Homology Modeling of Type-P5 ATPases from the Malaria Parasite: Insight into Their Functions and Evolution, and Implications About the Effect and Role of Intrinsically Disordered Protein Structure
Abstract
1. Introduction
1.1. P-Type ATPases
1.2. Type-P5 ATPases of Plasmodium
2. Materials and Methods
2.1. Identification of Subtype-P5A ATPases from Haemosporidians and the SAR Supergroup
2.2. Sequence Alignments
2.3. Prediction of 3-Dimensional Structures
3. Results
3.1. Subtype-P5A ATPase of Haemosporida
3.2. Sequence Homology Between Subtype-P5A and Subtype-P5B ATPases of Malaria Parasites
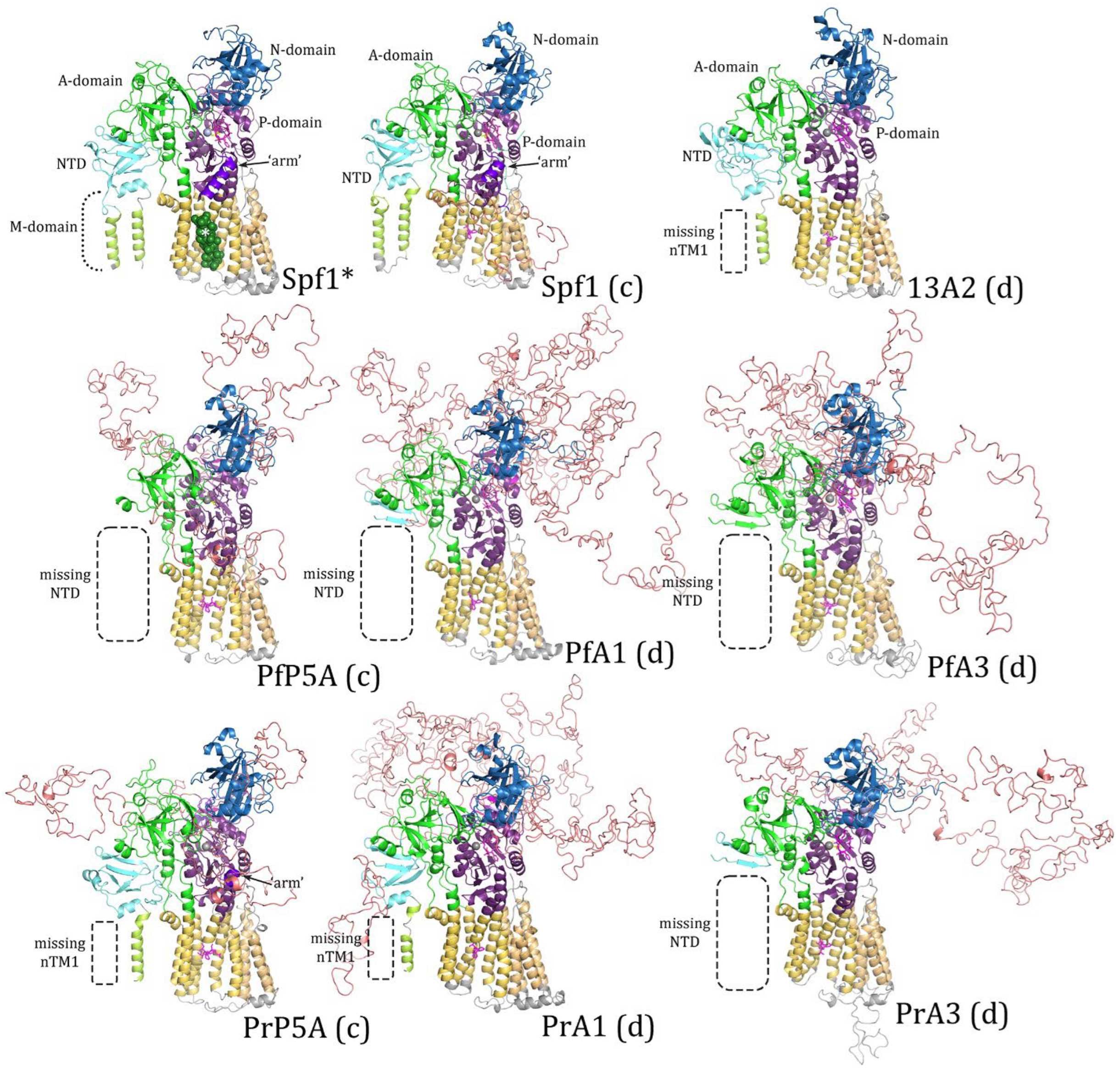
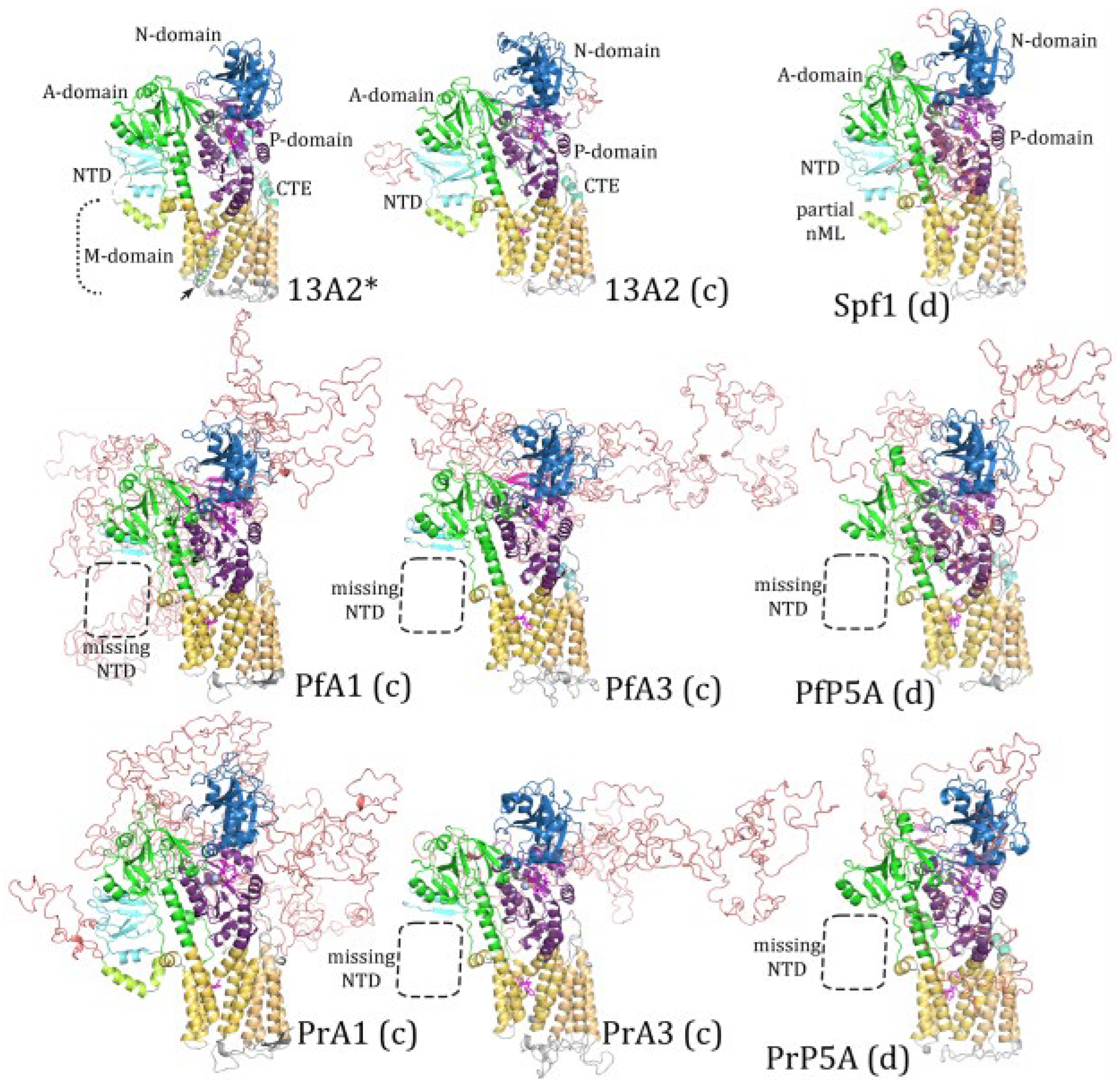
3.3. Homology Modeling and Structure Comparisons
3.4. Quality Assessment of Modeling
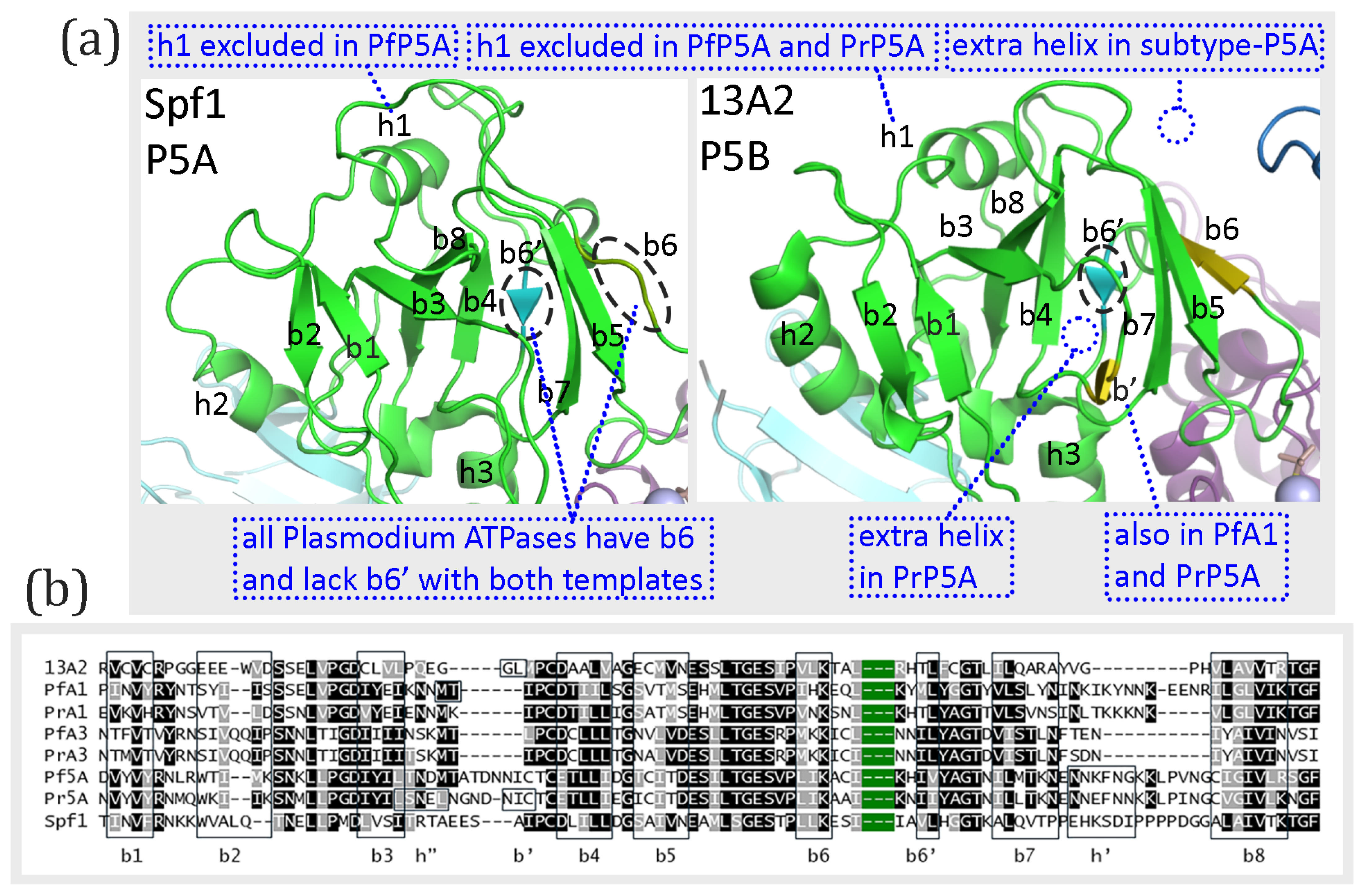
3.5. A-Domain
3.6. N-Domain
3.7. P-Domain
3.8. Variable Regions Effects
3.9. Substrate-Binding Site
3.10. AlphaFold Modeling
4. Discussion
4.1. Homology Modeling and Predicted Structures
4.2. Limitations of Homology Modeling
4.3. Possible Functions of IDL
4.4. Substrate Specificity
4.5. Divergent Evolution of Subtype-P5B ATPases
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cTM | Core transmembrane helix |
| CTE | C-terminal extension |
| IDL | Intrinsically disorganized loops |
| PEXEL | Plasmodium export element |
| PlP5A | Plasmodium subtype-P5A ATPase |
| SAR | Stramenopiles–alveolates–rhizarians |
| SERCA | Sarcoplasmic–endoplasmic reticulum ATPase |
References
- Palmgren, M. P-Type ATPases: Many More Enigmas Left to Solve. J. Biol. Chem. 2023, 299, 105352. [Google Scholar] [CrossRef]
- Martin, R.E.; Henry, R.I.; Abbey, J.L.; Clements, J.D.; Kirk, K. The “permeome” of the Malaria Parasite: An Overview of the Membrane Transport Proteins of Plasmodium falciparum. Genome Biol. 2005, 6, R26. [Google Scholar] [CrossRef]
- Arnou, B.; Montigny, C.; Morth, J.P.; Nissen, P.; Jaxel, C.; Møller, J.V.; le Maire, M. The Plasmodium Falciparum Ca2+-ATPase PfATP6: Insensitive to Artemisinin, but a Potential Drug Target. Biochem. Soc. Trans. 2011, 39, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Pulcini, S.; Fatih, F.; Staines, H. Artemisinins and the Biological Basis for the PfATP6/SERCA Hypothesis. Trends Parasitol. 2010, 26, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Fiser, A. Template-Based Protein Structure Modeling. Methods Mol. Biol. 2010, 673, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.L.; Green, N.M. Structure and Function of the Calcium Pump. Annu. Rev. Biophys. 2003, 32, 445–468. [Google Scholar] [CrossRef]
- Axelsen, K.B.; Palmgren, M.G. Evolution of Substrate Specificities in the P-Type ATPase Superfamily. J. Mol. Evol. 1998, 46, 84–101. [Google Scholar] [CrossRef]
- Sim, S.I.; Park, E. P5-ATPases: Structure, Substrate Specificities, and Transport Mechanisms. Curr. Opin. Struct. Biol. 2023, 79, 102531. [Google Scholar] [CrossRef]
