Abstract
The core perturbome is defined as a central response to multiple disturbances, functioning as a complex molecular network to overcome the disruption of homeostasis under stress conditions, thereby promoting tolerance and survival under stress conditions. Based on the biological and clinical relevance of Escherichia coli and Staphylococcus aureus, we characterized their molecular responses to multiple perturbations. Gene expression data from E. coli (8815 target genes—based on a pangenome—across 132 samples) and S. aureus (3312 target genes across 156 samples) were used. Accordingly, this study aimed to identify and describe the functionality of the core perturbome of these two prokaryotic models using a machine learning approach. For this purpose, feature selection and classification algorithms (KNN, RF and SVM) were implemented to identify a subset of genes as core molecular signatures, distinguishing control and perturbation conditions. After verifying effective dimensional reduction (with median accuracies of 82.6% and 85.1% for E. coli and S. aureus, respectively), a model of molecular interactions and functional enrichment analyses was performed to characterize the selected genes. The core perturbome was composed of 55 genes (including nine hubs) for E. coli and 46 (eight hubs) for S. aureus. Well-defined interactomes were predicted for each model, which are jointly associated with enriched pathways, including energy and macromolecule metabolism, DNA/RNA and protein synthesis and degradation, transcription regulation, virulence factors, and other signaling processes. Taken together, these results may support the identification of potential therapeutic targets and biomarkers of stress responses in future studies.
1. Introduction
Biological organisms require complex cellular and molecular interactions to ensure homeostasis and survival [1]. Several studies have revealed diverse molecular mechanisms that are coordinated through interaction networks and can explain the response to disturbances in many organisms [1,2,3,4,5]. Thus, developing new strategies for studying these interactions has been fundamental for understanding the biological processes and identifying different genotypic and phenotypic patterns, defined as consistent molecular changes, that serve as biomarkers of stress or biological states [6]. In this context, and given the importance of understanding central responses to cellular stress across organisms, the concept of the perturbome has been coined. Metabolic and signal transduction pathways are modulated following exposure to different perturbations, including a subset of shared or core pathways that are independent of the specific stressor, collectively referred to as the core perturbome (Figure 1), as shown in previous studies [1,7]. In one such study, a human cell model was used to describe diverse stress-response genes active upon drug exposure, suggesting the presence of a central control mechanism. The authors applied a framework to a large-scale imaging screen of cell morphology changes induced by diverse drugs and their combination, resulting in a network of 242 drugs and 1832 interactions [1]. In prokaryotes, the first perturbome was described for Pseudomonas aeruginosa, including a machine learning strategy implemented using a benchmarking strategy based on multiple data partition schemas and several classifiers to select genes guided by model performance metrics. The analysis identified 46 genes as part of the central response to perturbations, with biological functions related to biosynthesis, binding, and metabolism, DNA damage repair and aerobic respiration in the context of tolerance to stress [7].
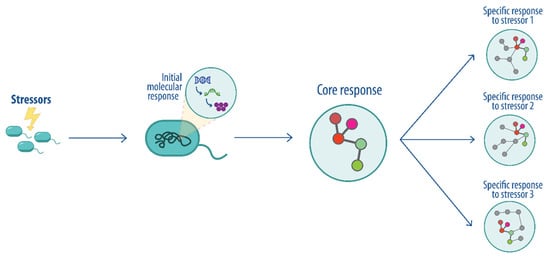
Figure 1.
Conceptualization of the core perturbome for biological systems. After exposure to a stressor, general molecular responses (core) are modulated jointly with other specific responses to the perturbation. The gray nodes indicate specific response to a given perturbation.
At the transcriptional level, multiple studies have shown that distinct molecular responses can be detected within gene networks that are specific to each perturbation. These responses are closely linked to the modulation of various metabolic pathways, ensuring functional redundancy and robustness in the face of diverse stress stimuli [1,3,7]. Key contributors to stress responses include genes related to the SOS system (lexA, recA, dinB, umuDC, etc.) [8,9,10] and the general stress response mediated by RpoS response (sigma factor rpoS, RNAP, xthA, etc.) [8,11,12], whose roles have been extensively documented.
Although studies explicitly using the term “perturbome” are still limited, the concept has been successfully applied in cellular models in both eukaryotes and prokaryotes. In eukaryotic systems, direct relationships between functionally similar drugs and a specific cellular response have been established [1]. Other studies have also shown neuronal responses through molecular networks triggered by defined stressors [3]. In prokaryotes, related investigations have been conducted in Escherichia coli [4,5].
On the other hand, therapeutic interventions, such as antibiotics and biocides, are among the most potent stressors acting on bacterial pathogens. These agents disrupt microbial homeostasis and impose strong selective pressures, ultimately threatening the survival of the microorganisms [13].
However, the emergence of pathogens resistant to antibiotics and biocides constitutes a public health concern and is currently among the most significant critical global challenges [14,15]. In this context, elucidating the central molecular response to perturbations offers a valuable opportunity not only to describe the physiological strategies that bacteria employ to survive under stress conditions but also to identify potential biomarkers and therapeutic targets [7].
In this work, we investigated the molecular determinants of the core perturbome in E. coli and Staphylococcus aureus models. E. coli is a gram-negative, facultative anaerobic bacterium commonly found in various environments and involved in various infections across distinct hosts [16,17]. Over several decades, a vast arsenal of resistance genes has been found in E. coli, suggesting that this genus serves as a critical reservoir of determinants related to antibiotic resistance [18]. The second model, S. aureus, is a ubiquitous, gram-positive, facultative anaerobe frequently implicated in both nosocomial and community-acquired infections in humans and animals [19]. Of particular concern are methicillin-resistant S. aureus strains (MRSA), which are associated with high morbidity and mortality rates worldwide [20].
