Risk Assessment of Avian Influenza Virus Subtype H7 Introduction and Spread in the Russian Federation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
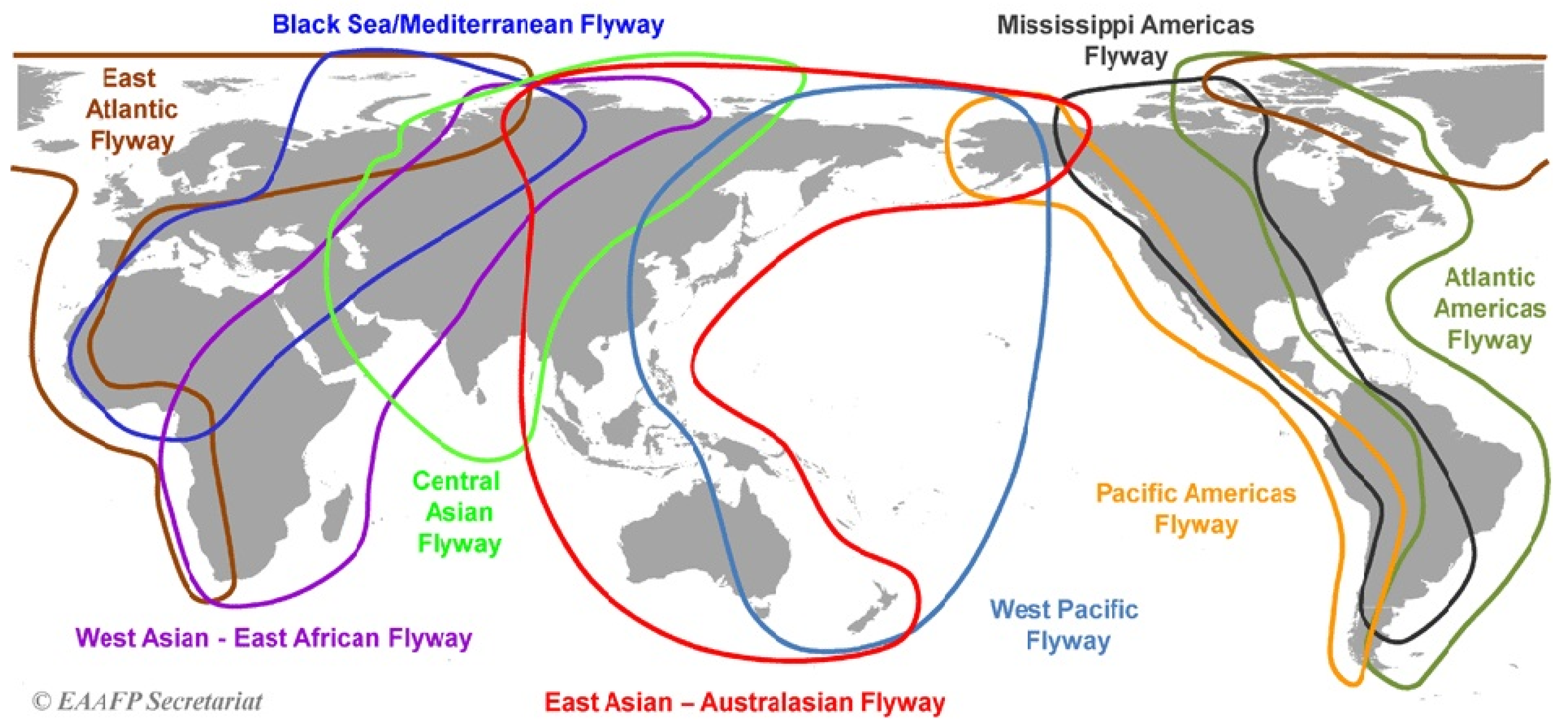
2.2. Data Sources
- (1)
- A temporal range from 2005 to 2025, selected because this 20-year period encompasses reported peaks of H7-subtype avian influenza epidemics [8];
- (2)
- Key bird species known to be involved in avian influenza spread;
- (3)
- Records of bird locations sourced from naturalists, ornithologists, and camera traps.