- McKenna, M.J.; Sim, S.I.; Ordureau, A.; Wei, L.; Harper, J.W.; Shao, S.; Park, E. The Endoplasmic Reticulum P5A-ATPase Is a Transmembrane Helix Dislocase. Science 2020, 369, eabc5809. [Google Scholar] [CrossRef]
- Li, P.; Wang, K.; Salustros, N.; Grønberg, C.; Gourdon, P. Structure and Transport Mechanism of P5B-ATPases. Nat. Commun. 2021, 12, 3973. [Google Scholar] [CrossRef]
- Tillinghast, J.; Drury, S.; Bowser, D.; Benn, A.; Lee, K.P.K. Structural Mechanisms for Gating and Ion Selectivity of the Human Polyamine Transporter ATP13A2. Mol. Cell 2021, 81, 4650–4662.e4. [Google Scholar] [CrossRef]
- Møller, A.B.; Asp, T.; Holm, P.B.; Palmgren, M.G. Phylogenetic Analysis of P5 P-Type ATPases, a Eukaryotic Lineage of Secretory Pathway Pumps. Mol. Phylogenet. Evol. 2008, 46, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Wiser, M.F. Duplication of a Type-P5B-ATPase in Laverania and Avian Malaria Parasites and Implications about the Evolution of Plasmodium. Parasitologia 2025, 5, 6. [Google Scholar] [CrossRef]
- Cortés, G.T.; Beltran, M.M.G.; Gómez-Alegría, C.J.; Wiser, M.F. Identification of a Protein Unique to the Genus Plasmodium That Contains a WD40 Repeat Domain and Extensive Low-Complexity Sequence. Parasitol. Res. 2021, 120, 2617–2629. [Google Scholar] [CrossRef]
- DePristo, M.A.; Zilversmit, M.M.; Hartl, D.L. On the Abundance, Amino Acid Composition, and Evolutionary Dynamics of Low-Complexity Regions in Proteins. Gene 2006, 378, 19–30. [Google Scholar] [CrossRef]
- Wiser, M.F. The Digestive Vacuole of the Malaria Parasite: A Specialized Lysosome. Pathogens 2024, 13, 182. [Google Scholar] [CrossRef]
- Krishna, S.; Cowan, G.; Meade, J.C.; Wells, R.A.; Stringer, J.R.; Robson, K.J. A Family of Cation ATPase-like Molecules from Plasmodium falciparum. J. Cell Biol. 1993, 120, 385–398. [Google Scholar] [CrossRef]
- Rozmajzl, P.J.; Kimura, M.; Woodrow, C.J.; Krishna, S.; Meade, J.C. Characterization of P-Type ATPase 3 in Plasmodium falciparum. Mol. Biochem. Parasitol. 2001, 116, 117–126. [Google Scholar] [CrossRef]
- Kimura, M.; Tanabe, K.; Krishna, S.; Tsuboi, T.; Saito-Ito, A.; Otani, S.; Ogura, H. Gametocyte-Dominant Expression of a Novel P-Type ATPase in Plasmodium yoelii. Mol. Biochem. Parasitol. 1999, 104, 331–336. [Google Scholar] [CrossRef]
- Wiser, M.F.; Lanners, H.N.; Bafford, R.A.; Favaloro, J.M. A Novel Alternate Secretory Pathway for the Export of Plasmodium Proteins into the Host Erythrocyte. Proc. Natl. Acad. Sci. USA 1997, 94, 9108–9113. [Google Scholar] [CrossRef]
- Aikawa, M. Parasitological Review. Plasmodium: The Fine Structure of Malarial Parasites. Exp. Parasitol. 1971, 30, 284–320. [Google Scholar] [CrossRef] [PubMed]