Given the public health relevance of these bacterial models and the increasing availability of high-throughput molecular technologies (e.g., microarrays and massively parallel sequencing), there is a pressing need for innovative computational strategies capable of handling and interpreting large-scale, complex datasets. In this regard, artificial intelligence (specifically machine learning) has emerged as a powerful tool for detecting and describing nontrivial patterns in massive molecular datasets [21]. Several studies have used machine learning algorithms to evaluate the impact of stressors on various biological organisms by identifying specific molecular responses, including models based on feature selection and classification tasks for accurate prediction of cellular states and the discovery of potential biomarkers from transcriptomic data [7]. Thus, machine learning has played a crucial role in uncovering patterns within molecular networks, enabling the identification of key and hub genes, pathways, and interactions that underlie complex biological responses in both eukaryotic organisms [22,23,24] and prokaryotes, such as Bacillus subtilis [25] and Listeria monocytogenes [26].
Overall, this study proposed to explore the perturbomes of E. coli and S. aureus through machine learning (specifically feature selection and classification) with transcriptomic data. Gene expression data from E. coli (8815 target genes based on pangenome, across 132 samples) and S. aureus (3312 target genes across 156 samples) were used. We hypothesized that the bacteria exposed to various perturbations would exhibit distinct transcriptomic signatures but would reveal a core molecular response characterized by the enrichment of metabolic and signal transduction pathways involved in stress responses. Based on this hypothesis, the specific goal was to identify and functionally characterize genes commonly associated with different perturbations in two prokaryotic models using machine learning.
2. Materials and Methods
The general strategy followed in this work is presented in Figure 2.
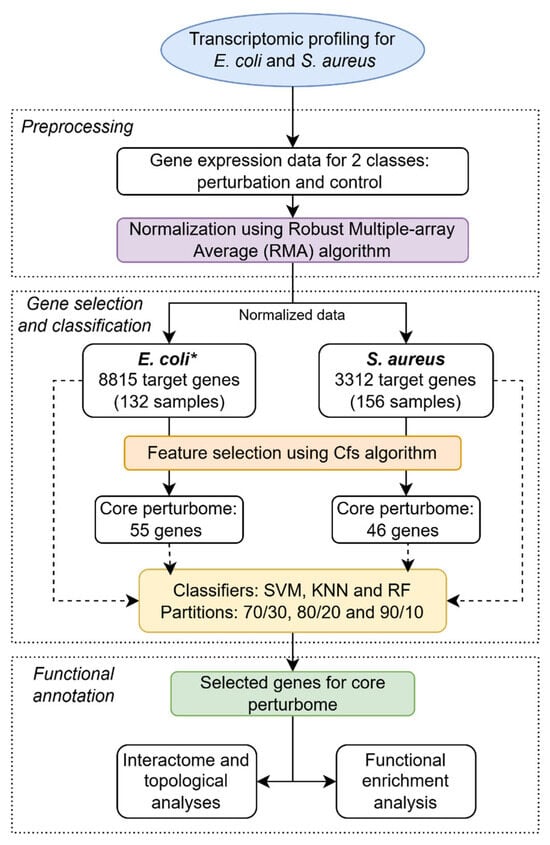
Figure 2.
General pipeline for identifying core perturbome in E. coli and S. aureus by a machine learning approach. * For E. coli, microarray was designed based on the pangenome with four strains.
2.1. Selection of Biological Models and Transcriptomic Data
Two biological models, E. coli and S. aureus, were selected for this study. Publicly available transcriptomic datasets were retrieved from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/, accessed on 5 March 2021). For each organism, the high-throughput molecular platforms with the largest number of experiments and samples with available data were chosen:
- E. coli—GPL3154: 8815 probes (target genes) after intergenic elements were excluded. Note: Annotated genomes of E. coli strains typically report > 4200 genes, but the Affymetrix microarray covers genes of the pangenome of four strains; details at the following website: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL3154;
- S. aureus—GPL1339: 3312 probes (target genes). Note: microarray based on a single genome; details at the following website: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL1339.
The inclusion criteria for experiments and samples in each platform were as follows: (i) having information available regarding the type of perturbation to which the bacteria were exposed (antibiotics, detergents, or chemicals), (ii) having similar culture conditions, and (iii) having control conditions (i.e., unexposed to perturbations).
For E. coli, the final gene expression dataset was composed of 9 series with 87 samples for perturbations and 45 controls. For S. aureus, the final dataset was composed of 15 series with 92 perturbation cases and 64 controls. Descriptions of the datasets, including accession numbers, types of perturbations, and numbers of cases, are presented in Table 1.

Table 1.
Description of datasets used to study the perturbomes of E. coli and S. aureus (from the NCBI-GEO platform).
2.2. Normalization
Transcriptomic data files (TAR format) were retrieved from the GEO database using Bioconductor (https://www.bioconductor.org/) in R software v4.2.2 (https://r-project.org/) with RStudio v2022.12.0 (https://rstudio.com) using classical functions for microarrays. Background correction, normalization, and summarization were performed with the Robust MultiArray Average algorithm (RMA) with the Affy package in Bioconductor [27].
2.3. Machine Learning Algorithms
Machine learning analyses were performed using the Caret package (caret.r-forge.r-project.org/) in RStudio/R software. In the first step, based on the complete dataset for each model, a feature selection approach was used to identify the most relevant genes contributing to each condition (control vs. perturbation). For this purpose, the correlation-based feature selection algorithm (Cfs) was used to reduce dimensionality [28], which identifies the most relevant features (genes) for distinguishing between classes. This approach selects a subset of features that are highly correlated with the target class (perturbations versus controls) but exhibit low intercorrelation among themselves.
