 | 1.8 | 8.1 | 0 | 5.6 | 2.2 |
| 31–40 | 5.2 | 4.2 | 4.0 | 16.8 | 1.5 | 2.0 | 6.0 | 4.4 | 2.5 | 2.1 | 4.0 | 5.7 |
| 41–50 | 11.4 | 7.5 | 5.8 | 11.2 | 13.0 | 14.2 | 7.7 | 6.2 | 9.1 | 9.6 | 9.3 | 10.0 |
| 51–60 | 14.0 | 12.5 | 18.8 | 15.0 | 17.5 | 8.8 | 19.0 | 9.7 | 11.7 | 11.7 | 16.3 | 11.3 |
| 61–70 | 18.8 | 22.5 | 25.1 | 11.2 | 30.0 | 16.9 | 26.6 | 21.2 | 19.3 | 25.5 | 24.0 | 19.2 |
| 71–80 | 22.3 | 25.8 | 26.0 | 19.6 | 18.5 | 30.4 | 20.6 | 37.2 | 16.8 | 24.5 | 21.0 | 27.8 |
| 81–90 | 16.6 | 19.2 | 11.2 | 18.7 | 12.0 | 20.3 | 11.3 | 17.7 | 21.3 | 24.5 | 14.3 | 19.9 |
| 91–100 | 0.4 | 1.7 | 0.4 | 1.9 | 1.0 | 0.7 | 1.6 | 0.9 | 0 | 2.1 | 0.7 | 1.4 |
| Total | 229 | 120 | 223 | 107 | 200 | 148 | 248 | 113 | 197 | 94 | 1097 | 582 |
| p-value | 0.442 | <0.001 | 0.003 | 0.02 | <0.001 | <0.001 | ||||||
| 2020 | 2021 | 2022 | 2023 | 2020–2023 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years | M (%) | F (%) | M (%) | F (%) | M (%) | F (%) | M (%) | F (%) | M (%) | F (%) |
| 0–10 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0.6 | 0 | 0.3 | 0 |
| 11–20 | 0.5 | 0 | 0.5 | 0 | 3.4 | 0 | 2.3 | 0 | 1.7 | 0 |
| 21–30 | 0.5 | 4.1 | 9.1 | 1.4 | 0 | 6.1 | 1.7 | 0 | 2.7 | 3.1 |
| 31–40 | 10.1 | 4.1 | 2.7 | 2.8 | 2.9 | 18.2 | 4.0 | 1.5 | 5.0 | 8.1 |
| 41–50 | 10.6 | 8.2 | 5.4 | 4.2 | 7.8 | 12.2 | 6.9 | 1.5 | 7.7 | 6.9 |
| 51–60 | 18.7 | 8.2 | 16.7 | 21.1 | 25.2 | 7.4 | 17.8 | 19.2 | 19.8 | 13.5 |
| 61–70 | 29.8 | 32.9 | 22.6 | 31.0 | 20.9 | 10.8 | 26.4 | 14.6 | 24.9 | 19.2 |
| 71–80 | 22.7 | 28.8 | 34.9 | 23.9 | 21.4 | 28.4 | 24.1 | 28.5 | 25.7 | 27.7 |
| 81–90 | 7.1 | 11.0 | 8.1 | 15.5 | 17.0 | 16.2 | 16.1 | 30.8 | 12.0 | 19.7 |
| 91–100 | 0 | 2.7 | 0 | 0 | 1.0 | 0.7 | 0 | 3.8 | 0.3 | 1.9 |
| Total | 198 | 73 | 186 | 71 | 206 | 148 | 174 | 130 | 764 | 422 |
| p-value | 0.02 | 0.07 | <0.001 | <0.001 | <0.001 | |||||
| Years | 2015–2019 | 2015 | 2016 | 2017 | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Isolates n. | Isolates % | Isolates n. | Isolates % | Isolates n. | Isolates % | Isolates n. | Isolates % | Isolates n. | Isolates % | Isolates n. | Isolates % |
| Respiratory | 782 | 46.6 | 161 | 46.1 | 144 | 43.6 | 151 | 43.4 | 166 | 46.0 | 160 | 55.0 |
| Wound swab | 248 | 14.8 | 56 | 16.0 | 52 | 15.8 | 45 | 12.9 | 52 | 14.4 | 43 | 14.8 |
| Blood culture | 197 | 11.7 | 39 | 11.2 | 32 | 9.7 | 47 | 13.5 | 52 | 14.4 | 27 | 9.3 |
