Low Neutrophil Counts in Milk Are Associated with an Increased Frequency of Antimicrobial Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd and Cow Selection
2.2. Sample Collection
2.3. Cellular Marker Analyses
2.4. Treatment Records
2.5. Definition and Description of Herd Immune Status
2.6. Statistical Analysis
- -
- Accuracy: expressed as the proportion of correctly classified subjects [true positive (TP) + true negative (TN)] among all subjects.
- -
- Sensitivity (Se): the proportion of TP/[TP + false positive (FP)]
- -
- Specificity (Sp): the proportion of TN/[false negative (FN) + TP]
- -
- Positive predictive value (PPV): TP/(TP + FN)
- -
- Negative predictive value (NPV): TN/(TN + FP)
- -
- Positive likelihood ratio (LR+): [TP/(TP + FN)/FP/(FP + TN)]
- -
- Negative likelihood ratio (LR−): [FN/(TP + FP)/[TN/(TN + FP)]
3. Results
3.1. Data Description
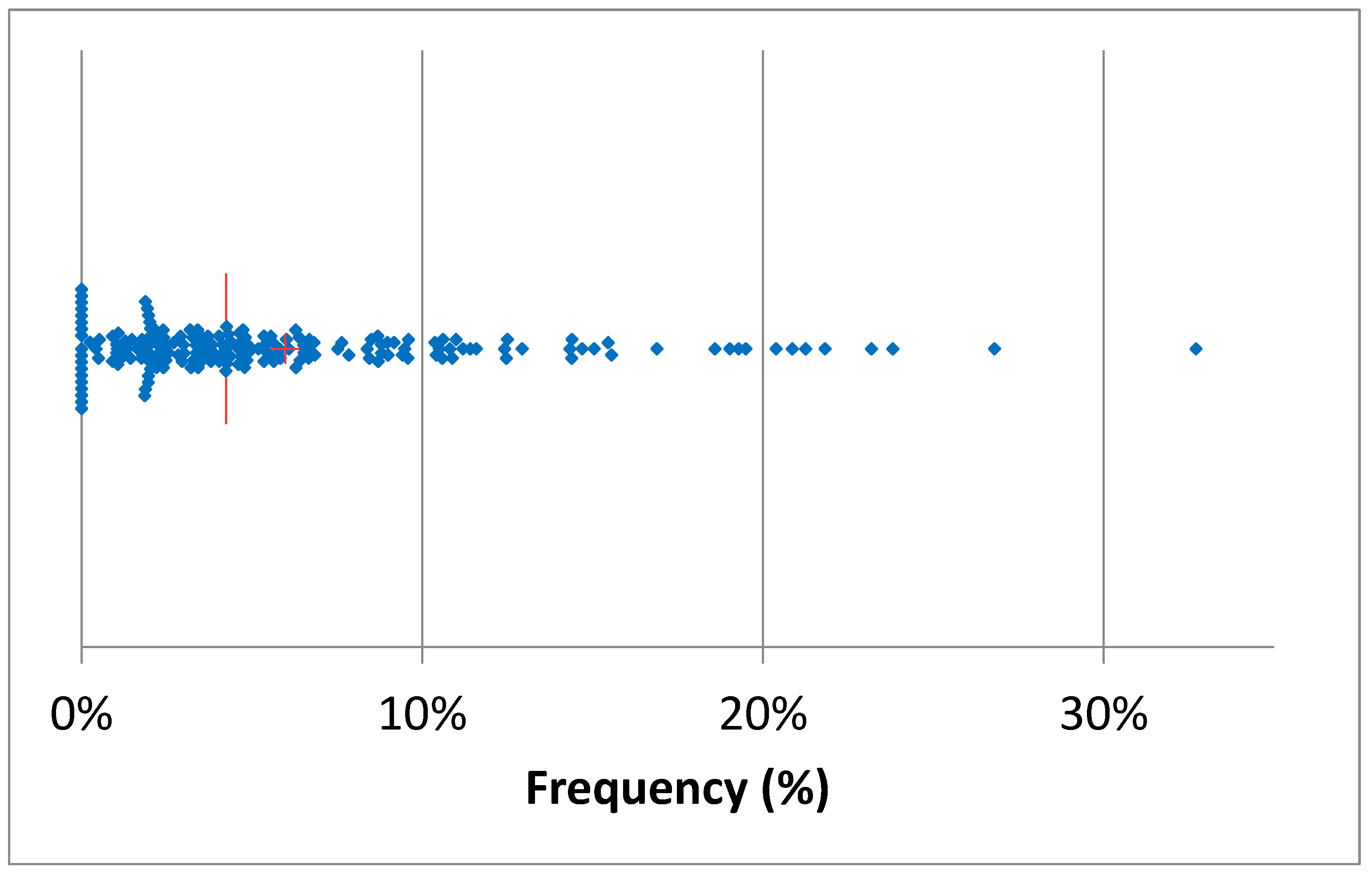
3.2. Antimicrobial Treatments
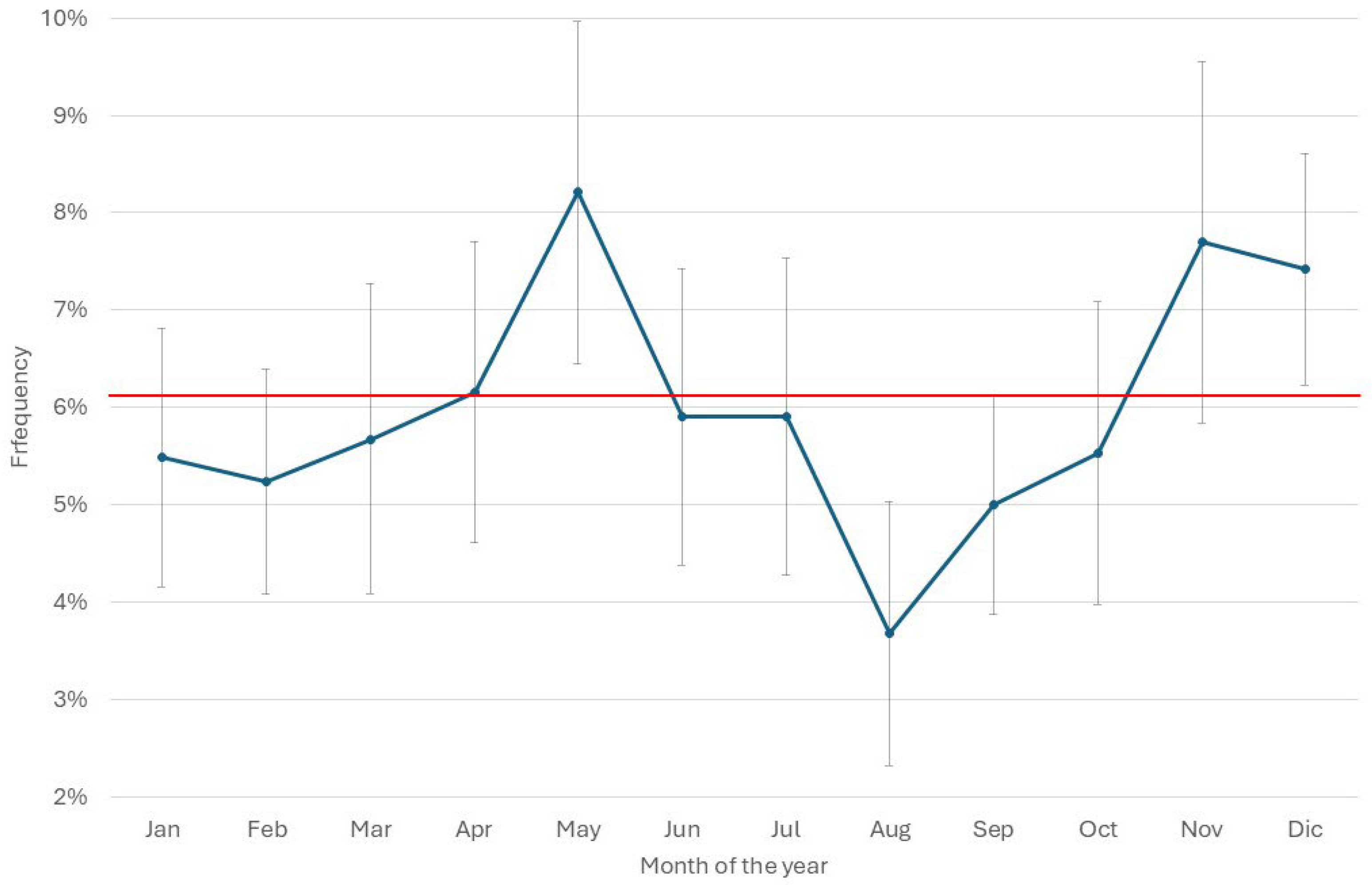
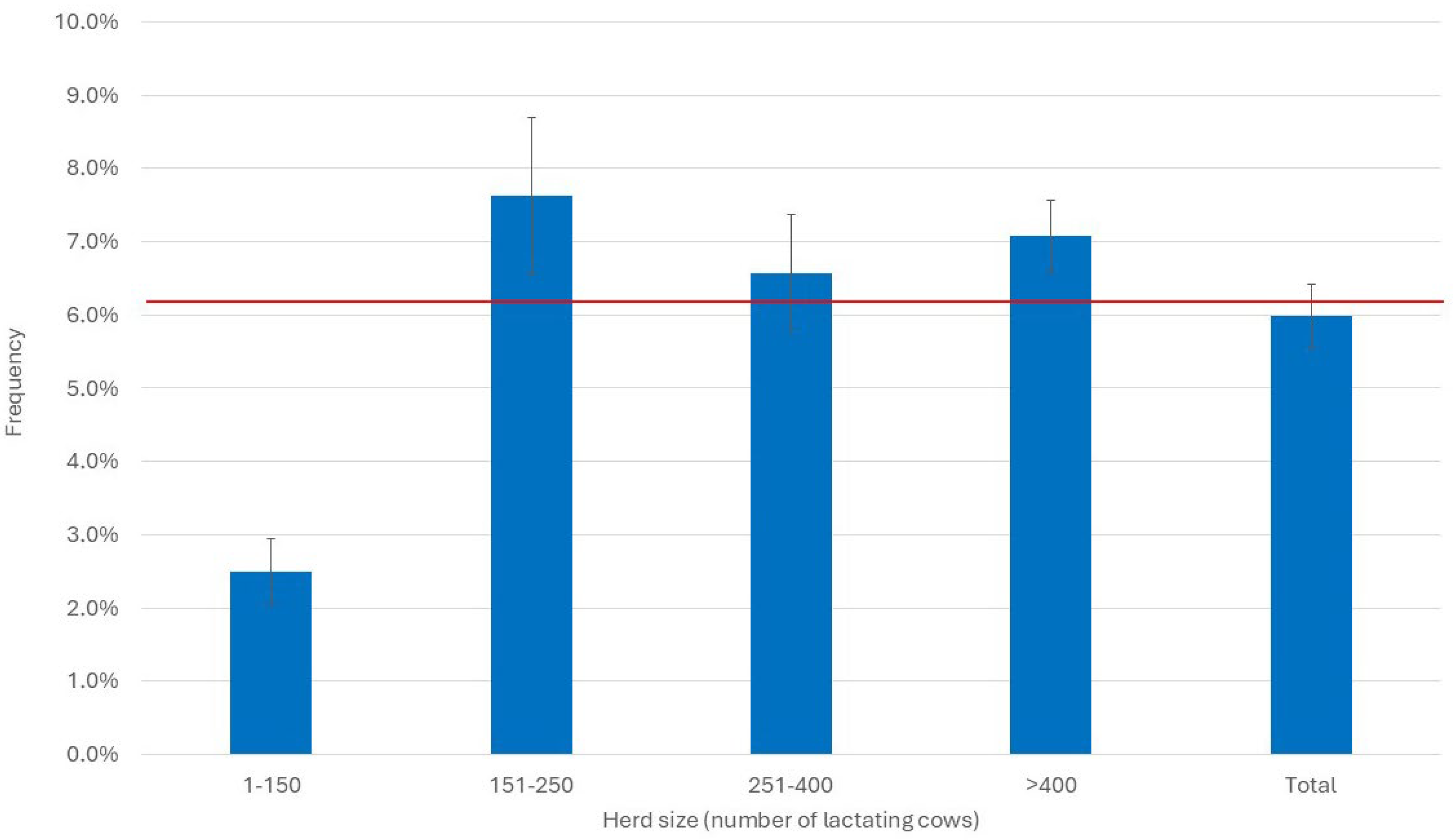
3.3. Herd Immune Status
3.4. Correlation Between Immune Status and Frequency of Treatments
4. Discussion
4.1. How to Measure Herd Immune Status
4.2. Antimicrobial Treatments
4.3. Correlation Between Herd Immune Status and Frequency of Antimicrobial Treatments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMT | Antimicrobial treatment |
| AMT/CH | Antimicrobial treatment/cows in the herd |
| DHI | Dairy herd improvement |
| DSCC | Differential somatic cell count |