In a second analysis, three classification algorithms were used to assess the effectiveness of dimensionality reduction based on the performance of the subset of genes in differentiating between the control and perturbation groups: support vector machine (SVM, kernel = “svmRadial”, epsilon = 0.1, complexity_C = 1.0, tolerance = 0.001) [29], K-nearest neighbors (KNN, algorithm = “LinearNNSearch”, Number_neighbours = 1) [30], and random forest (RF, num_slots = 1, bag% = 100, iterations = 100) [31]. Parameter tuning involved the use of the train() function and the “tuneGrid” option, in which specific model-dependent parameters were selected to be optimized. For RF, mtry (number of variables randomly sampled at each split) was tuned, while k (number of neighbors) was optimized for KNN. For SVM, cost (c) and sigma values were evaluated. Moreover, other classifiers were initially tested (logistic regression, rpart, logit-boost, and neural network) but were excluded after comparison (the three best cases at the training stage were selected for further analysis). All these algorithms considered a 10-fold cross-validation for training, similar to [7]. Due to the dependency of the results on data partitioning, three splits were applied for the training and testing steps: 70/30 (70% training and 30% testing), 80/20, and 90/10. These conditions were applied before and after gene selection. Performance metrics, including accuracy, kappa, precision, recall, true positives (TPs), false positives (FPs), and area under the receiver operating characteristic curve (AUC), were calculated. Selected genes were considered the key elements of the central response to multiple perturbations, i.e., the core perturbome members for each bacterial model.
2.4. Molecular Interactions and Functional Enrichment
Based on the list of candidate genes, corresponding identifiers, biological functions, and protein-level sequences were retrieved from the UniProt database (https://www.uniprot.org/id-mapping, accessed on 5 March 2021). Using a systems biology approach, sequences were employed to construct a model of molecular interactions (interactome) with the Search Tool for the Retrieval of Interacting Genes database (STRINGdb, https://string-db.org/) [32]. The interaction models were generated using default settings, incorporating evidence from experiments, co-expression, gene co-occurrence, text mining, and others, as well as a minimum required interaction score of 0.150.
The resulting graph was exported and visualized using Cytoscape software v 3.7.1 [33]. Hub genes were identified with the Cytohubba plugin [34] based on the top 5 nodes with the best values for degree, betweenness, and bottleneck topological metrics.
Finally, to investigate functional enrichment, protein sequences were analyzed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and the tool KOALA (KEGG Orthology And Links Annotation, https://www.kegg.jp/blastkoala/, accessed on 5 March 2021, version 3.0) [35], allowing the identification of functional modules among the selected genes.
3. Results
3.1. Core Perturbome Genes of E. coli and S. aureus Can Be Identified Using a Machine Learning Approach
Transcriptomic microarray data comprising 8815 genes from E. coli (based on the pangenome from four strains) and 3312 genes from S. aureus (based on a single genome) were preprocessed for machine learning analyses. The Cfs feature selection algorithm was implemented to identify key elements capable of distinguishing between experimental classes (control vs. perturbations). Following this dimensionality reduction, a substantial decrease in the number of genes was achieved: 55 genes (0.62%) for E. coli and 46 for S. aureus (1.39%).
Three classification algorithms (SVM, KNN, and RF) and three data partitions (70/30, 80/20, and 90/10) were applied before and after dimensionality reduction. The accuracy or percentage of correctly classified instances was determined for each case, as shown in Table 2.

Table 2.
Assessment of the performance of the classification models before and after dimensionality reduction of the transcriptomic data for E. coli and S. aureus.
Among all algorithm–partition combinations for E. coli, the median accuracy was 56.52% when using the full dataset (8815 genes), but this value drastically increased to 82.61% when using the selected subset of 55 genes. Similarly, for S. aureus, the median accuracy was 74.5% for the complete dataset (3312 genes), which increased to 85.1% after feature selection (46 genes). Furthermore, under partitioning variations and algorithms, for both biological models, the best combination was the KNN classifier with a 90/10 partition. Nonetheless, other classifiers also performed satisfactorily after dimensionality reduction, with most configurations achieving an accuracy of above 70%. More details are provided in Table 2.
Given that model performance should not rely solely on accuracy, additional evaluation metrics were used to compare the classification models after dimensionality reduction, as shown in Table 3. Depending on the metric, RF outperformed other classifiers in several cases. For instance, RF yielded superior values for the kappa value, TP rate, F score, and AUC across multiple partitions for both biological models. Again, the results for other conditions (partitions and algorithms) also showed acceptable performance after gene selection, reinforcing the robustness of the selected features and classification strategy.

Table 3.
Assessment of the performance of the classification models after dimensionality reduction of the transcriptomic data for E. coli and S. aureus using different metrics.
3.2. Biological Functions and Well-Defined Interactions Can Be Recognized for Genes of the Core Perturbome
Following gene selection, biological functions of the corresponding proteins were identified for each candidate gene, as shown in Table 4 and Table 5. Genes related to metabolic processes, transport, transcriptional regulators, and virulence factors were found in each model. More details are presented in the Supplementary Material. Subsequently, a systems biology approach was employed to construct molecular interaction networks for each model.

Table 4.
Gene annotation for elements of the core perturbome in E. coli.

Table 5.
Gene annotation for elements of the core perturbome in S. aureus.
As shown in Figure 3 and Figure 4 for E. coli and S. aureus, respectively, well-defined interactions were obtained. For E. coli, topological metrics indicated that 42 nodes out of 55 selected genes (76.4%) were connected with 67 edges in total (six nodes were not connected, and six were not mapped to the database); nine hub gene products were recognized in the network, namely, marR, cueR, ecsC, and ycfJ. For S. aureus, 42 nodes out of 46 genes (91.3%) were connected through 123 edges (four unconnected genes), and the gene products recA, guaA, and sleA were among the eight hub genes in this interactome.