- Langreth, S.G.; Jensen, J.B.; Reese, R.T.; Trager, W. Fine Structure of Human Malaria in Vitro. J. Protozool. 1978, 25, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A Better Web Interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Aurrecoechea, C.; Brestelli, J.; Brunk, B.P.; Dommer, J.; Fischer, S.; Gajria, B.; Gao, X.; Gingle, A.; Grant, G.; Harb, O.S.; et al. PlasmoDB: A Functional Genomic Database for Malaria Parasites. Nucleic Acids Res. 2009, 37, 539–543. [Google Scholar] [CrossRef]
- Bensch, S.; Canbäck, B.; DeBarry, J.D.; Johansson, T.; Hellgren, O.; Kissinger, J.C.; Palinauskas, V.; Videvall, E.; Valkiūnas, G. The Genome of Haemoproteus tartakovskyi and Its Relationship to Human Malaria Parasites. Genome Biol. Evol. 2016, 8, 1361–1373. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The Conserved Domain Database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Naylor, G.J.; Brown, W.M. Amphioxus Mitochondrial DNA, Chordate Phylogeny, and the Limits of Inference Based on Comparisons of Sequences. Syst. Biol. 1998, 47, 61–76. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Cepeda, A.S.; Mello, B.; Pacheco, M.A.; Luo, Z.; Sullivan, S.A.; Carlton, J.M.; Escalante, A.A. The Genome of Plasmodium Gonderi: Insights into the Evolution of Human Malaria Parasites. Genome Biol. Evol. Evol. 2024, 16, evae027. [Google Scholar] [CrossRef] [PubMed]
- Loy, D.E.; Liu, W.; Li, Y.; Learn, G.H.; Plenderleith, L.J.; Sundararaman, S.A.; Sharp, P.M.; Hahn, B.H. Out of Africa: Origins and Evolution of the Human Malaria Parasites Plasmodium falciparum and Plasmodium vivax. Int. J. Parasitol. 2017, 47, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Escalante, A.A. Origin and Diversity of Malaria Parasites and Other Haemosporida. Trends Parasitol. 2023, 39, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Böhme, U.; Otto, T.D.; Cotton, J.A.; Steinbiss, S.; Sanders, M.; Oyola, S.O.; Nicot, A.; Gandon, S.; Patra, K.P.; Herd, C.; et al. Complete Avian Malaria Parasite Genomes Reveal Features Associated with Lineage-Specific Evolution in Birds and Mammals. Genome Res. 2018, 28, 547–560. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cantini, F.; Inagaki, S.; Migliardi, M.; Rosato, A. The Binding Mode of ATP Revealed by the Solution Structure of the N-Domain of Human ATP7A. J. Biol. Chem. 2010, 285, 2537–2544. [Google Scholar] [CrossRef]
- Håkansson, K.O. The Crystallographic Structure of Na,K-ATPase N-Domain at 2.6Å Resolution. J. Mol. Biol. 2003, 332, 1175–1182. [Google Scholar] [CrossRef]
- Shin, W.-H.; Kihara, D. 55 Years of the Rossmann Fold. Methods Mol. Biol. 2019, 1958, 1–13. [Google Scholar] [CrossRef]
- Wiser, M.F. Protozoa. In Encyclopedia of Biodiversity; Scheiner, S.M.B.T., Ed.; Academic Press: Oxford, UK, 2024; Volume 2, pp. 802–817. ISBN 978-0-323-98434-8. [Google Scholar]