| Urinary | 183 | 10.9 | 34 | 9.7 | 38 | 11.5 | 43 | 12.4 | 40 | 11.1 | 28 | 9.6 |
| Cultural swab | 132 | 7.9 | 28 | 8.0 | 22 | 6.7 | 32 | 9.2 | 31 | 8.6 | 19 | 6.5 |
| Catheters | 93 | 5.5 | 25 | 7.2 | 25 | 7.6 | 18 | 5.2 | 20 | 5.5 | 5 | 1.7 |
| Liquor culture | 27 | 1.6 | 2 | 0.6 | 11 | 3.3 | 8 | 2.3 | 0 | 0 | 6 | 2.1 |
| Vaginal swab | 12 | 0.7 | 2 | 0.6 | 5 | 1.5 | 4 | 1.1 | 0 | 0 | 1 | 0.3 |
| Others | 5 | 0.3 | 2 | 0.6 | 1 | 0.3 | 0 | 0 | 0 | 0 | 2 | 0.7 |
| Total | 1679 | 100 | 349 | 100 | 330 | 100 | 348 | 100 | 361 | 100 | 291 | 100 |
| Years | 2020–2023 | 2020 | 2021 | 2022 | 2023 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Isolates n. | Isolates % | Isolates n. | Isolates % | Isolates n. | Isolates % | Isolates n. | Isolates % | Isolates n. | Isolates % |
| Respiratory | 536 | 45.2 | 137 | 50.6 | 129 | 50.2 | 147 | 41.5 | 123 | 40.5 |
| Blood culture | 178 | 15.0 | 60 | 22.1 | 43 | 16.7 | 47 | 13.3 | 28 | 9.2 |
| Urinary | 171 | 14.4 | 27 | 10.0 | 24 | 9.3 | 57 | 16.1 | 63 | 20.7 |
| Cultural swab | 127 | 10.7 | 20 | 7.4 | 21 | 8.2 | 49 | 13.8 | 37 | 12.2 |
| Wound swab | 115 | 9.7 | 14 | 5.2 | 27 | 10.5 | 35 | 9.9 | 39 | 12.8 |
| Catheters | 36 | 3.0 | 4 | 1.5 | 12 | 4.7 | 11 | 3.1 | 9 | 3.0 |
| Vaginal swab | 10 | 0.8 | 1 | 0.4 | 1 | 0.4 | 7 | 2.0 | 1 | 0.3 |
| Liquor culture | 8 | 0.7 | 4 | 1.5 | 0 | 0 | 0 | 0 | 4 | 1.3 |
| Others | 5 | 0.4 | 4 | 1.5 | 0 | 0 | 1 | 0.3 | 0 | 0 |
| Total | 1186 | 100 | 271 | 100 | 257 | 100 | 354 | 100 | 304 | 100 |
| Antimicrobials | Odds-Ratio | IC 95% | BH-Adjusted p-Value | Significant |
|---|---|---|---|---|
| Ciprofloxacin | 1.63 | 1.27–1.63 | 2.99 × 10−8 | TRUE |
| Colistin | 1.82 | 1.43–1.82 | 3.11 × 10−14 | TRUE |
| Gentamicin | 0.45 | 0.93–1.03 | 0.445 | FALSE |
| Imipenem | 0.17 | 0.90–1.01 | 0.167 | FALSE |
| Trimetoprim/Sulfam. | 2.52 | 0.89–0.98 | 0.0252 | TRUE |
| Antimicrobials | 2015 | 2016 | 2017 | 2018 | 2019 | 2015–2019 | p-Value | p-Value Trend | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n. | R% | n. | R% | n. | R% | n. | R% | n. | R% | n. | R% | |||
| Ciprofloxacin | 349 | 96.85 | 328 | 93.90 | 348 | 96.84 | 361 | 96.12 | 291 | 99.31 | 1677 | 96.54 | 0.007 | 0.04 |
| Colistin | 340 | 0 | 311 | 0.64 | 329 | 0.61 | 325 | 0.92 | 273 | 2.90 | 1578 | 0.95 | 0.004 | <0.001 |
| Imipenem | 346 | 96.24 | 327 | 92.66 | 348 | 96.26 | 313 | 95.53 | 34 | 94.12 | 1368 | 95.18 | 0.17 | 0.89 |