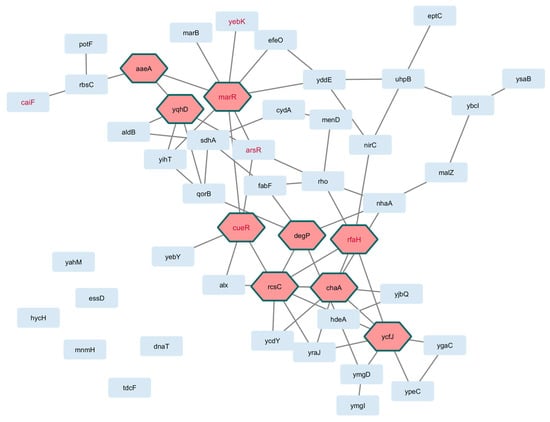
Figure 3.
Interactome of genes in the core perturbome of E. coli. Annotation of each gene product (light blue nodes) was used to model molecular interactions, resulting in 42 connected elements with 9 hub genes (pink nodes) as key determinants of the network. Red-label nodes identify transcription factors.
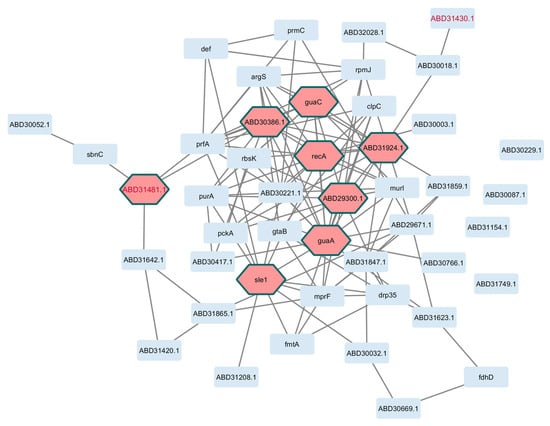
Figure 4.
Interactome based on genes of the core perturbome in S. aureus. Annotation of each gene product (light blue nodes) was used to model molecular interactions, resulting in 42 connected elements with 8 hub genes (pink nodes) as key determinants of the network. Red-label nodes identify transcription factors.
Finally, functional enrichment analysis based on KEGG ontologies revealed a diverse array of biological pathways shared across both models. These included energy and macromolecule metabolism, DNA/RNA and protein synthesis and degradation, transcription regulation, virulence factors, and pathways associated with human diseases (pathogenesis), and others, as depicted in Figure 5 and Table 6. These results were based on 35 (63.6%) annotated genes for E. coli and 41 (89.1%) annotated entries for S. aureus. Despite differences in the identities of the selected genes in the core perturbome in each model, both bacteria exhibited similar patterns of enriched biological modules (Table 6), suggesting conserved strategies in the bacterial response to stress.
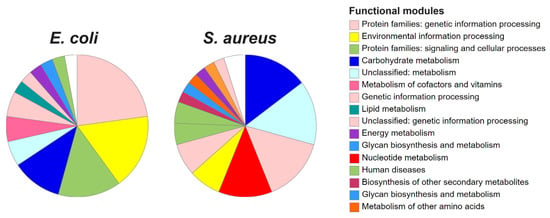
Figure 5.
Functional enrichment of genes in the core perturbome of E. coli and S. aureus. Annotation was based on KEGG ontologies, indicating modulation pathways related to metabolism, protein synthesis, transcription factors and others.

Table 6.
Functional enrichment of genes in the core perturbome of E. coli and S. aureus.
4. Discussion
The core perturbome is defined as the central molecular response of an organism to multiple external disturbances [1,7]. This response functions as a complex molecular network that counteracts disruptions in cellular homeostasis, thereby promoting tolerance and survival under stress conditions [36]. In this study, we focused on the core perturbomes of E. coli and S. aureus, two bacterial species frequently implicated in infections across several hosts, including critical cases in humans with multidrug-resistant strains [4,37,38]. Characterizing the molecular responses to perturbations in those pathogens can provide valuable insights into potential therapeutic targets and biomarkers [39].
As in other studies, transcriptomic data served as a powerful resource for determining key elements involved in stress response, based on changes in gene expression and associations with phenotypic outcomes [40,41,42]. Although gene expression patterns have been studied via machine learning in other biological contexts [24,43,44,45,46], reports on the central response to stimuli in prokaryotic models remain limited. Some reports exist for Bacillus subtilis [25] and Listeria monocytogenes [26], while systematic analyses under the core perturbome framework for E. coli and S. aureus are largely absent. Alternative approaches have explored responses to multiple stressors in E. coli [5] and S. aureus [47], but comprehensive perturbome-level investigations have yet to be reported.
In our analysis, machine learning was used to identify core molecular signatures distinguishing control and perturbation conditions. Feature selection was performed using the Cfs algorithm, which removed irrelevant and redundant features, thereby enhancing the classifiers’ performance. This approach yielded a reduced set of genes with high predictive power: 55 (with nine hubs) for E. coli and 46 (with eight hubs) for S. aureus. These results are in line with previous reports in terms of magnitude. For example, network analysis revealed 24 central genes in E. coli [5], 122 genes in the sigmaB regulon of S. aureus [48], and 46 genes in the perturbome of Pseudomonas aeruginosa [7].