- Grattepanche, J.-D.; Walker, L.M.; Ott, B.M.; Paim Pinto, D.L.; Delwiche, C.F.; Lane, C.E.; Katz, L.A. Microbial Diversity in the Eukaryotic SAR Clade: Illuminating the Darkness between Morphology and Molecular Data. BioEssays 2018, 40, 1700198. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Chakravarty, D. Intrinsically Disordered Proteins/Regions and Insight into Their Biomolecular Interactions. Biophys. Chem. 2022, 283, 106769. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Intrinsically Unstructured Proteins and Their Functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef]
- Uversky, V.N.; Kulkarni, P. Intrinsically Disordered Proteins: Chronology of a Discovery. Biophys. Chem. 2021, 279, 106694. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fuxreiter, M. The Structure and Dynamics of Higher-Order Assemblies: Amyloids, Signalosomes, and Granules. Cell 2016, 165, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.R.; Lwin, N.; Phelan, D.; Escalante, A.A.; Battistuzzi, F.U. Comparative Analysis of Low Complexity Regions in Plasmodia. Sci. Rep. 2018, 8, 335. [Google Scholar] [CrossRef]
- Viguera, E.; Canceill, D.; Ehrlich, S.D. Replication Slippage Involves DNA Polymerase Pausing and Dissociation. EMBO J. 2001, 20, 2587–2595. [Google Scholar] [CrossRef]
- Davies, H.M.; Nofal, S.D.; McLaughlin, E.J.; Osborne, A.R. Repetitive Sequences in Malaria Parasite Proteins. FEMS Microbiol. Rev. 2017, 41, 923–940. [Google Scholar] [CrossRef]
- Sørensen, D.M.; Holemans, T.; van Veen, S.; Martin, S.; Arslan, T.; Haagendahl, I.W.; Holen, H.W.; Hamouda, N.N.; Eggermont, J.; Palmgren, M. Parkinson Disease Related ATP13A2 Evolved Early in Animal Evolution. PLoS ONE 2018, 13, e0193228. [Google Scholar] [CrossRef]
- Cronin, S.R.; Rao, R.; Hampton, R.Y. Cod1p/Spf1p Is a P-Type ATPase Involved in ER Function and Ca2+ Homeostasis. J. Cell Biol. 2002, 157, 1017–1028. [Google Scholar] [CrossRef]
- Cohen, Y.; Megyeri, M.; Chen, O.C.W.; Condomitti, G.; Riezman, I.; Loizides-Mangold, U.; Abdul-Sada, A.; Rimon, N.; Riezman, H.; Platt, F.M. The Yeast P5 Type ATPase, Spf1, Regulates Manganese Transport into the Endoplasmic Reticulum. PLoS ONE 2013, 8, e85519. [Google Scholar] [CrossRef]
- Sørensen, D.M.; Holen, H.W.; Pedersen, J.T.; Martens, H.J.; Silvestro, D.; Stanchev, L.D.; Costa, S.R.; Günther Pomorski, T.; López-Marqués, R.L.; Palmgren, M. The P5A ATPase Spf1p Is Stimulated by Phosphatidylinositol 4-Phosphate and Influences Cellular Sterol Homeostasis. Mol. Biol. Cell 2019, 30, 1069–1084. [Google Scholar] [CrossRef]
- Feng, Z.; Zhao, Y.; Li, T.; Nie, W.; Yang, X.; Wang, X.; Wu, J.; Liao, J.; Zou, Y. CATP-8/P5A ATPase Regulates ER Processing of the DMA-1 Receptor for Dendritic Branching. Cell Rep. 2020, 32, 108101. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, S.; Sørensen, D.M.; Holemans, T.; Holen, H.W.; Palmgren, M.G.; Vangheluwe, P. Cellular Function and Pathological Role of ATP13A2 and Related P-Type Transport ATPases in Parkinson’s Disease and Other Neurological Disorders. Front. Mol. Neurosci. 2014, 7, 48. [Google Scholar] [CrossRef]