| Trim./Sulfam. | 349 | 93.98 | 327 | 91.44 | 348 | 95.69 | 361 | 94.46 | 291 | 90.72 | 1676 | 93.38 | 0.06 | 0.51 |
| Gentamicin | 349 | 92.8 | 328 | 90.2 | 348 | 87.1 | 361 | 92.2 | 291 | 93.1 | 1677 | 91.0 | 0.03 | 0.7 |
| Antimicrobials | 2020 | 2021 | 2022 | 2023 | 2020–2023 | p-Value | p-Value Trend | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n. | R% | n. | R% | n. | R% | n. | R% | n. | R% | |||
| Ciprofloxacin | 270 | 98.1 | 256 | 100.0 | 354 | 100.0 | 303 | 100.0 | 1183 | 99.6 | <0.001 | 0.001 |
| Colistin | 237 | 2.5 | 221 | 2.3 | 334 | 8.4 | 251 | 10.0 | 1043 | 6.1 | <0.001 | <0.001 |
| Imipenem | 231 | 95.2 | 257 | 92.2 | 354 | 94.6 | 302 | 93.0 | 1144 | 93.8 | 0.45 | 0.58 |
| Trim./Sulfam. | 270 | 93.7 | 257 | 88.7 | 355 | 91.0 | 277 | 91.0 | 1159 | 91.1 | 0.25 | 0.44 |
| Gentamicin | 270 | 93.7 | 256 | 90.6 | 355 | 89.3 | 305 | 89.2 | 1186 | 90.6 | 0.21 | 0.05 |
| Years | 2015–2019 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Bacterial Species in Co-Infection | Isolates n. (%) | Isolates n. (%) | Isolates n. (%) | Isolates n. (%) | Isolates n. (%) | Isolates n. (%) |
| Klebsiella pneumoniae | 109 (28.8) | 29 (29.3) | 34 (45.9) | 20 (25.3) | 14 (17.5) | 12 (26.1) |
| Staphylococcus aureus | 84 (22.2) | 20 (20.2) | 10 (13.5) | 22 (27.8) | 18 (22.5) | 14 (30.4) |
| Pseudomonas aeruginosa | 56 (14.8) | 16 (16.2) | 10 (13.5) | 9 (11.4) | 15 (18.8) | 6 (13.0) |
| Proteus mirabilis | 32 (8.5) | 3 (3.0) | 3 (4.1) | 8 (10.1) | 12 (15.0) | 6 (13.0) |
| Others | 28 (7.4) | 8 (8.1) | 7 (9.5) | 8 (10.1) | 4 (5.0) | 1 (2.2) |
| Escherichia coli | 23 (6.1) | 2 (2.0) | 6 (8.1) | 4 (5.1) | 6 (7.5) | 5 (10.9) |
| Providencia stuartii | 20 (5.3) | 10 (10.1) | 1 (1.4) | 5 (6.3) | 3 (3.8) | 1 (2.2) |
| Enterococcus faecalis | 8 (2.1) | 5 (5.1) | 1 (1.4) | 0 (0) | 1 (1.3) | 1 (2.2) |
| Enterobacter cloacae | 7 (1.9) | 0 (0) | 1 (1.4) | 1 (1.3) | 5 (6.3) | 0 (0) |
| Stenotrophomonas maltophilia | 6 (1.6) | 2 (2.0) | 0 (0) | 2 (2.5) | 2 (2.5) | 0 (0) |
| Candida albicans | 5 (1.3) | 4 (4.0) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) |
| Total | 378 | 99 | 74 | 79 | 80 | 46 |
| Years | 2020–2023 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|
| Bacterial Species in Co-Infection | Isolates n. (%) | Isolates n. (%) | Isolates n. (%) | Isolates n. (%) | Isolates n. (%) |
| Staphylococcus aureus | 67 (28.6) | 24 (50) | 21 (26.9) | 9 (16.7) | 13 (24.1) |
