Long-Term Epidemiological Trends of Human Adenovirus Infection in South Korea: A Single-Center Study (2007–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
2.3. DNA Extraction and Real-Time PCR
2.4. Statistical Analysis
3. Results
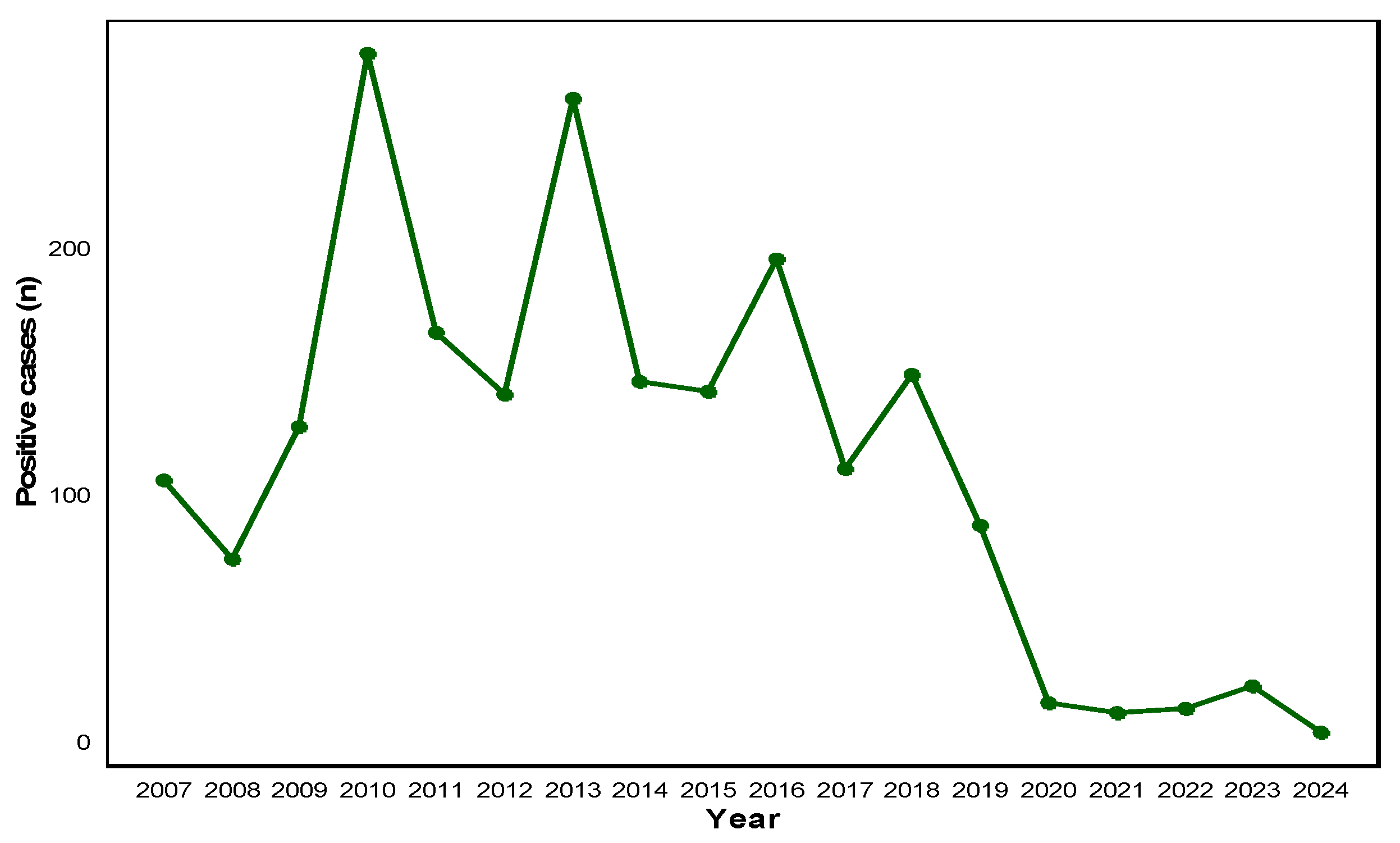
3.1. Annual HAdV Positivity Trend (2007–2024)
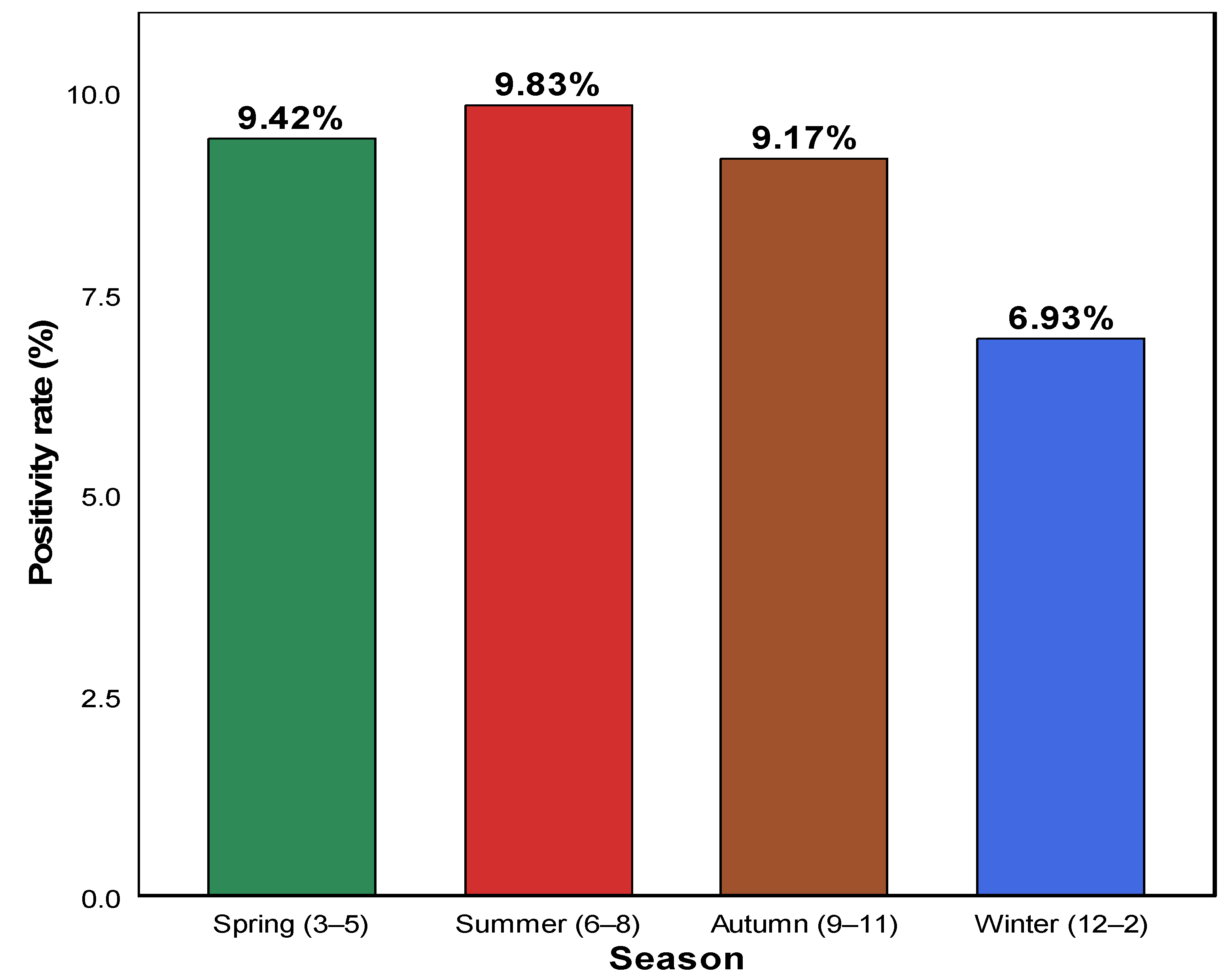
3.2. Seasonal HAdV Positivity Rate
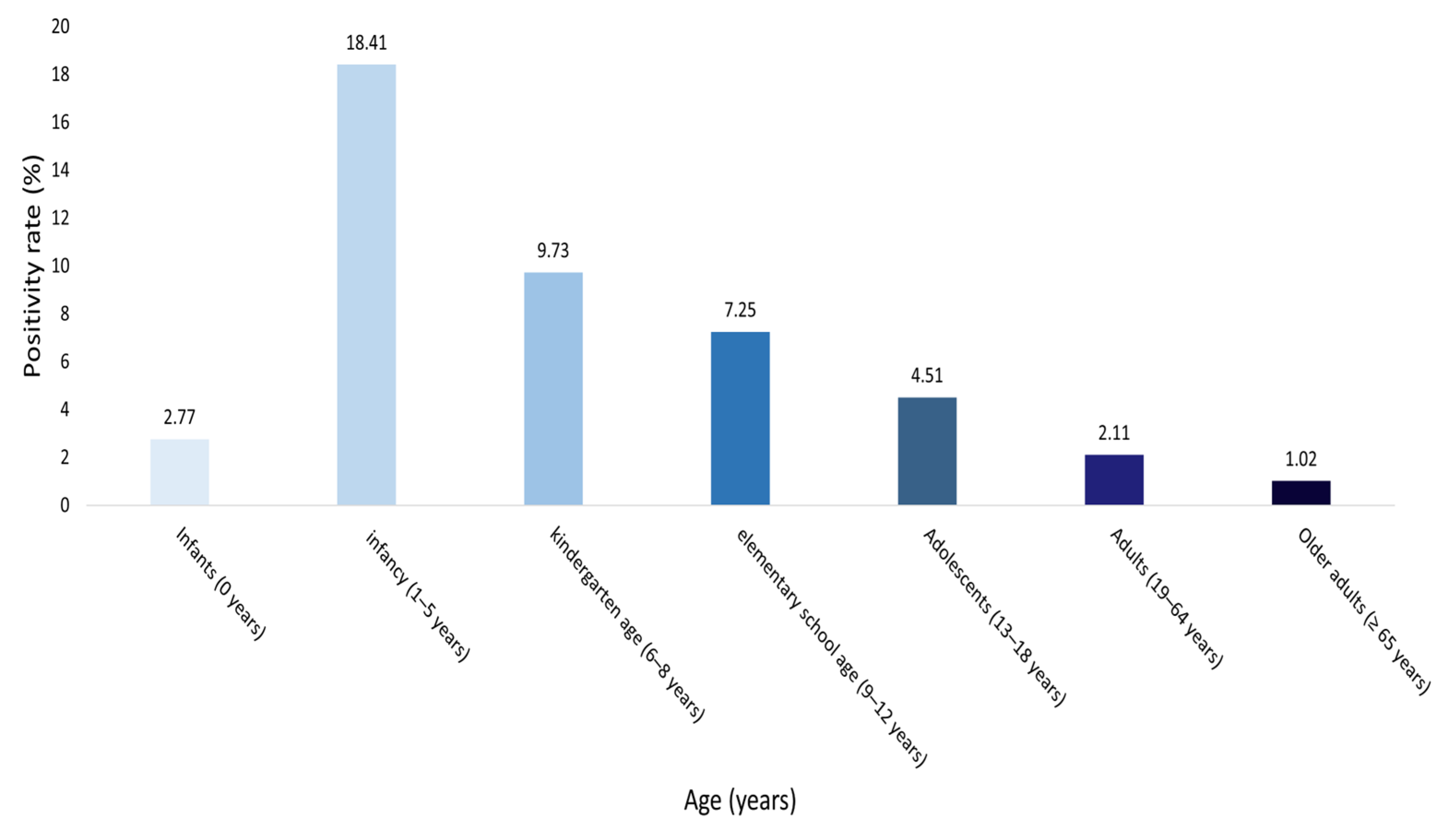
3.3. HAdV Positivity Rate by Age Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HAdVs | Human adenoviruses |
| COVID-19 | Coronavirus disease 2019 |
| NPI | Non-pharmaceutical interventions |
| RSV | respiratory syncytial virus |
References
- Urmi, T.J.; Rahman, J.M.; Dewan, S.M.R. Addressing the health risks posed by adenovirus: A perspective on strategies for prevention and management. Health Sci. Rep. 2025, 8, e71010. [Google Scholar] [CrossRef]
- Zhang, R.; Goto, T.; Zhang, X. Association of human adenovirus load and viral genotype diversity with respiratory disease severity in children: A systematic review and meta-analysis. Transl. Pediatr. 2025, 14, 671–682. [Google Scholar] [CrossRef]
- Soler Wenglein, J.S.; Scarsella, L.; Kotlewski, C.; Heim, A.; Aydin, M. Current trends of human adenovirus types among hospitalized children—A systematic review. Viruses 2025, 17, 914. [Google Scholar] [CrossRef]
- Jin, R.; Qin, T.; Li, P.; Yuan, J.; Li, H.; Liu, Y.; Wang, M.; Xu, J.; Sun, Y. Increased circulation of adenovirus in China during 2023–2024: Association with an increased prevalence of species B and school-associated transmission. J. Infect. 2025, 90, 106475. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qiu, S.; Zhang, L.; Wu, H.; Tian, X.; Li, X.; Xu, D.; Dai, J.; Gu, S.; Liu, Q.; et al. Analysis of severe human adenovirus infection outbreak in Guangdong Province, southern China in 2019. Virol. Sin. 2022, 37, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Woo, S.; Rhee, J.E.; Lee, J.; Lee, S.; Kim, E.J. Increased trend of adenovirus activity after the COVID-19 pandemic in South Korea: Analysis of national surveillance data. Ann. Lab. Med. 2024, 44, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Togashi, A.; Hirakawa, S.; Yamamoto, M.; Fukumura, S.; Nawa, T.; Kushima, N.; Nakamura, S.; Kunizaki, J.; Nishino, K.; et al. A significant outbreak of respiratory human adenovirus infections among children aged 3–6 years in Hokkaido, Japan, in 2023. J. Med. Virol. 2024, 96, e29780. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, T.; Wang, C.B.; Liang, W.L.; Lian, G.W.; Zanin, M.; Wong, S.S.; Tian, X.G.; Zhong, J.Y.; Zhang, Y.Y.; et al. Human adenovirus (HAdV) infection in children with acute respiratory tract infections in Guangzhou, China, 2010–2021: A molecular epidemiology study. World J. Pediatr. 2022, 18, 545–552. [Google Scholar] [CrossRef]
- Abdullah, O.; Fall, A.; Klein, E.; Mostafa, H.H. Increased circulation of human adenovirus in 2023: An investigation of the circulating genotypes, upper respiratory viral loads, and hospital admissions in a large academic medical center. J. Clin. Microbiol. 2024, 62, e0123723. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Aganovic, A.; Bi, Y.; Cao, G.; Kurnitski, J.; Wargocki, P. Modeling the impact of indoor relative humidity on the infection risk of five respiratory airborne viruses. Sci. Rep. 2022, 12, 11481. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, X.; Wang, J.; Ye, C.; Zhao, S. The epidemiological analysis of respiratory virus infections in children in Hangzhou from 2019 to 2023. Virus Res. 2025, 355, 199558. [Google Scholar] [CrossRef]
- Richter, V.P.; de-Paris, F.; Pires, M.R.; Bock, H. Epidemiology of respiratory viruses before and during the COVID-19 pandemic in a tertiary care hospital in Southern Brazil. J. Clin. Virol. Plus 2024, 4, 100190. [Google Scholar] [CrossRef]