- Sim, S.I.; von Bülow, S.; Hummer, G.; Park, E. Structural Basis of Polyamine Transport by Human ATP13A2 (PARK9). Mol. Cell 2021, 81, 4635–4649.e8. [Google Scholar] [CrossRef]
- Van Veen, S.; Martin, S.; Van den Haute, C.; Benoy, V.; Lyons, J.; Vanhoutte, R.; Kahler, J.P.; Decuypere, J.-P.; Gelders, G.; Lambie, E. ATP13A2 Deficiency Disrupts Lysosomal Polyamine Export. Nature 2020, 578, 419–424. [Google Scholar] [CrossRef]
- Azfar, M.; van Veen, S.; Houdou, M.; Hamouda, N.N.; Eggermont, J.; Vangheluwe, P. P5B-ATPases in the Mammalian Polyamine Transport System and Their Role in Disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2022, 1869, 119354. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, M.; Zhang, S.; Yin, J.; Zhang, P.; Xuan, X.; Wang, P.; Liu, Z.; Zhou, B.; Yang, M. Cryo-EM Structures and Transport Mechanism of Human P5B Type ATPase ATP13A2. Cell Discov. 2021, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, D.M.; Holen, H.W.; Holemans, T.; Vangheluwe, P.; Palmgren, M.G. Towards Defining the Substrate of Orphan P5A-ATPases. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 524–535. [Google Scholar] [CrossRef]
- Palmgren, M.; Sørensen, D.M.; Hallström, B.M.; Säll, T.; Broberg, K. Evolution of P2A and P5A ATPases: Ancient Gene Duplications and the Red Algal Connection to Green Plants Revisited. Physiol. Plant. 2020, 168, 630–647. [Google Scholar] [CrossRef]
- Lamarque, M.; Tastet, C.; Poncet, J.; Demettre, E.; Jouin, P.; Vial, H.; Dubremetz, J.-F. Food Vacuole Proteome of the Malarial Parasite Plasmodium falciparum. Proteom. Clin. Appl. 2008, 2, 1361–1374. [Google Scholar] [CrossRef]
- Phillips, M.A. Polyamines in Protozoan Pathogens. J. Biol. Chem. 2018, 293, 18746–18756. [Google Scholar] [CrossRef]
- Niemand, J.; Louw, A.I.; Birkholtz, L.; Kirk, K. Polyamine Uptake by the Intraerythrocytic Malaria Parasite, Plasmodium falciparum. Int. J. Parasitol. 2012, 42, 921–929. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, W.; Zhou, J.; Li, H.; Xu, X.; Zhang, B.; Gao, X. Repetitive DNA Sequence Detection and Its Role in the Human Genome. Commun. Biol. 2023, 6, 954. [Google Scholar] [CrossRef] [PubMed]
- Wiser, M.F. Unique Endomembrane Systems and Virulence in Pathogenic Protozoa. Life 2021, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Bullen, H.E.; Crabb, B.S.; Gilson, P.R. Recent Insights into the Export of PEXEL/HTS-Motif Containing Proteins in Plasmodium Parasites. Curr. Opin. Microbiol. 2012, 15, 699–704. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Hiller, N.L.; Liolios, K.; Win, J.; Kanneganti, T.-D.; Young, C.; Kamoun, S.; Haldar, K. The Malarial Host-Targeting Signal Is Conserved in the Irish Potato Famine Pathogen. PLoS Pathog. 2006, 2, e50. [Google Scholar] [CrossRef]