2.3. Hazard Identification
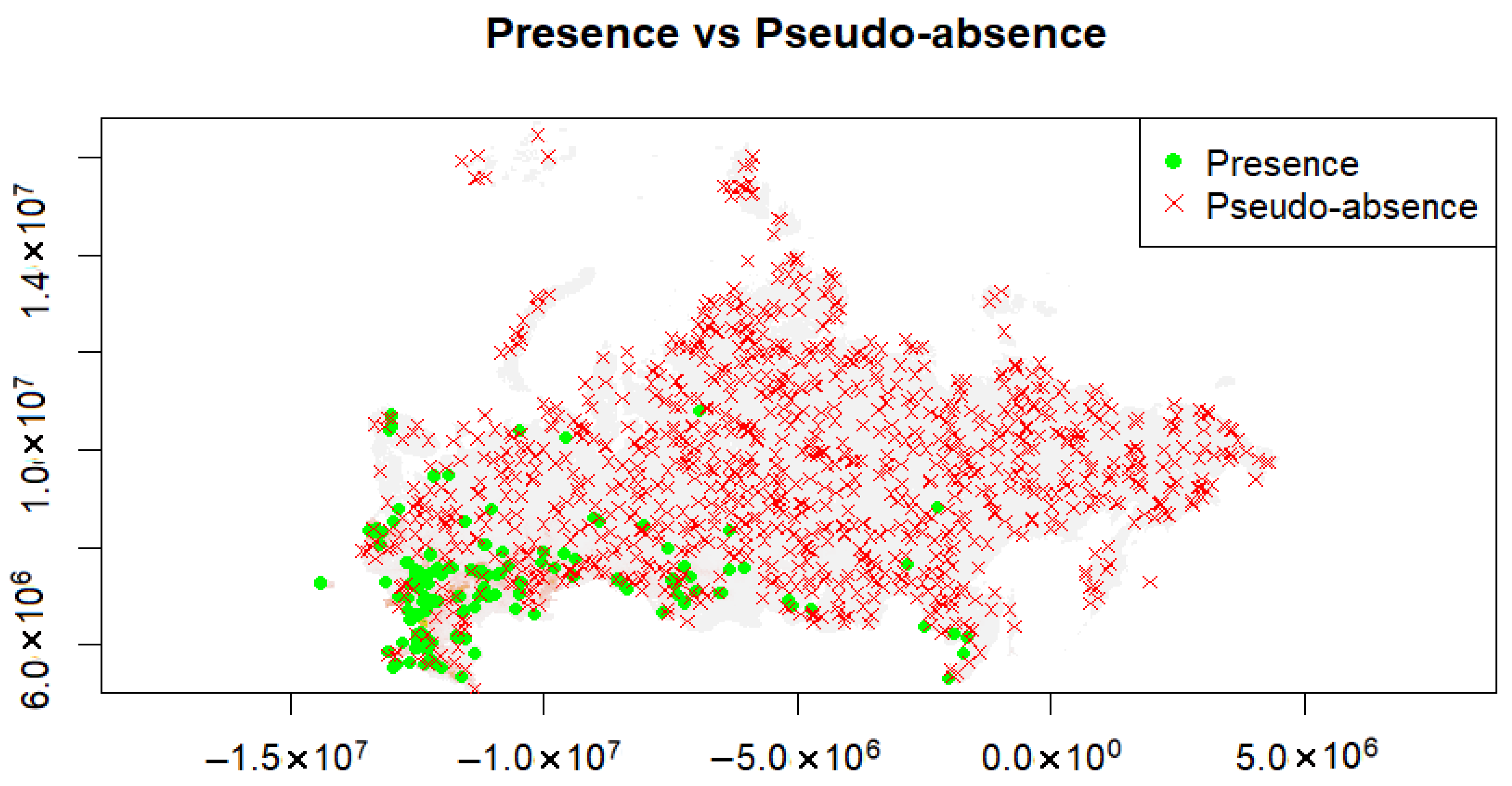
2.4. Ecological Niche Modeling
2.5. Model Construction and Validation
2.6. Software
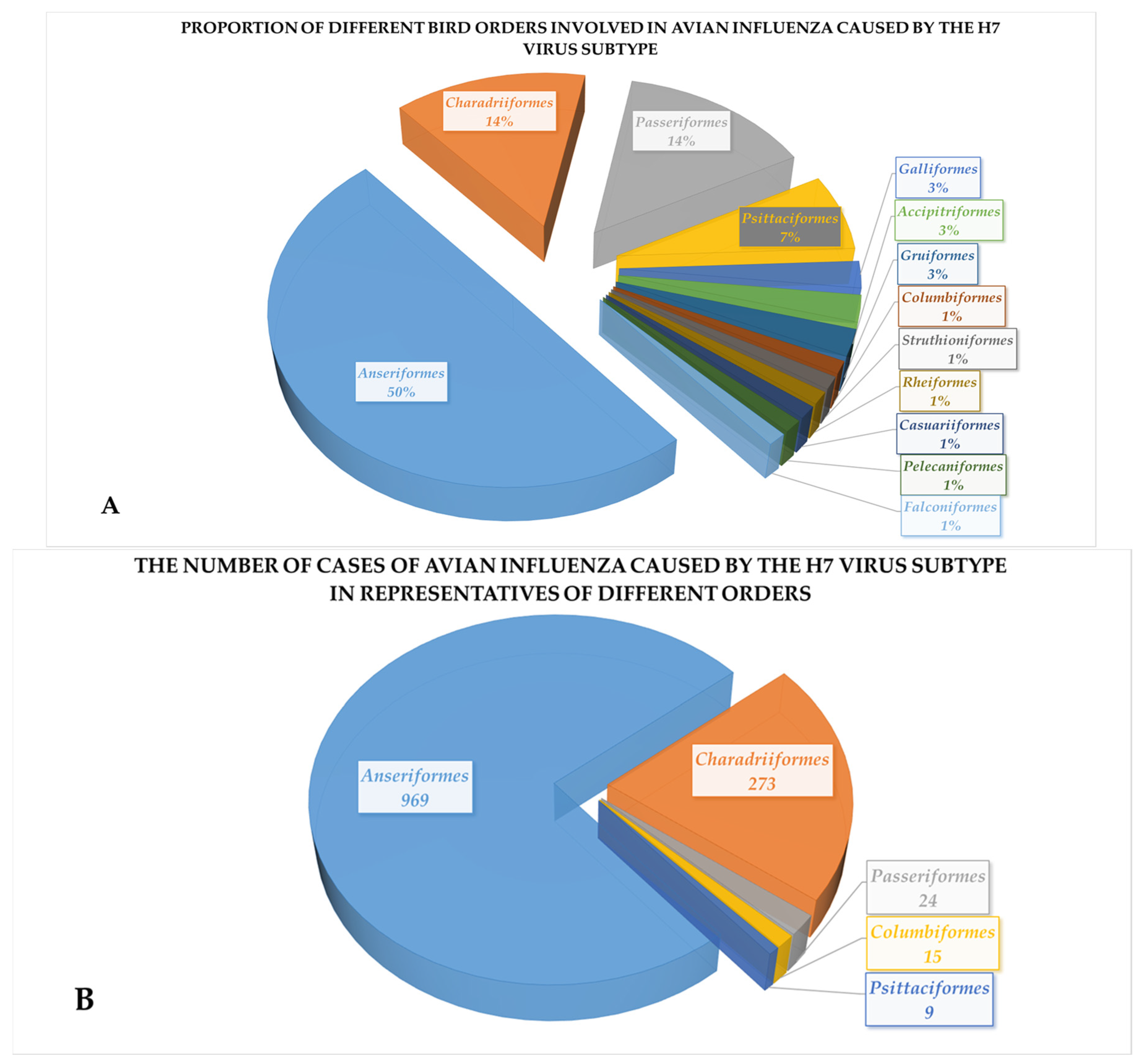
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Committee on Taxonomy of Viruses: ICTV—Electronic Resource. Available online: https://ictv.global/taxonomy (accessed on 30 May 2025).