The assessment of selected genes using classification algorithms (SVM, RF, and KNN) and data partitions (70/30, 80/20, and 90/10) demonstrated a substantial improvement in model performance after feature selection in both prokaryotic models. The median accuracy was 82.6% for E. coli and 85.1% for S. aureus after dimensional reduction, in contrast to the median accuracies of 56.52% and 74.5%, respectively, for complete datasets. These results suggest that the selected subset of genes not only retained sufficient discriminatory power to classify the samples accurately but reduced noise effectively—an expected outcome of successful dimensional reduction. In transcriptomic profiling using massive amounts of molecular data, the extraction of relevant information and reducing noise by selecting a subset of relevant genes are still open problems [49]. Our approach, combining feature selection with robust machine learning classifiers, effectively addressed this challenge. The use of SVM, RF, and KNN, which usually outperform other classifiers in comparative strategies [50,51,52], was key to this success. In contrast, the other four classifiers (logistic regression, rpart, logit-boost, and neural network) that demonstrated suboptimal performance were excluded early in the analysis. For the selected classifiers, performance showed some differences across bacterial species. Based on all the metrics, and despite not large differences among classifiers, RF outperformed other algorithms for E. coli, while SVM showed superior performance for S. aureus. This situation is a common and expected behavior for different datasets (from two very different models, distinct microarray platforms, and diverse wet lab experiments used to generate transcriptomic data), as previously reported [30,46,53]. Given the data variability introduced by processing assays across different laboratories (different GEO projects), traditional approaches like differential expression analysis were unsuitable. A more robust method was, therefore, required to account for this variability, and machine learning proved effective in identifying meaningful patterns under these conditions.
Regarding the biological functionality of the selected genes, an orchestrated response was observed to work synergistically based on the modulation of metabolic pathways with interrelated genes, supported by gene annotation, network analysis, and functional enrichment analyses. In the case of hub genes, these are decisive regulators for transferring regulatory information through signaling, functioning as activators or repressors of case-specific operons/genes. Hubs not only have many connections with other genes within the network but also influence the expression and function of many other genes, acting as control centers in the network. Notably, transcription factors played a central role: six in E. coli (ybbI -hub-, caiF, RfaH -hub-, HexR, arsR, and marR -hub) and two in S. aureus (mtlR/SACOL2147 and MerR/SACOL2193 -hub-). The regulatory functions of these genes are associated with the control of efflux pump activity, porin expression, DNA repair mechanisms, and macromolecule transport, which together counteract the effects of antibiotics and genotoxic agents [54,55,56]. These regulated elements were found in our analysis. Furthermore, genes linked to protein synthesis, modulation of growth, and virulence factors were also identified in both bacterial models. These biological functions are consistent with other studies, indicating that these routes are involved in the physiological and metabolic changes that contribute to the tolerance, resisting stress and ensuring only essential functions to survive under stressful conditions [56,57].
Although no specific genes are directly linked to classical stress responses, such as the SOS or RpoS responses, several key pathways functionally related to these responses were enriched in the core perturbomes of E. coli and S. aureus [5,58,59]. For example, functional and enriched pathways associated with “DNA damage repair”, “energy and macromolecule metabolism”, “DNA/RNA and protein synthesis and degradation”, “transcription regulation”, “virulence factors”, and other signaling processes were consistently enriched in both models. These biological modules are well-documented components of bacterial adaptation under stress conditions [4,54,55,60]. Although part of the transcriptomic data is from non-pathogenic strains (E. coli K12, for example, as well as the consideration of genes from the pangenome with four strains), the selected genes largely belong to the conserved core genome, implying that these determinants likely play similar roles in pathogenic lineages.
Interestingly, the number of enriched pathways was relatively limited, which may reflect redundancy and robustness in the general response to stress [4,7]. This has been reported in other works. For instance, our previous study of the core perturbome of P. aeruginosa revealed 46 genes and a reduced number of pathways associated with biosynthesis, protein binding, and metabolism, many of which are related to DNA damage repair and aerobic respiration in the context of tolerance to stress [7]. In the study by [5] with E. coli, interactome analysis of 24 central proteins revealed the role of RNA binding, virulence factors, transporters, and DNA repair as important processes during the stress response.
Furthermore, the biological significance of perturbome analysis was underscored in our prior research with P. aeruginosa strain AG1 [61]. There, we identified core genes and those exclusively induced by ciprofloxacin exposure. Most of the genes that were not part of the core perturbome were resident prophages of the genome. These determinants were expressed in response to ciprofloxacin but not to other antibiotics. This observation, validated phenotypically, supports the potential utility of phages as endogenous modulators in therapeutic strategies, including phage therapy [61].
Moreover, a preliminary ortholog comparison among the studied models (P. aeruginosa from the previous study and E. coli and S. aureus from the present work) using OrthoFinder [62] revealed six shared genes (rho, fabF, tdcF, argS, sle1, and gtaB), suggesting a conserved role in the molecular stress response across these species. This is considered a robust selection from different bacteria and diverse experimental conditions to obtain transcriptomic data within each bacterial species. We are currently studying them by molecular docking to evaluate their druggability and eventually predict the in silico and in vitro effects using chemical compounds on the modulation of stress tolerance. These genes are being structurally modeled using PDB or AlphaFold, and in collaboration with a structural biology team, we are working to identify chemical inhibitors. We are also standardizing PCR protocols to quantify gene expression of the core perturbome for E. coli and S. aureus, as it was established for P. aeruginosa recently [63].
Regarding the limitations, this study was primarily affected by data availability, in which specific platforms were used to obtain complete data with comparable information. For example, for E. coli, the microarray data used were from a non-pathogenic strain (K-12 substrain MG1655) and the microarray platform was designed based on the pangenome with four strains; selection was based on data availability rather than relevance as a pathogen or obtained with more recent and advanced technologies such as RNA sequencing. Future analyses would benefit from incorporating RNA-seq data from pathogenic strains, ideally obtained using the same platform and under comparable experimental conditions to enhance consistency and biological relevance. Furthermore, the included transcriptomic datasets were generated under heterogeneous experimental setups, which may introduce variability. Although strict inclusion criteria were applied to minimize this source of bias, the lack of uniform conditions remains a limitation. Finally, while this study provides insights into gene expression responses at the transcriptomic level, additional validation of the identified genes through proteomic analyses and comprehensive phenotypic assays is necessary to confirm their functional roles in bacterial stress responses.