| MTR | Milk test record |
| PLCC | Polymorphonuclear neutrophil leukocyte cell count |
| PMN | Polymorphonuclear neutrophils |
| SCC | Somatic cell count |
References
- Davis, M.; Lohm, D.; Flowers, P.; Whittaker, A. The immune self, hygiene and performative virtue in general public narratives on antibiotics and antimicrobial resistance. Health 2023, 27, 491–507. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Di Martino, G.; Moscati, L.; Buniolo, F.; Cibin, V.; Bonfanti, L. Natural immunity in conventionally and organically reared turkeys and its relation with antimicrobial resistance. Poult. Sci. 2020, 99, 763–771. [Google Scholar] [CrossRef]
- Gani, Z.; Kumar, A.; Raje, M.; Raje, C. Antimicrobial peptides: An alternative strategy to combat antimicrobial resistance. Drug Discov. Today 2025, 30, 14. [Google Scholar] [CrossRef]
- Shapira, T.; Christofferson, M.; Av-Gay, Y. The antimicrobial activity of innate host-directed therapies: A systematic review. Int. J. Antimicrob. Agents 2024, 63, 17. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Riollet, C. Innate immunity of the bovine mammary gland. Vet. Res. 2006, 37, 369–400. [Google Scholar] [CrossRef]
- Alluwaimi, A.M. The cytokines of bovine mammary gland: Prospects for diagnosis and therapy. Res. Vet. Sci. 2004, 77, 211–222. [Google Scholar] [CrossRef]
- Trevisi, E.; Zecconi, A.; Bertoni, G.; Piccinini, R. Blood and milk immune and inflammatory profiles in periparturient dairy cows showing a different liver activity index. J. Dairy Res. 2010, 77, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Smith, K.L. Ruminant Mammary Gland Immunity; FIL-IDF: Bruxelles, Belgium, 2003; p. 128. [Google Scholar]