- Kessler, S.; Burke, B.; Andrieux, G.; Schinköthe, J.; Hamberger, L.; Kacza, J.; Zhan, S.; Reasoner, C.; Dutt, T.S.; Kaukab Osman, M.; et al. Deciphering bat influenza H18N11 infection dynamics in male Jamaican fruit bats on a single-cell level. Nat. Commun. 2024, 15, 4500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Byrne, A.M.P.; Reid, S.M.; Seekings, A.H.; Núñez, A.; Obeso Prieto, A.B.; Ridout, S.; Warren, C.J.; Puranik, A.; Ceeraz, V.; Essen, S.; et al. H7N7 Avian Influenza Virus Mutation from Low to High Pathogenicity on a Layer Chicken Farm in the UK. Viruses 2021, 13, 259. [Google Scholar] [CrossRef]
- Nunez, A.; Brookes, S.M.; Reid, S.M.; Rueda, G.C.; Hicks, D.J.; Seekings, J.M.; Spencer, Y.I.; Brown, I.H. Highly Pathogenic Avian Influenza H5N8 Clade 2.3.4.4 Virus: Equivocal Pathogenicity and Implications for Surveillance Following Natural Infection in Breeder Ducks in the United Kingdom. Transbound. Emerg. Dis. 2016, 63, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Seekings, A.H.; Slomka, M.J.; Russell, C.; Howard, W.A.; Choudhury, B.; Nunez, A.; Londt, B.Z.; Cox, W.; Ceeraz, V.; Thoren, P.; et al. Direct evidence of H7N7 avian influenza virus mutation from low to high virulence on a single poultry premises during an outbreak in free range chickens in the UK, 2008. Infect. Genet. Evol. 2018, 64, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.M.; Núnez, A.; Seekings, A.H.; Thomas, S.S.; Slomka, M.J.; Mahmood, S.; Clark, J.R.; Banks, J.; Brookes, S.M.; Brown, I.H. Two Single Incursions of H7N7 and H5N1 Low Pathogenicity Avian Influenza in U.K. Broiler Breeders During 2015 and 2016. Avian Dis. 2019, 63, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J.; Brown, I.H. History of Highly Pathogenic Avian Influenza. Rev. Sci. Tech. 2009, 28, 19–38. [Google Scholar] [CrossRef]
- World Animal Health Information System (WAHIS Interface). Available online: https://wahis.oie.int/ (accessed on 21 May 2025).
- Zhao, C.; Guo, J.; Zeng, X.; Shi, J.; Deng, G.; Zhang, Y.; Wang, Y.; Ma, Q.; Gao, X.; Cui, P.; et al. Novel H7N7 avian influenza viruses detected in migratory wild birds in eastern China between 2018 and 2020. Microb. Infect. 2022, 24, 105013. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (WOAH). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Chapter 3.3.4; OIE: Paris, France, 2021. [Google Scholar]
- Scott, A.B.; Toribio, J.A.; Singh, M.; Groves, P.; Barnes, B.; Glass, K.; Moloney, B.; Black, A.; Hernandez-Jover, M. Low Pathogenic Avian Influenza Exposure Risk Assessment in Australian Commercial Chicken Farms. Front. Vet. Sci. 2018, 5, 68. [Google Scholar] [CrossRef]
- Beato, M.S.; Capua, I. Transboundary spread of highly pathogenic avian influenza through poultry commodities and wild birds: A review. Rev. Sci. Tech. 2011, 30, 51–61. [Google Scholar] [CrossRef] [PubMed]
- L’vov, D.K.; Iamnikova, S.S.; Fediakina, I.T.; Aristova, V.A.; L’vov, D.N.; Lomakina, N.F.; Petrova, E.S.; Zlobin, V.I.; Khasnatinov, M.A.; Chepurgina, E.A.; et al. Ecology and evolution of influenza viruses in Russia (1979–2002). Issues Virol. 2004, 49, 17–25. [Google Scholar]
- Lviv, D.K.; Ilyichev, V.D. Bird Migrations and Transmission of Infectious Agents; Nauka: Moscow, Russia, 1979; 270p. [Google Scholar]
- Shchelkanov, M.Y.; Kirillov, I.M.; Shestopalov, A.M.; Litvin, K.E.; Deryabin, P.G.; Lviv, D.C. Evolution of the influenza A/H5N1 virus (1996–2016). Issues Virol. 2016, 61, 245–256. [Google Scholar] [CrossRef]
- Matrosov, A.N.; Kuznetsov, A.A.; Sludsky, A.A.; Tarasov, M.A.; Udovikov, A.I.; Yakovlev, S.A.; Kologorov, A.I.; Karavaeva, T.B.; Merkulova, T.K.; Popov, N.V.; et al. Epizootology of avian influenza and the likelihood of secondary outbreaks in the Lower Volga region. Probl. Highly Danger. Infect. 2005, 2, 27–32. Available online: https://cyberleninka.ru/article/n/epizootologiya-grippa-ptits-i-veroyatnost-formirovaniya-vtorichnyh-ochagov-etoy-infektsii-na-territorii-nizhnego-povolzhya (accessed on 27 May 2025).