- Xuan, M.; Yan, S.; Xiao, C.; Zhong, X.; Zhang, S. High humidity exposures and mechanisms in respiratory disease. Ecotoxicol. Environ. Saf. 2025, 305, 119234. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, R.; Qian, Y.; Sun, Y.; Chen, D.; Wang, F.; Zhou, Y.; Guo, Q.; Liu, L.; Xu, Y.; et al. The changed endemic pattern of human adenovirus from species B to C among pediatric patients under the pressure of non-pharmaceutical interventions against COVID-19 in Beijing, China. Virol. J. 2023, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lin, Y.; Xiong, W.; Liu, C.; Gao, H.; Ho, F.; Zhou, J.; Zhang, R.; Wong, J.Y.; Cheung, J.K.; et al. Comparison of control and transmission of COVID-19 across epidemic waves in Hong Kong: An observational study. Lancet Reg. Health West. Pac. 2024, 43, 100969. [Google Scholar] [CrossRef]
- Tempia, S.; Walaza, S.; Bhiman, J.N.; McMorrow, M.L.; Moyes, J.; Mkhencele, T.; Meiring, S.; Quan, V.; Bishop, K.; McAnerney, J.M.; et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Eurosurveillance 2021, 26, 2001600. [Google Scholar] [CrossRef]
- Liang, D.F.; Guo, W.L.; Zhu, D.P.; Li, S.Y.; Zhu, W.D.; Li, Y.; Huang, L.; Shen, J.; Li, P.Q. Changes in the epidemic patterns of respiratory pathogens of children in Guangzhou, China during the COVID-19 pandemic. BMC Infect. Dis. 2025, 25, 833. [Google Scholar] [CrossRef]
- Cho, H.J.; Rhee, J.E.; Kang, D.; Choi, E.H.; Lee, N.J.; Woo, S.; Lee, J.; Lee, S.W.; Kim, E.J.; Yun, K.W. Epidemiology of respiratory viruses in Korean children before and after the COVID-19 pandemic: A prospective study from national surveillance system. J. Korean Med. Sci. 2024, 39, e171. [Google Scholar] [CrossRef]