- Wiser, M.F. Knobs, Adhesion, and Severe Falciparum Malaria. Trop. Med. Infect. Dis. 2023, 8, 353. [Google Scholar] [CrossRef] [PubMed]
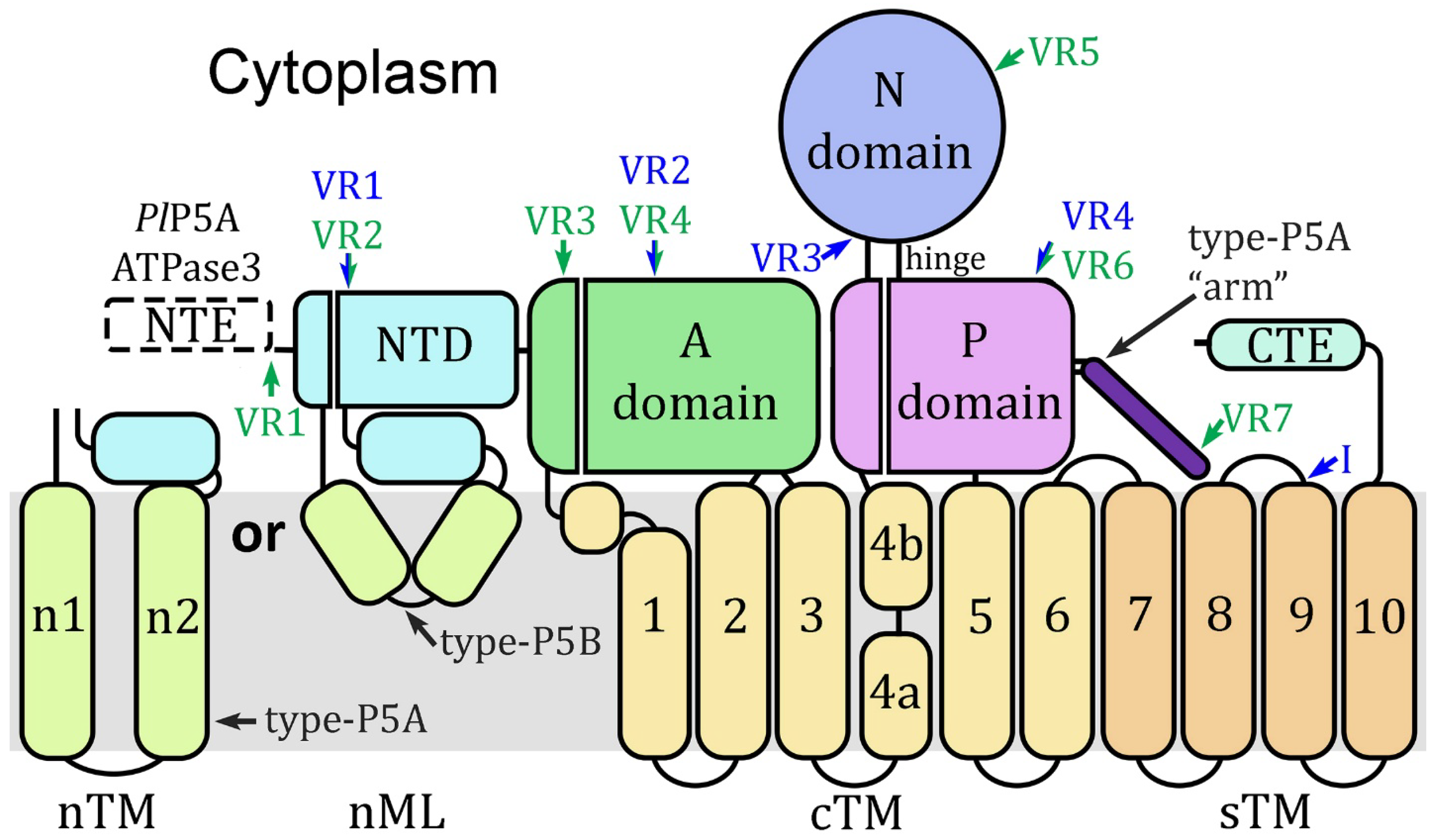
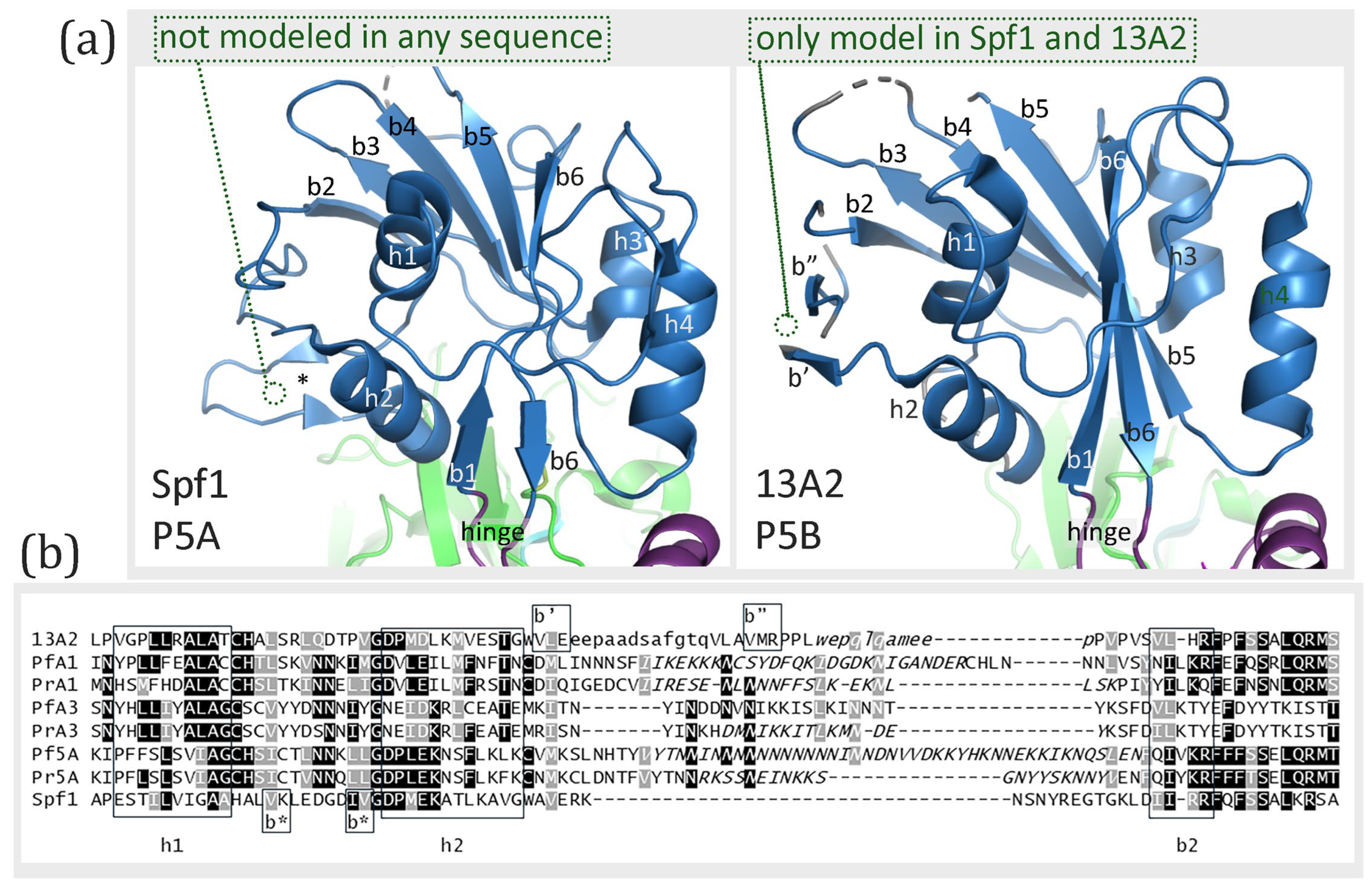
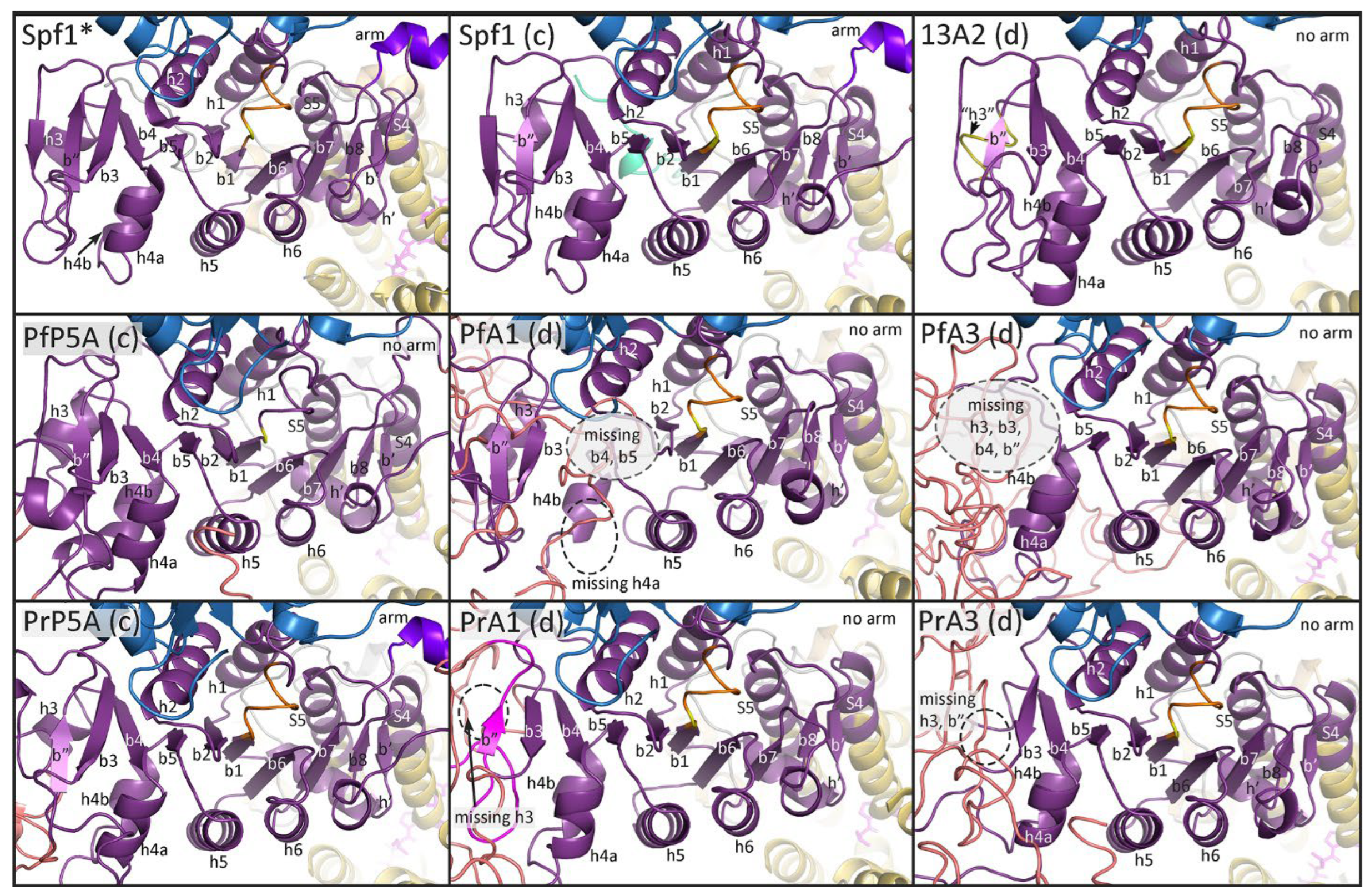


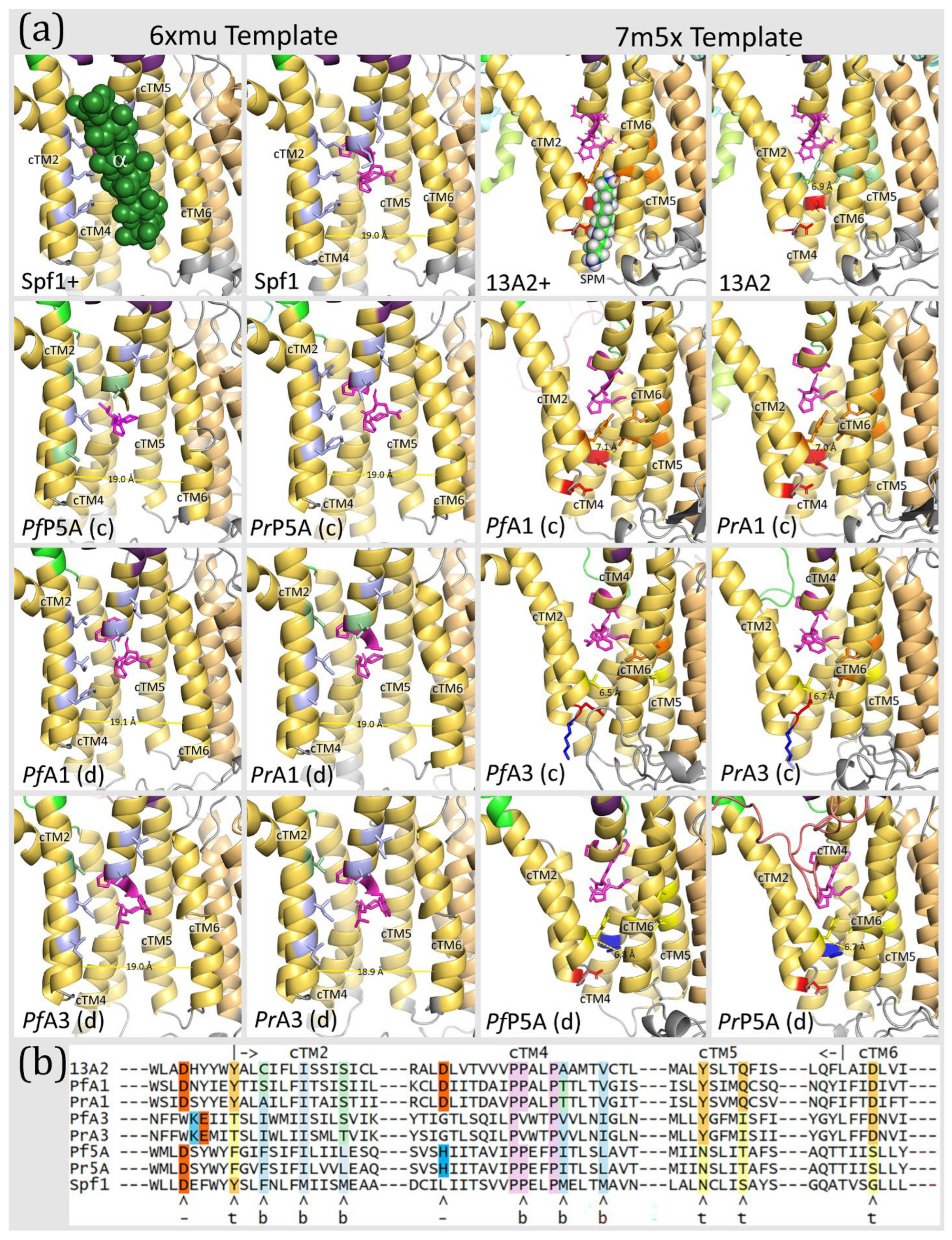
| Protein | Species | Abbr | Gene ID | Activity/Description | Ref. |
|---|---|---|---|---|---|
| Spf1 | Saccharomyces cerevisiae | Spf1 | AAB64508.1 | Transmembrane helix dislocase | [9] |
| Uncharacterized subtype-P5A | Plasmodium falciparum | PfP5A | PF3D7_0727800 | Only identified in sequence databases | [12,2] |
| Uncharacterized subtype-P5A | Plasmodiumrelictum | PrP5A | PRELSG_0216200 | ||
| ATP13A2 | Homo sapiens | 13A2 | NP_071372.1 | Polyamine transporter | [11] |
| ATPase3 | Plasmodium falciparum | PfA3 | PF3D7_0504000 | Apicomplexan subtype-P5B of unknown substrate specificity | [13] |
| ATPase3 | Plasmodiumrelictum | PrA3 | PRELSG_1028500 | ||
| ATPase1 | Plasmodium falciparum | PfA1 | PF3D7_0516100 | Paralogue of ATPase3 only found in Laverania and avian Haemosporida | |
| ATPase1 | Plasmodiumrelictum | PrA1 | PRELSG_1015800 |
| Domain | NTE | NTD | A1 | A2 | N1 | N2 | P1 | P2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| P5B-VR | VR1 | VR2 | VR3 | VR4 | |||||
| 13A2 | n.a. | 23 | 0 | 0 | 0 | 0 | 56 | 0 | 79 (7%) |
| PfA1 | n.a. | 126 | 0 | 168 | 281 | 37 | 664 | 0 | 1276 (53%) |
| PrA1 | n.a. | 45 | 0 | 136 | 145 | 0 | 681 | 0 | 1007 (48%) |
| PfA3 | 0 | 288 | 0 | 127 | 478 | 0 | 327 | 0 | 1220 (51%) |
| PrA3 | 0 | 89 | 0 | 52 | 332 | 0 | 280 | 0 | 753 (39%) |
| P5A-VR | VR1 | VR2 | VR3 | VR4 | VR5 | VR6 | VR7 | ||
| PfP5A | 79 | 25 | 180 | 107 | 2 | 147 | 158 | 103 | 801 (42%) |
| PrP5A | 79 | 24 | 119 | 15 | 2 | 43 | 152 | 91 | 525 (32%) |
| Spf1 | n.a. | 0 | 0 | 0 | 0 | 0 | 65 | 119 | 184 (15%) |
| 13A2 | PfA1 | PrA1 | PfA3 | PrA3 | Pf5A | Pr5A | |
|---|---|---|---|---|---|---|---|
| PfA1 | 1.32 | ||||||
| PrA1 | 1.24 | 0.42 | |||||
| PfA3 | 1.49 | 1.39 | 1.41 | ||||
| PrA3 | 1.56 | 1.40 | 1.40 | 0.13 | |||
| Pf5A | 1.43 | 1.37 | 1.38 | 1.51 | 1.47 | ||
| Pr5A | 1.45 | 1.36 | 1.40 | 1.48 | 1.44 | 0.25 | |
| Spf1 | 1.39 | 1.44 | 1.41 | 1.59 | 1.58 | 1.14 | 1.16 |
| 6xmu (aHS, BeF, Mg) | 6xmq (ACP, Mg) | 7m5x (spm, BeF, Mg) | 7m5v (ANP, Mg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATPase | GM | QM | RF | Ligand | GM | QM | RF | Ligand | GM | QM | RF | Ligand | GM | QM | RF | Ligand |
| Spf1 | 0.72 | 0.78 | 92.4 | Mg | 0.78 | 0.82 | 95.5 | ACP, Mg | 0.47 | 0.55 | 86.4 | Mg | 0.50 | 0.59 | 86.6 | Mg |
| ATP13A2 | 0.54 | 0.59 | 88.9 | Mg | 0.54 | 0.60 | 89.4 | Mg | 0.67 | 0.71 | 91.9 | BeF, Mg | 0.69 | 0.74 | 91.8 | ANP, Mg |