- Gulyaeva, M.A.; Sharshov, K.A.; Sobolev, I.A.; Yurlov, A.K.; Gadzhiev, A.A.; Rabazanov, N.I.; Shestopalova, L.V.; Shestopalov, A.M. Isolation of the influenza A virus from the plumage of waterfowl during autumn migration. S. Russ. Ecol. Dev. 2018, 13, 134–141. [Google Scholar] [CrossRef]
- International Wader Study Group. Available online: https://www.waderstudygroup.org/ (accessed on 26 April 2025).
- Yurnov, A.K.; Chernyshev, V.M.; Yanovskiy, A.P. Novel data on migration routes and wintering grounds of some bird species in the south of Western Siberia. In Distribution Patterns of Birds of the Urals and the Urals and Western Siberia; Ekaterinburg, 1998; Volume 3, pp. 189–192. Available online: https://cyberleninka.ru/article/n/novye-svedeniya-o-putyah-proleta-i-rayonah-zimovki-nekotoryh-vidov-ptits-iz-yuzhnoy-chasti-zapadnoy-sibiri (accessed on 27 May 2025).
- EAAFP Secretariat (The East Asian—Australasian Flyway Partnership). Available online: https://eaaflyway.net/the-flyway/ (accessed on 26 April 2025).
- Fries, A.C.; Nolting, J.M.; Danner, A.; Webster, R.G.; Bowman, A.S.; Krauss, S.; Slemons, R.D. Evidence for the circulation and inter-hemispheric movement of the H14 subtype influenza A virus. PLoS ONE 2013, 8, e59216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fries, A.C.; Nolting, J.M.; Bowman, A.S.; Lin, X.; Halpin, R.A.; Wester, E.; Fedorova, N.; Stockwell, T.B.; Das, S.R.; Dugan, V.G.; et al. Spread and persistence of influenza A viruses in waterfowl hosts in the North American Mississippi migratory flyway. J. Virol. 2015, 89, 5371–5381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackwood, M.W.; Stallknecht, D.E. Molecular epidemiologic studies on North American H9 avian influenza virus isolates from waterfowl and shorebirds. Avian Dis. 2007, 51 (Suppl. S1), 448–450. [Google Scholar] [CrossRef]
- Koehler, A.V.; Pearce, J.M.; Flint, P.L.; Franson, J.C.; Ip, H.S. Genetic evidence of intercontinental movement of avian influenza in a migratory bird: The northern pintail (Anas acuta). Mol. Ecol. 2008, 17, 4754–4762. [Google Scholar] [CrossRef]
- Makarova, N.V.; Kaverin, N.V.; Krauss, S.; Senne, D.; Webster, R.G. Transmission of Eurasian avian H2 influenza virus to shorebirds in North America. J Gen Virol. 1999, 80 Pt 12, 3167–3171. [Google Scholar] [CrossRef]
- Wahlgren, J.; Waldenström, J.; Sahlin, S.; Haemig, P.D.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Pinhassi, J.; Bonnedahl, J.; Pisareva, M.; Grudinin, M.; et al. Gene segment reassortment between American and Asian lineages of avian influenza virus from waterfowl in the Beringia area. Vector Borne Zoonotic Dis. 2008, 8, 783–790. [Google Scholar] [CrossRef]
- Shestopalov, A.M.; Alekseev, A.Y.; Glupov, V.V.; Voevoda, M.I. Wild Animal Migration as a Potential Threat of Introduction of New Viruses into Russia. Her. Russ. Acad. Sci. 2022, 92, 497–504. [Google Scholar] [CrossRef]
- Lapshin, N.V. General characteristics of bird migrations in the European North of Russia according to ringing data. In Monitoring and Conservation of Biodiversity of Taiga Ecosystems of the European North of Russia; Karelian Scientific Center of the Russian Academy of Sciences: Petrozavodsk, Russia, 2010; pp. 156–158. [Google Scholar]
- Li, C.; Chen, H. H7N9 Influenza Virus in China. Cold Spring Harb. Perspect. Med. 2021, 11, a038349. [Google Scholar] [CrossRef] [PubMed]
- Phillips Steven, J.; Dudík, M.; Schapire Robert, E. MaxEnt Software for Niche Modeling and Distributions (Version 3.4.1). Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 25 May 2025).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Lisovsky, A.A.; Dudov, S.V. Advantages and limitations of ecological habitat modeling methods. 2. Maxent. J. General. Biol. 2020, 81, 135–146. [Google Scholar]