- Choi, Y.J.; Chung, E.H.; Lee, E.; Kim, C.H.; Lee, Y.J.; Kim, H.B.; Kim, B.S.; Kim, H.Y.; Cho, Y.; Seo, J.H.; et al. Clinical characteristics of macrolide-refractory Mycoplasma pneumoniae pneumonia in Korean children: A multicenter retrospective study. J. Clin. Med. 2022, 11, 306. [Google Scholar] [CrossRef]
- Billard, M.N.; van de Ven, P.M.; Baraldi, B.; Kragten-Tabatabaie, L.; Bont, L.J.; Wildenbeest, J.G. International changes in respiratory syncytial virus (RSV) epidemiology during the COVID-19 pandemic: Association with school closures. Influ. Other Respir. Viruses 2022, 16, 926–936. [Google Scholar] [CrossRef]
- Kim, K.R.; Won, J.; Kim, H.; Kim, B.I.; Kim, M.J.; Kim, J.Y.; Gwack, J.; Kim, Y.J. The changes in respiratory and enteric adenovirus epidemiology in Korea from 2017 to June 2022. J. Korean Med. Sci. 2023, 38, e71. [Google Scholar] [CrossRef] [PubMed]
- Thindwa, D.; Li, K.; Cooper-Wootton, D.; Zheng, Z.; Pitzer, V.E.; Weinberger, D.M. Global patterns of rebound to normal RSV dynamics following COVID-19 suppression. BMC Infect. Dis. 2024, 24, 635. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Tseng, H.Y.; Chen, C.L.; Lin, Y.C.; Liang, S.J.; Tu, C.Y.; Chen, W.C.; Hsueh, P.R. The real-world impact of the BioFire FilmArray blood culture identification 2 panel on antimicrobial stewardship among patients with bloodstream infections in intensive care units with a high burden of drug-resistant pathogens. J. Microbiol. Immunol. Infect. 2024, 57, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, X.; Zhang, L.; Li, Q.; Feng, G.; Zeng, Y.; Wang, R.; Xie, Z. Clinical epidemiology and disease burden of adenoviral encephalitis in hospitalized children in China: A nationwide cross-sectional study. Pediatr. Investig. 2023, 7, 247–253. [Google Scholar] [CrossRef]
- Endo, A.; CMMID COVID-19 Working Group; Uchida, M.; Liu, Y.; Atkins, K.E.; Kucharski, A.J.; Funk, S. Simulating respiratory disease transmission within and between classrooms to assess pandemic management strategies at schools. Proc. Natl. Acad. Sci. USA 2022, 119, e2203019119. [Google Scholar] [CrossRef]