| PfP5A | 0.27 | 0.50 | 81.6 | BeF, Mg | 0.28 | 0.50 | 80.2 | Mg | 0.19 | 0.46 | 82.9 | BeF, Mg | 0.20 | 0.46 | 80.6 | Mg |
| PrP5A | 0.39 | 0.51 | 83.5 | Mg | 0.39 | 0.50 | 84.1 | Mg | 0.27 | 0.48 | 82.9 | Mg | 0.29 | 0.51 | 86.9 | Mg |
| PfA1 | 0.12 | 0.37 | 73.8 | Mg | 0.13 | 0.38 | 73.7 | Mg | 0.11 | 0.38 | 69.4 | BeF, Mg | 0.12 | 0.39 | 72.2 | - |
| PrA1 | 0.20 | 0.38 | 76.0 | Mg | 0.18 | 0.38 | 76.7 | - | 0.18 | 0.38 | 72.7 | Mg | 0.18 | 0.38 | 73.0 | Mg |
| PfA3 | 0.15 | 0.41 | 75.5 | Mg | 0.15 | 0.41 | 74.0 | Mg | 0.13 | 0.41 | 72.9 | Mg | 0.14 | 0.43 | 75.4 | Mg |
| PrA3 | 0.24 | 0.41 | 79.3 | Mg | 0.24 | 0.41 | 78.3 | Mg | 0.21 | 0.41 | 77.2 | Mg | 0.21 | 0.42 | 78.0 | Mg |
| PfP5Avrr | 0.54 | 0.58 | 91.4 | Mg | 0.54 | 0.58 | 91.6 | Mg | not analyzed | |||||||
| PrP5Avrr | 0.54 | 0.59 | 91.2 | Mg | 0.54 | 0.59 | 91.2 | Mg | ||||||||
| PfA1vrr | not analyzed | 0.49 | 0.53 | 89.6 | BeF, Mg | 0.51 | 0.55 | 91.0 | - | |||||||
| PrA1vrr | 0.52 | 0.56 | 87.1 | BeF, Mg | 0.54 | 0.56 | 90.4 | - | ||||||||
| PfA3vrr | 0.46 | 0.52 | 86.7 | Mg | 0.47 | 0.53 | 89.3 | Mg | ||||||||
| PrA3vrr | 0.49 | 0.52 | 88.0 | Mg | 0.49 | 0.53 | 89.7 | Mg | ||||||||
| 6xmu Template (Discordant) | 7m5x Template (Concordant) | |||||||
|---|---|---|---|---|---|---|---|---|
| Domain | ATPase | VR | Discrepancy | VRR Effect | Discrepancy | VRR Effect | ||
| NTD (VR1) | PfA1 | 126 | missing NTD | partial restoration of nTM2 | + | missing NTD | restoration of nML | + |
| PrA1 | 45 | missing nTM1 | none | 0 | none | none | 0 | |
| PfA3 | 288 | missing NTD | missing NTD | 0 | missing NTD | partial restoration of nML | + | |
| PrA3 | 89 | missing NTD | partial restoration of nTM2 | + | missing NTD | partial restoration of nML | + | |
| A (VR2) | PfA1 | 168 | none | none | 0 | extra β-strand | loss of extra β-strand | + |
| PrA1 | 136 | none | none | 0 | none | none | 0 | |
| PfA3 | 127 | none | none | 0 | none | none | 0 | |
| PrA3 | 52 | none | none | 0 | none | none | 0 | |
| N (VR3) | PfA1 | 278 | none | none | 0 | none | none | 0 |
| PrA1 | 153 | none | none | 0 | none | none | 0 | |
| PfA3 | 469 | none | none | 0 | none | none | 0 | |
| PrA3 | 323 | none | none | 0 | none | none | 0 | |
| P (VR4) | PfA1 | 558 | b4 and b5 missing, extra helix derived from VR4 | b4 and b5 restored, loss of extra helix | + | b4 and h3 generated from VR4, extra β-strand derived from VR4 | b4 and h3 generated from expected sequence, loss of extra β-strand | + |
| PrA1 | 568 | extra β-strand derived from VR4 | loss of extra β-strand | + | none | none | 0 | |
| PfA3 | 217 | b3 and b4 missing | b3 and b4 restored | + | b3 generated from VR4, extra β-strand derived from VR4 | b3 generated from expected sequence, loss of extra β-strand | + | |
| PrA3 | 172 | none | b3 missing | − | b3 missing | additional loss of b4 and b5 | − | |