- Sun, Z.; Li, Y.P.; An, Q.; Gao, X.; Wang, H. Bin Risk Factors Contributing to Highly Pathogenic Avian Influenza H5N6 in China, 2014–2021: Based on a Maxent Model. Transbound. Emerg. Dis. 2023, 2023, 6449392. [Google Scholar] [CrossRef]
- Alkhamis, M.; Hijmans, R.J.; Al-Enezi, A.; Martínez-López, B.; Peres, A.M. The Use of Spatial and Spatiotemporal Modeling for Surveillance of H5N1 Highly Pathogenic Avian Influenza in Poultry in the Middle East. Avian Dis. 2016, 60, 146–155. [Google Scholar] [CrossRef]
- Belkhiria, J.; Hijmans, R.J.; Boyce, W.; Crossley, B.M.; Martínez-López, B. Identification of High Risk Areas for Avian Influenza Outbreaks in California Using Disease Distribution Models. PLoS ONE 2018, 13, e0190824. [Google Scholar] [CrossRef]
- Kyuyoung, L.; Yu, D.; Martínez-López, B.; Belkhiria, J.; Kang, S.; Yoon, H.; Hong, S.-K.; Lee, I.; Son, H.-M.; Lee, K. Identification of Highly Pathogenic Avian Influenza Suitable Areas for Wild Birds Using Species Distribution Models in South Korea. Front. Vet. Sci. 2019, 6, 34. [Google Scholar] [CrossRef]
- Abenova, A.Z.; Mukhanbetkaliyev, Y.Y.; Kadyrov, A.S.; Sytnik, I.I.; Shevtsov, A.B.; Korennoy, F.I.; Martin, I.I.; Perez, A.M.; Abdrakhmanov, S.K. Environmental Suitability of Kazakhstan to Highly Pathogenic Avian Influenza Using Data on Eurasian Outbreaks, 2020–2024. Viruses 2025, 17, 574. [Google Scholar] [CrossRef]
- FAO EMPRES-i—Global Animal Disease Information System. Available online: http://empres-i.fao.org (accessed on 25 May 2025).
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.vjb3hx (accessed on 3 June 2025).
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.vp4tut (accessed on 1 June 2025).
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.wwjrn8 (accessed on 3 June 2025).
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.92w4yc (accessed on 1 June 2025).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Lehner, B.; Anand, M.; Fluet-Chouinard, E.; Tan, F.; Aires, F.; Allen, G.H.; Bousquet, P.; Canadell, J.G.; Davidson, N.; Finlayson, C.M.; et al. Mapping the World’s Inland Waters: An Update to the Global Lakes and Wetlands Database (GLWD v2). Available online: https://www.hydrosheds.org/products/glwd (accessed on 21 May 2025).
- ESA DUE GlobCover. Available online: http://due.esrin.esa.int/page_globcover.php (accessed on 1 June 2025).
- FAO Livestock Density. Global Distribution Data. Available online: https://data.apps.fao.org/catalog//iso/9d1e149b-d63f-4213-978b-317a8eb42d02 (accessed on 25 May 2025).
- Kennedy, C.M.; Oakleaf, J.R.; Theobald, D.M.; Baruch-Mordo, S.; Kiesecker, J. Managing the middle: A shift in conservation priorities based on the global human modification gradient. Glob. Change Biol. 2019, 25, 811–826. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of Maxent for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Zhou, X.; Chen, R.; Zhao, G.; Zhang, F. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef] [PubMed]
- QGIS Geographic Information System Development Team. QGIS Open Source Geospatial Foundation Project 2009. Available online: http://qgis.osgeo.org (accessed on 31 May 2025).
- Grillo, V.L.; Arzey, K.E.; Hansbro, P.M.; Hurt, A.C.; Warner, S.; Bergfeld, J.; Burgess, G.W.; Cookson, B.; Dickason, C.J.; Ferenczi, M. Avian influenza in Australia: A summary of 5 years of wild bird surveillance. Aust. Vet. J. 2015, 93, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.L.; Saatchi, S.S.; Curd, E.E.; Toffelmier, E.; Thomassen, H.A.; Buermann, W.; DeSante, D.F.; Nott, M.P.; Saracco, J.F.; Ralph, C.J.; et al. Mapping the risk of avian influenza in wild birds in the US. BMC Infect. Dis. 2010, 10, 187. [Google Scholar] [CrossRef]
- Pugachev, O.N.; Krylov, N.V.; Belova, L.M. Natural reservoir of influenza A viruses. Int. Bull. Vet. Med. 2008, 2, 12–17. Available online: https://www.zin.ru/Administration/files/Prirodn_res_virA_2008.pdf (accessed on 27 May 2025).