- Abbas, A.K.; Lichtman, A.K.; Pillai, S. Cellular and Molecular Immunology, 6th ed.; Elsevier: Philadelphia, PA, USA, 2007. [Google Scholar]
- Vlasova, A.; Saif, L. Bovine Immunology: Implications for Dairy Cattle. Front. Immunol. 2021, 12, 18. [Google Scholar] [CrossRef]
- Merle, R.; Schroder, A.; Hamann, J. Cell function in the bovine mammary gland: A preliminary study on interdependence of healthy and infected udder quarters. J. Dairy Res. 2007, 74, 174–179. [Google Scholar] [CrossRef]
- Rivas, A.L.; Quimby, F.W.; Blue, J.; Coksaygan, O. Longitudinal evaluation of bovine mammary gland health status by somatic cell counting, flow cytometry, and cytology. J. Vet. Diagn. Investig. 2001, 13, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.L.; Quimby, F.W.; Coksaygan, O.; Olmstead, L.; Lein, D.H. Longitudinal evaluation of CD4+and CD8+peripheral blood and mammary gland lymphocytes in cows experimentally inoculated with Staphylococcus aureus. Can. J. Vet. Res. Rev. Can. Rech. Vet. 2000, 64, 232–237. [Google Scholar]
- Wall, S.K.; Wellnitz, O.; Bruckmaier, R.M.; Schwarz, D. Differential somatic cell count in milk before, during, and after lipopolysaccharide- and lipoteichoic-acid-induced mastitis in dairy cows. J. Dairy Sci. 2018, 101, 5362–5373. [Google Scholar] [CrossRef] [PubMed]