| Score | pTM | %Dis | IDL Hallucination | Arm | nML | SBG | P-Domain | |
|---|---|---|---|---|---|---|---|---|
| Spf1 | 0.88 | 0.86 | 0.03 | Moderate | Full helix | Expected structure | 4.9 Å | Expected structure |
| Pf5A | 0.80 | 0.65 | 0.31 | Extensive | Partial helix | Extracytoplasmic IDL | 4.6 Å | Expected structure |
| Pr5A | 0.83 | 0.74 | 0.19 | Extensive | Quasi-helix | Extracytoplasmic IDL | 6.5 Å | Expected structure |
| ATP13A2 | 0.88 | 0.84 | 0.08 | Minor | n.a. | Expected structure | 6.0 Å | Expected structure |
| PfA1 | 0.78 | 0.56 | 0.43 | Extensive | n.a. | Expected structure | 5.6 Å | Partially generated from VR4 |
| PfA1_vrr | 0.87 | 0.83 | 0.06 | Minor | n.a. | Expected structure | 6.1 Å | Missing structural elements |
| PrA1 | 0.81 | 0.61 | 0.41 | Extensive | n.a. | Expected structure | 5.9 Å | Partially generated from VR4 |
| PrA1_vrr | 0.87 | 0.83 | 0.09 | Moderate | n.a. | Expected structure | 6.3 Å | Missing structural elements |
| PfA3 | 0.81 | 0.57 | 0.47 | Extensive with a TM helix | n.a. | Two transmembrane helices instead of triangular loop | 5.9 Å | Partially generated from VR4 |
| PfA3_vrr | 0.89 | 0.86 | 0.05 | Minor with a TM helix | n.a. | Two transmembrane helices instead of triangular loop | 5.9 Å | Missing structural elements |
| PrA3 | 0.83 | 0.65 | 0.35 | Extensive with a TM helix | n.a. | Two transmembrane helices instead of triangular loop | 5.7 Å | Partially generated from VR4 |
| PrA3_vrr | 0.89 | 0.86 | 0.06 | Minor with a TM helix | n.a. | Two transmembrane helices instead of triangular loop | 6.0 Å | Missing structural elements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiser, M.F. Homology Modeling of Type-P5 ATPases from the Malaria Parasite: Insight into Their Functions and Evolution, and Implications About the Effect and Role of Intrinsically Disordered Protein Structure. Pathogens 2025, 14, 1164. https://doi.org/10.3390/pathogens14111164
Wiser MF. Homology Modeling of Type-P5 ATPases from the Malaria Parasite: Insight into Their Functions and Evolution, and Implications About the Effect and Role of Intrinsically Disordered Protein Structure. Pathogens. 2025; 14(11):1164. https://doi.org/10.3390/pathogens14111164
Chicago/Turabian StyleWiser, Mark F. 2025. "Homology Modeling of Type-P5 ATPases from the Malaria Parasite: Insight into Their Functions and Evolution, and Implications About the Effect and Role of Intrinsically Disordered Protein Structure" Pathogens 14, no. 11: 1164. https://doi.org/10.3390/pathogens14111164
APA StyleWiser, M. F. (2025). Homology Modeling of Type-P5 ATPases from the Malaria Parasite: Insight into Their Functions and Evolution, and Implications About the Effect and Role of Intrinsically Disordered Protein Structure. Pathogens, 14(11), 1164. https://doi.org/10.3390/pathogens14111164