- Zhang, J.L.; Chen, Z.Y.; Lin, S.L.; King, C.C.; Chen, C.C.; Chen, P.S. Airborne Avian Influenza Virus in Ambient Air in the Winter Habitats of Migratory Birds. Environ. Sci. Technol. 2022, 56, 15365–15376. [Google Scholar] [CrossRef]
- Claes, G.; Welby, S.; van den Berg, T.; Van der Stede, Y.; Dewulf, J.; Lambrecht, B.; Marché, S. The impact of viral tropism and housing conditions on the transmission of three H5/H7 low pathogenic avian influenza viruses in chickens. Epidemiol. Infect. 2013, 141, 2428–2443. [Google Scholar] [CrossRef]
- Velkers, F.C.; Manders, T.T.M.; Vernooij, J.C.M.; Stahl, J.; Slaterus, R.; Stegeman, J.A. Association of Wild Bird Densities around Poultry Farms with the Risk of Highly Pathogenic Avian Influenza Virus Subtype H5N8 Outbreaks in the Netherlands, 2016. Transbound. Emerg. Dis. 2021, 68, 76–87. [Google Scholar] [CrossRef]

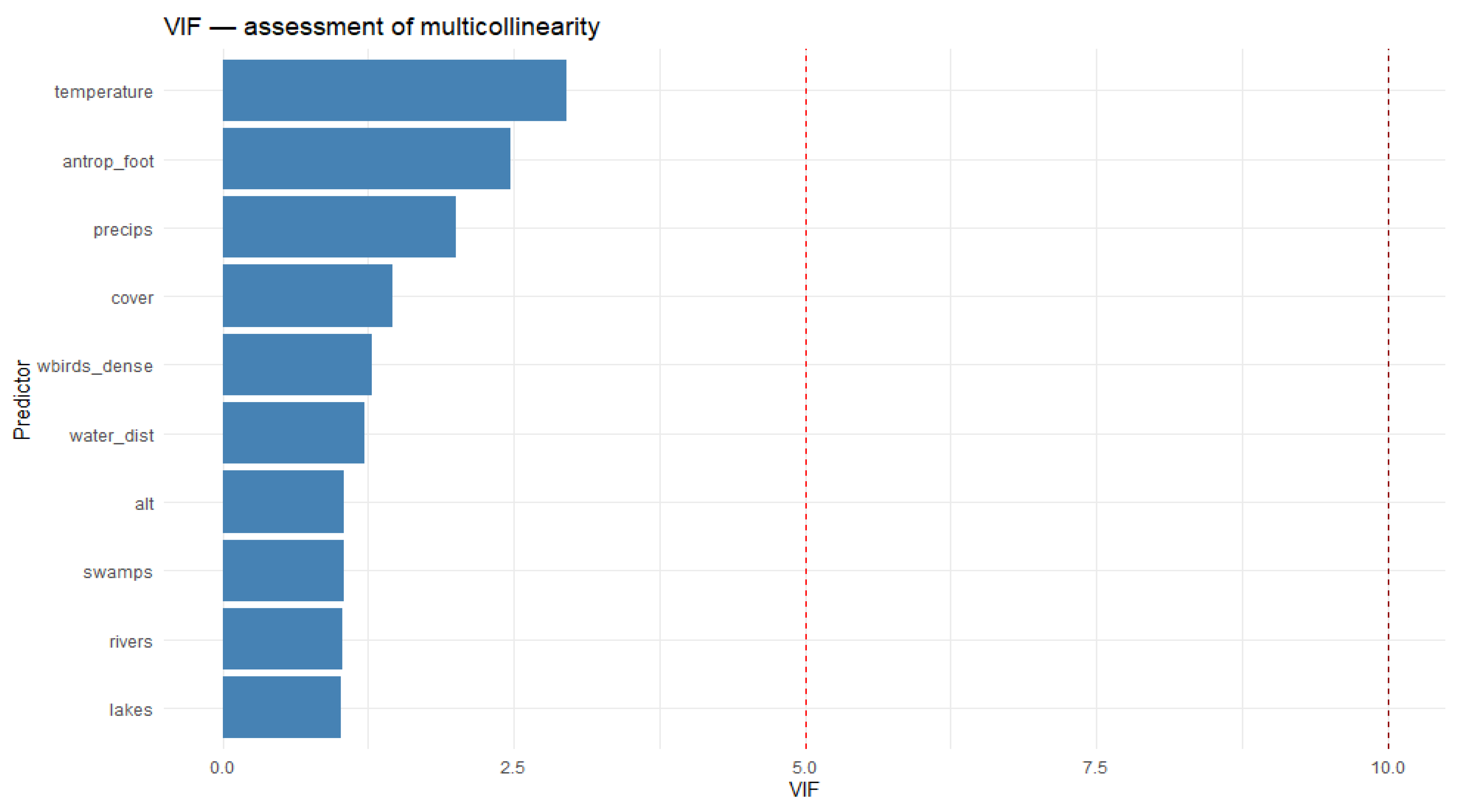


| Variable | Meaning | Factor | Unit of Measurement | Reference |
|---|---|---|---|---|
| temperature | average annual air temperature | climatic | °C | https://www.worldclim.org/ (accessed on 25 May 2025) [43] |
| precips | average annual precipitation | climatic | millimeters | https://www.worldclim.org/ (accessed on 25 May 2025) [43] |
| lakes | distribution or presence of lakes | environmental | % | https://www.hydrosheds.org/ (accessed on 21 May 2025) [44] |
| swamps | presence and distribution of swamps | environmental | % | https://www.hydrosheds.org/ (accessed on 21 May 2025) [44] |
| rivers | river network or proximity to rivers | environmental | kilometers | https://www.hydrosheds.org/ (accessed on 21 May 2025) [44] |
| water_dist | distance to the nearest water bodies | environmental | kilometers | modeled in QGIS |
| alt | digital relief model (height above mean sea level) | environmental | meters | https://www.worldclim.org/ (accessed on 25 May 2025) [43] |
| cover | types of land cover | environmental | category | https://due.esrin.esa.int/ (accessed on 25 May 2025) [45] |