5. Conclusions
In conclusion, this study identified the core perturbomes of E. coli and S. aureus through a machine learning-based approach with transcriptomic data. Feature selection enabled effective dimensionality reduction, improving classification performance to median accuracies of 82.6% and 85.1% for E. coli and S. aureus, respectively, across multiple data partitions (70/30, 80/20, and 90/10). The core perturbomes comprised 55 genes (including nine hubs) for E. coli and 46 genes (including eight hubs) for S. aureus, including both old-acquaintance regulators (such as transcription factors) and new possible determinants of the response to stress. Functional and network analyses revealed enrichment in key biological processes, including pathways related to energy and macromolecule metabolism, DNA/RNA and protein synthesis and degradation, transcription regulation, virulence, and other signaling processes. These results provide new insights into the conserved and strain-specific molecular mechanisms that underpin bacterial adaptation to diverse stressors, offering a foundation for future research on antimicrobial targets and stress resilience in prokaryotes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14080788/s1, Tables S1 and S2: Gene annotation for elements of the core perturbome; Tables S3 and S4: Correlation of genes and class (control or perturbation); Tables S5 and S6: Gene annotations for the complete dataset in the microarray.
Author Contributions
J.A.M.-M. participated in the funding, the conception and the design of the study. J.F.C.-G., M.V.-C. and J.A.M.-M. performed the experimental assays and data analysis. J.F.C.-G. and J.A.M.-M. drafted the manuscript. J.F.C.-G., M.V.-C. and J.A.M.-M. were involved in the revision and final approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Vicerrectoría de Investigación, Universidad de Costa Rica with the projects “C1163 pro-NGS 2.0: Protocolos operativos estandarizados de análisis de datos moleculares obtenidos por NGS o afines y de algoritmos de inteligencia artificial en modelos biológicos”, “C4604 iPAT: Plataforma genómica, bioinformática y de inteligencia artificial para la vigilancia de patógenos”, and “C5027 PAM-IA Patrones moleculares y clínico-demográficos en bases de datos masivos del cihata asociadas a tres patologías estudiadas con Inteligencia Artificial”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Public raw data used in this study can be retrieved from the GEO database (https://www.ncbi.nlm.nih.gov/geo/, accessed on 5 March 2025) based on the Series ID reported in Table 1. Pipelines to access data, normalization analysis, machine learning methods, and normalized data are available at: https://github.com/josemolina6/Perturbome.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AUC | Area under the receiver operating characteristic curve |
| Cfs | Correlation-based feature selection |
| KNN | K-nearest neighbors |
| RF | Random forest |
| SVM | Support vector machine |
References
- Caldera, M.; Müller, F.; Kaltenbrunner, I.; Licciardello, M.P.; Lardeau, C.H.; Kubicek, S.; Menche, J. Mapping the perturbome network of cellular perturbations. Nat. Commun. 2019, 10, 5140. [Google Scholar] [CrossRef]
- Bermingham, M.L.; Pong-Wong, R.; Spiliopoulou, A.; Hayward, C.; Rudan, I.; Campbell, H.; Wright, A.F.; Wilson, J.F.; Agakov, F.; Navarro, P. Application of high-dimensional feature selection: Evaluation for genomic prediction in man. Sci. Rep. 2015, 5, 10312. [Google Scholar] [CrossRef]
- Sadeh, S.; Clopath, C. Theory of Neuronal Perturbome: Linking Connectivity to Coding via Perturbations. bioRxiv 2020. bioRxiv: 2020.02.20.954222. [Google Scholar] [CrossRef]
- Dragosits, M.; Mozhayskiy, V.; Quinones-Soto, S.; Park, J.; Tagkopoulos, I. Evolutionary potential, cross-stress behavior and the genetic basis of acquired stress resistance in Escherichia coli. Mol. Syst. Biol. 2014, 9, 643. [Google Scholar] [CrossRef]
- Nagar, S.D.; Aggarwal, B.; Joon, S.; Bhatnagar, R.; Bhatnagar, S. A Network Biology Approach to Decipher Stress Response in Bacteria Using Escherichia coli As a Model. OMICS 2016, 20, 310–324. [Google Scholar] [CrossRef]
- KC, K.; Li, R.; Cui, F.; Yu, Q.; Haake, A.R. GNE: A deep learning framework for gene network inference by aggregating biological information. BMC Syst. Biol. 2019, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.A.M.; Montero-Manso, P.; García-Batán, R.; Campos-Sánchez, R.; Fernández, J.V.; García, F. A first perturbome of Pseudomonas aeruginosa: Identification of core genes related to multiple perturbations by a machine learning approach. Biosystems 2021, 205, 104411. [Google Scholar] [CrossRef]
- Trastoy, R.; Manso, T.; Fernández-García, L.; Blasco, L.; Ambroa, A.; del Molino, M.L.P.; Bou, G.; García-Contreras, R.; Wood, T.K.; Tomás, M. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Am. Soc. Microbiol. 2018, 31, e00023-18. [Google Scholar] [CrossRef]