- Lv, Q.; Luo, R.; Li, X.; Deng, S.; Liu, K.; Liu, L.; Zhu, K.; Wen, Y.; Ma, X.; Ci, R.; et al. Risk assessment of communicable respiratory diseases transmission based on social contact networks: A primary school contact data survey conducted with portable high-precision devices. BMC Public Health 2025, 25, 3192. [Google Scholar] [CrossRef]
- Chedid, K.; Arts, P.; Blair, C.; Hashikawa, A.; Clack, H.; Wigginton, K.; Lauring, A.S.; Marr, L.; Prussin, A.; Lakdawala, S.; et al. 890. Environmental air and surface sampling of respiratory viruses in child care centers. Open Forum Infect. Dis. 2023, 10, ofad500-935. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.J.; Jia, N.; Richardson, S.; Huang, C. Viral respiratory infections in a rapidly changing climate: The need to prepare for the next pandemic. eBioMedicine 2023, 93, 104593. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Ohyama, M.; Ito, E.; Akiyoshi, K.; Onishi, Y.; Mori, A.; Nomoto, R. Whole genome-based surveillance for human adenovirus-related diseases in Kobe City, Japan, 2018–2022. BMC Res. Notes 2025, 18, 170. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, Y.; Zhang, J.; Ren, L.; Li, J.; Xie, Z.; Xu, B.; Yang, Y.; Qian, S.; Wang, J.; et al. Adenovirus infection in children with acute lower respiratory tract infections in Beijing, China, 2007 to 2012. BMC Infect. Dis. 2015, 15, 408. [Google Scholar] [CrossRef]
| Age Group | Total Individuals (n) | Male (n) | Female (n) | Percentage (%) |
|---|---|---|---|---|
| Infants (0 years) | 4543 | 2704 | 1839 | 19.5 |
| Infancy (1–5 years) | 8886 | 5055 | 3831 | 38.2 |
| Kindergarten age (6–8 years) | 976 | 555 | 421 | 4.2 |
| Elementary school age (9–12 years) | 675 | 382 | 293 | 2.8 |
| Adolescents (13–18 years) | 577 | 336 | 241 | 2.5 |
| Adults (19–64 years) | 2935 | 1893 | 1042 | 12.6 |