| wbirds_dense | population distribution or density of migrating birds in the study area | population | birds/km2 | https://www.gbif.org/ru/ (accessed on 3 June 2025) [39,41] |
| wbirds_dense_Ru | population distribution or density of migrating birds in Russia | population | birds/km2 | modeled in QGIS |
| sinantrop_dense_Ru | population distribution or density of synanthropic birds in Russia | population | birds/km2 | https://www.gbif.org/ru/ (accessed on 1 June 2025) [40,42] |
| chicken_dense | population density of chickens | population | birds/km2 | https://www.fao.org/ (accessed on 25 May 2025) [46] |
| antrop_foot | human footprint (impact of humans on the global environment) | Population | Index | https://doi.org/10.1111/gcb.14549 (accessed on 3 June 2025) [47] |
| antrop_foot_Ru | human footprint (impact of humans on the global environment) for Russia | Population | Index | modeled in QGIS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varvashenko, D.; Shcherbinin, S.; Varkentin, A.; Irza, V.; Chvala, I.; Sprygin, A.; Volkov, M. Risk Assessment of Avian Influenza Virus Subtype H7 Introduction and Spread in the Russian Federation. Pathogens 2025, 14, 1142. https://doi.org/10.3390/pathogens14111142
Varvashenko D, Shcherbinin S, Varkentin A, Irza V, Chvala I, Sprygin A, Volkov M. Risk Assessment of Avian Influenza Virus Subtype H7 Introduction and Spread in the Russian Federation. Pathogens. 2025; 14(11):1142. https://doi.org/10.3390/pathogens14111142
Chicago/Turabian StyleVarvashenko, Dmitry, Sergey Shcherbinin, Andrey Varkentin, Viktor Irza, Ilya Chvala, Alexander Sprygin, and Mikhail Volkov. 2025. "Risk Assessment of Avian Influenza Virus Subtype H7 Introduction and Spread in the Russian Federation" Pathogens 14, no. 11: 1142. https://doi.org/10.3390/pathogens14111142
APA StyleVarvashenko, D., Shcherbinin, S., Varkentin, A., Irza, V., Chvala, I., Sprygin, A., & Volkov, M. (2025). Risk Assessment of Avian Influenza Virus Subtype H7 Introduction and Spread in the Russian Federation. Pathogens, 14(11), 1142. https://doi.org/10.3390/pathogens14111142