- Harmon, R.J. Physiology of mastitis and factors affecting somatic cell counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Damm, M.; Holm, C.; Blaabjerg, M.; Bro, M.N.; Schwarz, D. Differential somatic cell count-A novel method for routine mastitis screening in the frame of Dairy Herd Improvement testing programs. J. Dairy Sci. 2017, 100, 4926–4940. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Zaghen, F.; Meroni, G.; Sommariva, F.; Ferrari, S.; Sora, V. Machine Learning Approach for Early Lactation Mastitis Diagnosis Using Total and Differential Somatic Cell Counts. Animals 2025, 15, 1125. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Zaghen, F.; Meroni, G.; Sora, V.; Martino, P.A.; Laterza, G.; Zanini, L. Early Milk Total and Differential Cell Counts as a Diagnostic Tool to Improve Antimicrobial Therapy Protocols. Animals 2023, 13, 1143. [Google Scholar] [CrossRef]
- Stocco, G.; Cipolat-Gotet, C.; Stefanon, B.; Zecconi, A.; Francescutti, M.; Mountricha, M.; Summer, A. Herd and animal factors affect the variability of total and differential somatic cell count in bovine milk. J. Anim. Sci. 2023, 101, skac406. [Google Scholar] [CrossRef]
- Zecconi, A.; Zanini, L.; Cipolla, M.; Stefanon, B. Factors Affecting the Patterns of Total Amount and Proportions of Leukocytes in Bovine Milk. Animals 2020, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Soto, E.; Maltecca, C.; Anderson, K.; Tiezzi, F. Analysis of milk leukocyte differential measures for use in management practices for decreased mastitis incidence. J. Dairy Sci. 2020, 103, 572–582. [Google Scholar] [CrossRef]