| Older adults (≥65 years) | 4692 | 3036 | 1656 | 20.2 |
| Total | 23,284 | 13,961 | 9323 | 100 |
| Season | Total Individuals (n) | Positive Case (n) | Positivity Rate (%) |
|---|---|---|---|
| Spring (3–5) | 6391 | 602 | 9.42% |
| Summer (6–8) | 4810 | 473 | 9.83% |
| Autumn (9–11) | 5607 | 514 | 9.17% |
| Winter (12–2) | 6476 | 449 | 6.93% |
| Age Group | Total Individuals (n) | Positive (n) | Negative (n) | Positivity Rate (%) |
|---|---|---|---|---|
| Infants (0 years) | 4543 | 125 | 4418 | 2.77 |
| Infancy (1–5 years) | 8886 | 1636 | 7250 | 18.41 |
| Kindergarten age (6–8 years) | 976 | 95 | 881 | 9.73 |
| Elementary school age (9–12 years) | 675 | 49 | 626 | 7.25 |
| Adolescents (13–18 years) | 577 | 25 | 552 | 4.51 |
| Adults (19–64 years) | 2935 | 61 | 2874 | 2.11 |
| Older adults (≥ 65 years) | 4692 | 47 | 4645 | 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Jang, S.H.; Han, J.S.; Jeon, J.-S.; Kim, J.K. Long-Term Epidemiological Trends of Human Adenovirus Infection in South Korea: A Single-Center Study (2007–2024). Pathogens 2025, 14, 1143. https://doi.org/10.3390/pathogens14111143
Kim YJ, Jang SH, Han JS, Jeon J-S, Kim JK. Long-Term Epidemiological Trends of Human Adenovirus Infection in South Korea: A Single-Center Study (2007–2024). Pathogens. 2025; 14(11):1143. https://doi.org/10.3390/pathogens14111143
Chicago/Turabian StyleKim, Yu Jeong, Sung Hun Jang, Jeong Su Han, Jae-Sik Jeon, and Jae Kyung Kim. 2025. "Long-Term Epidemiological Trends of Human Adenovirus Infection in South Korea: A Single-Center Study (2007–2024)" Pathogens 14, no. 11: 1143. https://doi.org/10.3390/pathogens14111143
APA StyleKim, Y. J., Jang, S. H., Han, J. S., Jeon, J.-S., & Kim, J. K. (2025). Long-Term Epidemiological Trends of Human Adenovirus Infection in South Korea: A Single-Center Study (2007–2024). Pathogens, 14(11), 1143. https://doi.org/10.3390/pathogens14111143