| Pseudomonas aeruginosa | 43 (18.4) | 6 (12.5) | 17 (21.8) | 11 (20.4) | 9 (16.7) |
| Klebsiella pneumoniae | 35 (15.0) | 10 (20.8) | 8 (10.3) | 11 (20.4) | 6 (11.1) |
| Others | 21 (9.0) | 2 (4.2) | 11 (14.1) | 5 (9.3) | 3 (5.6) |
| Providencia stuartii | 13 (5.6) | 0 (0) | 3 (3.8) | 3 (5.6) | 7 (13) |
| Escherichia coli | 10 (4.3) | 1 (2.1) | 1 (1.3) | 2 (3.7) | 6 (11.1) |
| Proteus mirabilis | 10 (4.3) | 1 (2.1) | 2 (2.6) | 4 (7.4) | 3 (5.6) |
| Staphylococcus haemolyticus | 10 (4.3) | 0 (0) | 4 (5.1) | 3 (5.6) | 3 (5.6) |
| Staphylococcus epidermidis | 10 (4.3) | 0 (0) | 7 (9) | 2 (3.7) | 1 (1.9) |
| Enterococcus faecalis | 9 (3.8) | 3 (6.3) | 4 (5.1) | 1 (1.9) | 1 (1.9) |
| Enterococcus faecium | 6 (2.6) | 1 (2.1) | 0 (0) | 3 (5.6) | 2 (3.7) |
| Total | 234 | 48 | 78 | 54 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serretiello, E.; De Prisco, M.; Di Siervi, G.; Cosimato, I.; Dell’Annunziata, F.; Santoro, E.; Vozzella, E.A.; Boccia, G.; Folliero, V.; Franci, G. A Nine-Year Review of Acinetobacter baumannii Infections Frequency and Antimicrobial Resistance in a Single-Center Study in Salerno, Italy. Pathogens 2025, 14, 1165. https://doi.org/10.3390/pathogens14111165
Serretiello E, De Prisco M, Di Siervi G, Cosimato I, Dell’Annunziata F, Santoro E, Vozzella EA, Boccia G, Folliero V, Franci G. A Nine-Year Review of Acinetobacter baumannii Infections Frequency and Antimicrobial Resistance in a Single-Center Study in Salerno, Italy. Pathogens. 2025; 14(11):1165. https://doi.org/10.3390/pathogens14111165
Chicago/Turabian StyleSerretiello, Enrica, Mariagrazia De Prisco, Giuseppe Di Siervi, Ilaria Cosimato, Federica Dell’Annunziata, Emanuela Santoro, Emilia Anna Vozzella, Giovanni Boccia, Veronica Folliero, and Gianluigi Franci. 2025. "A Nine-Year Review of Acinetobacter baumannii Infections Frequency and Antimicrobial Resistance in a Single-Center Study in Salerno, Italy" Pathogens 14, no. 11: 1165. https://doi.org/10.3390/pathogens14111165
APA StyleSerretiello, E., De Prisco, M., Di Siervi, G., Cosimato, I., Dell’Annunziata, F., Santoro, E., Vozzella, E. A., Boccia, G., Folliero, V., & Franci, G. (2025). A Nine-Year Review of Acinetobacter baumannii Infections Frequency and Antimicrobial Resistance in a Single-Center Study in Salerno, Italy. Pathogens, 14(11), 1165. https://doi.org/10.3390/pathogens14111165