- Kirkeby, C.; Toft, N.; Schwarz, D.; Farre, M.; Nielsen, S.S.; Zervens, L.; Hechinger, S.; Halasa, T. Differential somatic cell count as an additional indicator for intramammary infections in dairy cows. J. Dairy Sci. 2020, 103, 1759–1775. [Google Scholar] [CrossRef]
- Zecconi, A.; Vairani, D.; Cipolla, M.; Rizzi, N.; Zanini, L. Assessment of Subclinical Mastitis Diagnostic Accuracy by Differential Cell Count in Individual Cow Milk. Ital. J. Anim. Sci. 2018, 18, 435–440. [Google Scholar] [CrossRef]
- Schwarz, D.; Lipkens, Z.; Piepers, S.; De Vliegher, S. Investigation of differential somatic cell count as a potential new supplementary indicator to somatic cell count for identification of intramammary infection in dairy cows at the end of the lactation period. Prev. Vet. Med. 2019, 172, 7. [Google Scholar] [CrossRef] [PubMed]
- Mehrzad, J.; Duchateau, L.; Burvenich, C. Viability of milk neutrophils and severity of bovine coliform mastitis. J. Dairy Sci. 2004, 87, 4150–4162. [Google Scholar] [CrossRef]
- Burton, J.L.; Erskine, R.J. Immunity and mastitis: Some new ideas for an old disease. (Mastitis). Vet. Clin. N. Am. Food Anim. Pract. 2003, 19, 1–45. [Google Scholar] [CrossRef]
- Zecconi, A.; Bronzo, V.; Piccinini, R.; Spreafico, G.; Ruffo, G. Phagocytic activity of bovine polymorphonuclear neutrophil leucocytes. J. Dairy Res. 1994, 61, 271–279. [Google Scholar] [CrossRef]