- Vollmer, A.C.; Belkin, S.; Smulski, D.R.; Van Dyk, T.K.; Larossa, R.A. Detection of DNA damage by use of Escherichia coli carrying recA’::lux, uvrA’::lux, or alkA’::lux reporter plasmids. Appl. Environ. Microbiol. 1997, 63, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Valencia, E.Y.; Esposito, F.; Spira, B.; Blázquez, J.; Galhardo, R.S. Ciprofloxacin-mediated mutagenesis is suppressed by subinhibitory concentrations of amikacin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 61, e02107-16. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Polen, T.; Heuveling, J.; Wendisch, V.F.; Hengge, R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. Society 2005, 187, 1591–1603. [Google Scholar] [CrossRef]
- Galhardo, R.S.; Do, R.; Yamada, M.; Friedberg, E.C.; Hastings, P.J.; Nohmi, T.; Rosenberg, S.M. DinB upregulation is the sole role of the SOS response in stress-induced mutagenesis in Escherichia coli. Genetics 2009, 182, 55–68. [Google Scholar] [CrossRef]
- Khodaparast, L.; Wu, G.; Khodaparast, L.; Schmidt, B.Z.; Rousseau, F.; Schymkowitz, J. Bacterial Protein Homeostasis Disruption as a Therapeutic Intervention. Front. Mol. Biosci. 2021, 8, 681855. [Google Scholar] [CrossRef]
- Nwobodo, D.C.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef]
- Jenkins, C.; Rentenaar, R.J.; Landraud, L.; Brisse, S. 180—Enterobacteriaceae. In Infectious Diseases; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1565–1578.e2. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Li, L.; Yeaman, M.R.; Bayer, A.S.; Xiong, Y.Q. Phenotypic and Genotypic Characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA) Related to Persistent Endovascular Infection. Antibiotics 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Rağbetli, C.; Parlak, M.; Bayram, Y.; Guducuoglu, H.; Ceylan, N. Evaluation of Antimicrobial Resistance in Staphylococcus aureus Isolates by Years. Interdiscip. Perspect. Infect. Dis. 2016, 2016, 9171395. [Google Scholar] [CrossRef] [PubMed]
- Molina-Mora, J.A.; Herrera-Hidalgo, M.L. Inteligencia Artificial en Ciencias de Laboratorio: Conceptos, Aplicaciones y Escenario Actual en Costa Rica. Rev. Del. Col. De. Microbiól. Quím. Clín. 2025, 29, 1–13. Available online: https://revista.microbiologos.cr/wp-content/uploads/2025/01/Articulo-MOLINA-MORA-IA.pdf (accessed on 5 February 2025).
- Gupta, C.; Ramegowda, V.; Basu, S.; Pereira, A. Using Network-Based Machine Learning to Predict Transcription Factors Involved in Drought Resistance. Front. Genet. 2021, 12, 652189. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Niazi, A.; Akrami, S. Integration of meta-analysis, machine learning and systems biology approach for investigating the transcriptomic response to drought stress in Populus species. Sci. Rep. 2023, 13, 847. [Google Scholar] [CrossRef]
- Ma, C.; Xin, M.; Feldmann, K.A.; Wang, X. Machine Learning-Based Differential Network Analysis: A Study of Stress-Responsive Transcriptomes in Arabidopsis. Plant Cell 2014, 26, 520–537. [Google Scholar] [CrossRef]
- Huang, Y.; Sinha, N.; Wipat, A.; Bacardit, J. A knowledge integration strategy for the selection of a robust multi-stress biomarkers panel for Bacillus subtilis. Synth. Syst. Biotechnol. 2023, 8, 97–106. [Google Scholar] [CrossRef]
- Hanes, R.; Zhang, F.; Huang, Z. Protein Interaction Network Analysis to Investigate Stress Response, Virulence, and Antibiotic Resistance Mechanisms in Listeria monocytogenes. Microorganisms 2023, 11, 930. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, Normalization, and Summaries of High Density Oligonucleotide Array Probe Level Data. Biostatistics 2018, 4, 249–264. [Google Scholar]
- Hall, M.A. Correlation-Based Feature Selection for Machine Learning. Ph.D. Thesis, University of Waikato, Hamilton, New Zealand, 1999. Available online: https://ml.cms.waikato.ac.nz/publications/1999/99MH-Thesis.pdf (accessed on 5 March 2021).
- Vapnik, V. Estimation of Dependences Based on Empirical Data; Springer: Berlin/Heidelberg, Germany, 1982; Available online: https://dl.acm.org/citation.cfm?id=1098680 (accessed on 16 November 2018).
- Li, L.; Weinberg, C.R.; Darden, T.A.; Pedersen, L.G. Gene selection for sample classification based on gene expression data: Study of sensitivity to choice of parameters of the GA/KNN method. Bioinformatics 2001, 17, 1131–1142. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11751221 (accessed on 16 November 2018). [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- DeLong, E.F. Prokaryotes: Prokaryotic Physiology and Biochemistry; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- McVicker, G.; Prajsnar, T.K.; Williams, A.; Wagner, N.L.; Boots, M.; Renshaw, S.A.; Foster, S.J. Clonal Expansion during Staphylococcus aureus Infection Dynamics Reveals the Effect of Antibiotic Intervention. PLoS Pathog. 2014, 10, e1003959. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/handle/10665/259462/9789241550178-eng.pdf?sequence=1&ua=1 (accessed on 21 January 2020).