- Mehrzad, J.; Paape, M.; Burvenich, C. Role of neutrophils in protection of udder from infection in high yielding dairy cows. Iran. J. Vet. Res. 2010, 11, 102–118. [Google Scholar]
- Adewuyi, A.A.; Gruys, E.; van Eerdenburg, F. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet. Q. 2005, 27, 117–126. [Google Scholar] [CrossRef]
- Chapinal, N.; Carson, M.; Duffield, T.F.; Capel, M.; Godden, S.; Overton, M.; Santos, J.E.P.; LeBlanc, S.J. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 2011, 94, 4897–4903. [Google Scholar] [CrossRef]
- Goff, J.P. Major advances in our understanding of nutritional influences on bovine health. J. Dairy Sci. 2006, 89, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, L.; Scali, F.; Formenti, N.; Alborali, G.L.; Aragrande, M.; Canali, M.; Romanelli, C.; Suprani, V.; De Meneghi, D. Evaluation of ‘ClassyFarm’, the Italian integrated surveillance system of livestock farms, in the context of antimicrobial use and antimicrobial resistance. Ital. J. Anim. Sci. 2024, 23, 1426–1438. [Google Scholar] [CrossRef]
- Sharkey, L.C.; Heinrich, D.A. Chapter 25—Alterations in the Leukogram. In Large Animal Internal Medicine, 6th ed.; Smith, B.P., Van Metre, D.C., Pusterla, N., Eds.; Mosby: St. Louis, MO, USA, 2020; pp. 429–434.e421. [Google Scholar]
- Frater, J.L. How I investigate neutropenia. Int. J. Lab. Hematol. 2020, 42, 121–132. [Google Scholar] [CrossRef]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34, S93–S106. [Google Scholar] [CrossRef]