- Pinto, A.C.; de Sá, P.H.C.G.; Ramos, R.T.J.; Barbosa, S.; Barbosa, H.P.M.; Ribeiro, A.C.; Silva, W.M.; Rocha, F.S.; Santana, M.P.; de Paula Castro, T.L.; et al. Differential transcriptional profile of Corynebacterium pseudotuberculosis in response to abiotic stresses. BMC Genom. 2014, 15, 14. [Google Scholar] [CrossRef]
- Blasdel, B.G.; Chevallereau, A.; Monot, M.; Lavigne, R.; Debarbieux, L. Comparative transcriptomics analyses reveal the conservation of an ancestral infectious strategy in two bacteriophage genera. ISME J. 2017, 11, 1988–1996. [Google Scholar] [CrossRef]
- Chung, M.; Bruno, V.M.; Rasko, D.A.; Cuomo, C.A.; Muñoz, J.F.; Livny, J.; Shetty, A.C.; Mahurkar, A. Best practices on the differential expression analysis of multi-species RNA-seq. Genome Biol. 2021, 22, 121. [Google Scholar] [CrossRef]
- Li, L.; Tetu, S.G.; Paulsen, I.T.; Hassan, K.A. A transcriptomic approach to identify novel drug efflux pumps in bacteria. Methods Mol. Biol. 2018, 1700, 221–235. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, J.J.; Perkins, R.; Wang, Y.; Liu, Z.; Hong, H.; Tong, W.; Zou, W. A novel procedure on next generation sequencing data analysis using text mining algorithm. BMC Bioinform. 2016, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, D.M.; Dees, J.L.; Ibberson, C.B.; Huse, H.K.; Mathiesen, I.H.; Kirketerp-Møller, K.; Wolcott, R.D.; Rumbaugh, K.P.; Bjarnsholt, T.; Whiteley, M. Pseudomonas aeruginosa transcriptome during human infection. Proc. Natl. Acad. Sci. USA 2018, 115, E5125–E5134. [Google Scholar] [CrossRef] [PubMed]
- Glaab, E.; Bacardit, J.; Garibaldi, J.M.; Krasnogor, N. Using rule-based machine learning for candidate disease gene prioritization and sample classification of cancer gene expression data. PLoS ONE 2012, 7, e39932. [Google Scholar] [CrossRef]
- Raza, K.; Hasan, A. A Comprehensive Evaluation of Machine Learning Techniques for Cancer Class Prediction Based on Microarray Data. Int. J. Bioinform. Res. Appl. 2015, 11, 397–416. [Google Scholar] [CrossRef]
- Ranganathan, N.; Johnson, R.; Edwards, A.M. The general stress response of Staphylococcus aureus promotes tolerance of antibiotics and survival in whole human blood. Microbiology 2020, 166, 1088. [Google Scholar] [CrossRef]
- Pané-Farré, J.; Jonas, B.; Förstner, K.; Engelmann, S.; Hecker, M. The σB regulon in Staphylococcus aureus and its regulation. Int. J. Med. Microbiol. 2006, 296, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Lee, D.; Selvarajoo, K. ScatLay: Utilizing transcriptome-wide noise for identifying and visualizing differentially expressed genes. Sci. Rep. 2020, 10, 17483. [Google Scholar] [CrossRef]
- Leung, R.K.K.; Wang, Y.; Ma, R.C.; Luk, A.O.; Lam, V.; Ng, M.; So, W.Y.; Tsui, S.K.; Chan, J.C. Using a multi-staged strategy based on machine learning and mathematical modeling to predict genotype-phenotype risk patterns in diabetic kidney disease: A prospective case-control cohort analysis. BMC Nephrol. 2013, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Noi, P.T.; Kappas, M. Comparison of random forest, k-nearest neighbor, and support vector machine classifiers for land cover classification using sentinel-2 imagery. Sensors 2018, 18, 18. [Google Scholar] [CrossRef]
- Park, H.; Shimamura, T.; Imoto, S.; Miyano, S. Adaptive NetworkProfiler for Identifying Cancer Characteristic-Specific Gene Regulatory Networks. J. Comput. Biol. 2017, 25, 130–145. [Google Scholar] [CrossRef]
- Tabe-Bordbar, S.; Emad, A.; Zhao, S.D.; Sinha, S. A closer look at cross-validation for assessing the accuracy of gene regulatory networks and models. Sci. Rep. 2018, 8, 6620. [Google Scholar] [CrossRef]
- Sharma, P.; Haycocks, J.R.; Middlemiss, A.D.; Kettles, R.A.; Sellars, L.E.; Ricci, V.; Piddock, L.J.; Grainger, D.C. The multiple antibiotic resistance operon of enteric bacteria controls DNA repair and outer membrane integrity. Nat. Commun. 2017, 8, 1444. [Google Scholar] [CrossRef]
- Poole, K. Bacterial stress responses as determinants of antimicrobial resistance. J. Antimicrob. Chemother. 2012, 67, 2069–2089. [Google Scholar] [CrossRef]
- Andersson, D. The biological cost of mutational antibiotic resistance: Any practical conclusions? Curr. Opin. Microbiol. 2006, 9, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Wiesch, P.S.Z.; Engelstädter, J.; Bonhoeffer, S. Compensation of fitness costs and reversibility of antibiotic resistance mutations. Antimicrob. Agents Chemother. 2010, 54, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Storvik, K.A.M.; Foster, P.L. RpoS, the stress response sigma factor, plays a dual role in the regulation of Escherichia coli’s error-prone DNA polymerase IV. J. Bacteriol. 2010, 192, 3639–3644. [Google Scholar] [CrossRef]
- Cirz, R.T.; O’Neill, B.M.; Hammond, J.A.; Head, S.R.; Romesberg, F.E. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J. Bacteriol. 2006, 188, 7101–7110. [Google Scholar] [CrossRef] [PubMed]
- Vihervaara, A.; Duarte, F.M.; Lis, J.T. Molecular mechanisms driving transcriptional stress responses. Nat. Rev. Genet. 2018, 19, 385–397. [Google Scholar] [CrossRef]
- Molina-Mora, J.A.; García, F. Molecular Determinants of Antibiotic Resistance in the Costa Rican Pseudomonas aeruginosa AG1 by a Multi-omics Approach: A Review of 10 Years of Study. Phenomics 2021, 1, 3. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Molina-Mora, J.A.; Sibaja-Amador, M.; Rivera-Montero, L.; Chacón-Arguedas, D.; Guzmán, C.; García, F. Assessment of Mathematical Approaches for the Estimation and Comparison of Efficiency in qPCR Assays for a Prokaryotic Model. DNA 2024, 4, 189–200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).