- Hudson, J.A.; Frewer, L.J.; Jones, G.; Brereton, P.A.; Whittingham, M.J.; Stewart, G. The agri-food chain and antimicrobial resistance: A review. Trends Food Sci. Technol. 2017, 69, 131–147. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- Halasa, T.; Kirkeby, C. Differential Somatic Cell Count: Value for Udder Health Management. Front. Vet. Sci. 2020, 7, 7. [Google Scholar] [CrossRef]
- Stocco, G.; Summer, A.; Cipolat-Gotet, C.; Zanini, L.; Vairani, D.; Dadousis, D.; Zecconi, A. Differential cell count as a novel indicator of milk quality in dairy cows. Animals 2020, 10, 753. [Google Scholar] [CrossRef]
- Fusi, V. Correlazione Tra Uso Del Farmaco E Tasso Di Riforma Negli Allevamenti Di Bovine Da Latte Del Lodigiano; Università Degli Studi di Milano: Milan, Italy, 2025. [Google Scholar]
- Dahl, G.E.; McFadden, T.B. Symposium review: Environmental effects on mammary immunity and health. J. Dairy Sci. 2022, 105, 8586–8589. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, A.; Joy, A.; Dunshea, F.R.; Chauhan, S.S. The Impact of Heat Stress on Immune Status of Dairy Cattle and Strategies to Ameliorate the Negative Effects. Animals 2023, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- George, J.W.; Snipes, J.; Lane, V.M. Comparison of bovine hematology reference intervals from 1957 to 2006. Vet. Clin. Pathol. 2010, 39, 138–148. [Google Scholar] [CrossRef] [PubMed]
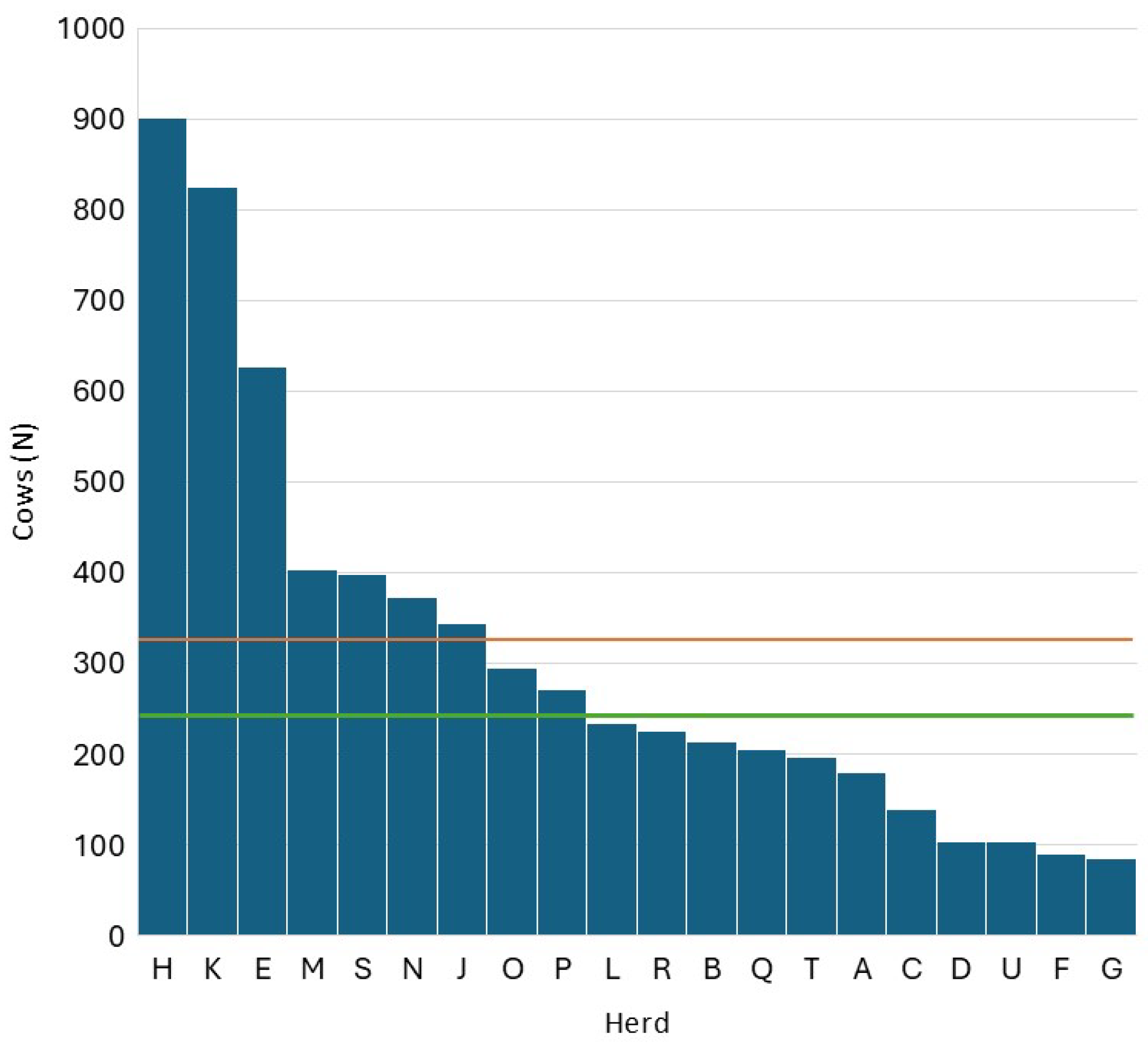
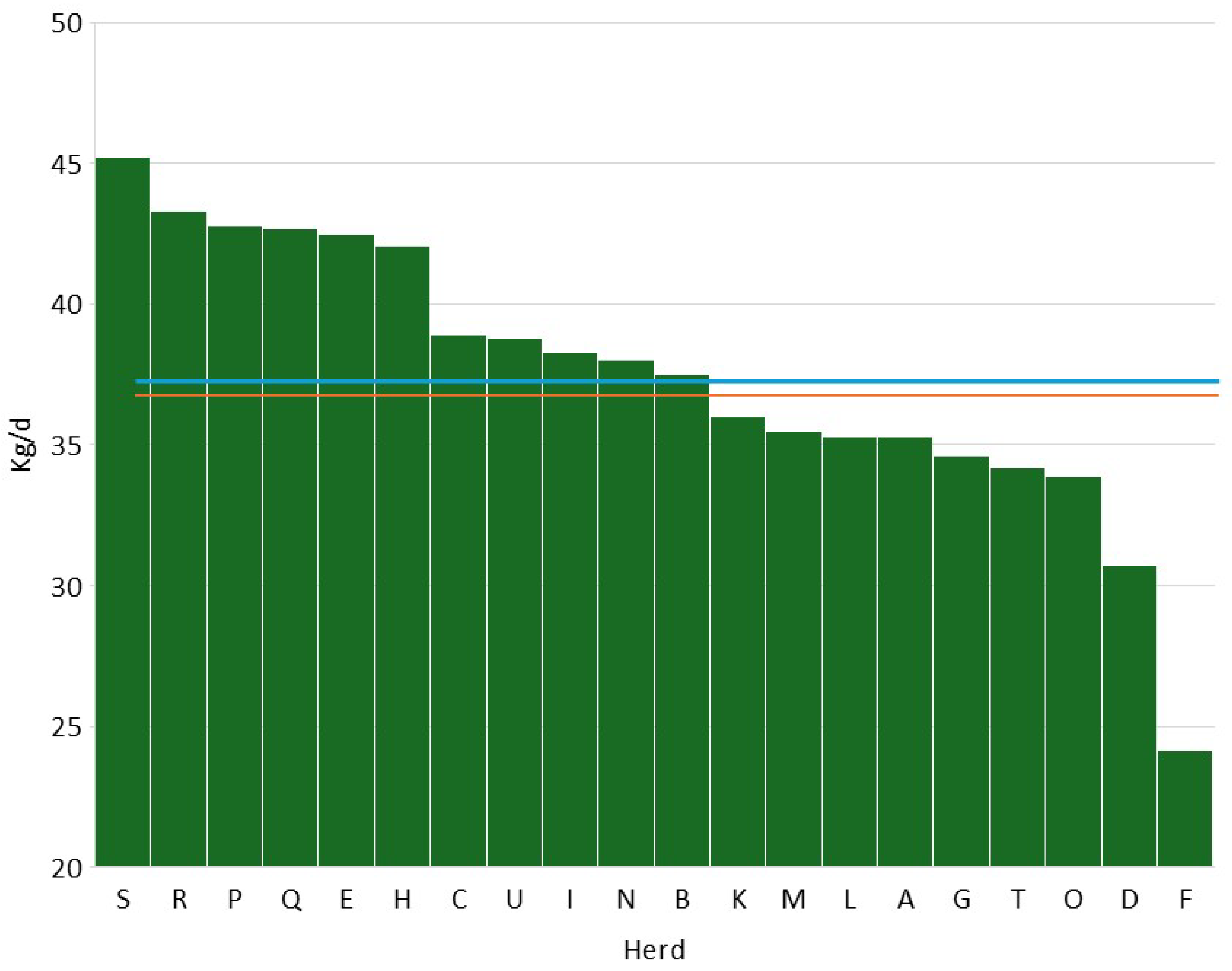
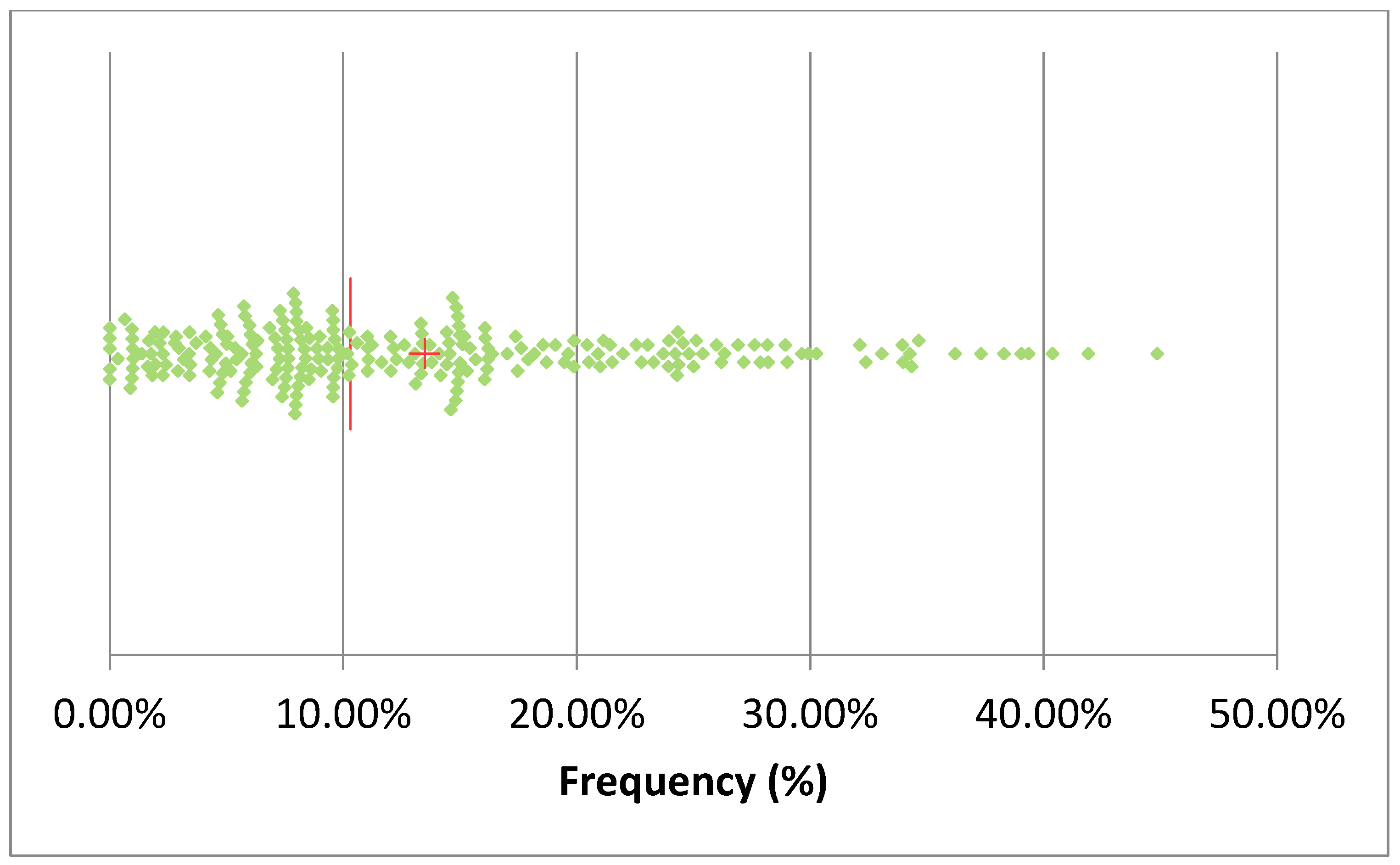

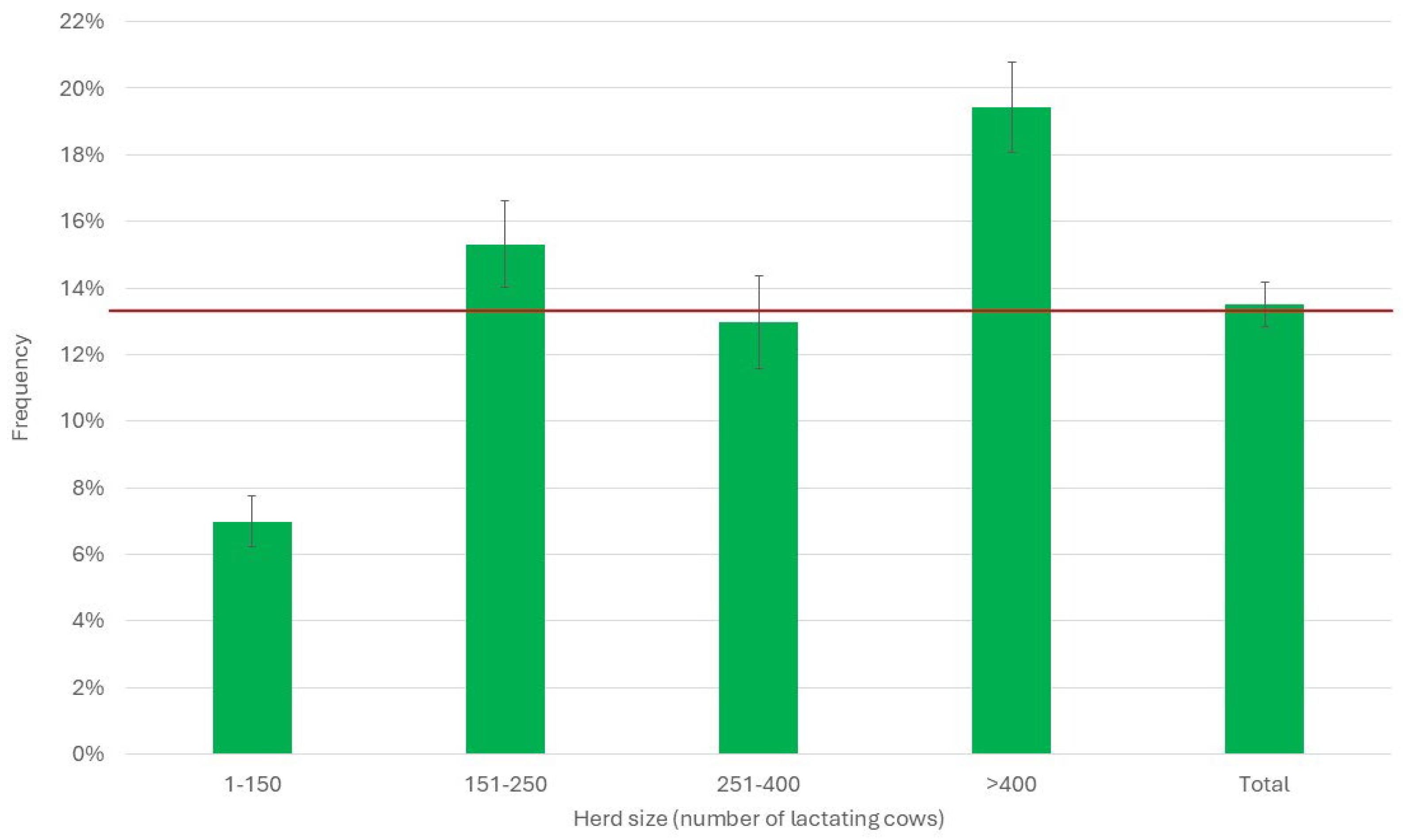

| Herd | SCC (Log10/mL) | DSCC (%) | PLCC (Log10/mL) | |||
|---|---|---|---|---|---|---|
| Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | |
| A | 5.07 | 0.61 | 61.50 | 17.42 | 4.83 | 0.71 |
| B | 4.87 | 0.63 | 60.55 | 17.53 | 4.63 | 0.73 |
| C | 4.68 | 0.56 | 57.30 | 18.55 | 4.43 | 0.68 |
| D | 4.99 | 0.62 | 59.92 | 17.54 | 4.74 | 0.72 |
| E | 4.85 | 0.63 | 61.61 | 17.97 | 4.62 | 0.73 |
| F | 5.13 | 0.63 | 64.59 | 17.32 | 4.92 | 0.74 |
| G | 5.06 | 0.59 | 66.42 | 15.43 | 4.85 | 0.68 |
| H | 4.95 | 0.63 | 63.44 | 18.08 | 4.73 | 0.74 |
| K | 4.72 | 0.58 | 61.07 | 17.54 | 4.48 | 0.68 |
| J | 5.02 | 0.67 | 65.18 | 18.64 | 4.81 | 0.77 |
| L | 5.04 | 0.67 | 66.44 | 17.45 | 4.85 | 0.76 |
| M | 5.04 | 0.69 | 66.09 | 17.12 | 4.84 | 0.80 |
| N | 4.84 | 0.62 | 63.15 | 17.53 | 4.62 | 0.72 |
| O | 5.00 | 0.62 | 64.93 | 16.23 | 4.80 | 0.72 |
| P | 4.74 | 0.63 | 58.55 | 19.44 | 4.48 | 0.75 |
| Q | 4.52 | 0.55 | 52.89 | 18.88 | 4.22 | 0.66 |
| R | 4.70 | 0.57 | 55.94 | 18.42 | 4.43 | 0.67 |
| S | 4.74 | 0.55 | 56.15 | 18.08 | 4.46 | 0.65 |
| T | 5.13 | 0.63 | 72.17 | 14.77 | 4.98 | 0.70 |
| U | 4.97 | 0.60 | 63.73 | 16.25 | 4.76 | 0.69 |
| Total | 4.88 | 0.64 | 61.84 | 18.10 | 4.64 | 0.74 |
| Herd | Total Antimicrobial Treatments (N) | Mastitis Treatments Proportion |
|---|---|---|
| A | 531 | 27% |
| B | 308 | 56% |
| C | 242 | 58% |
| D | 86 | 79% |
| E | 1865 | 13% |
| F | 41 | 37% |
| G | 81 | 68% |
| H | 1829 | 40% |
| K | 2695 | 4% |
| J | 339 | 74% |
| L | 152 | 84% |
| M | 618 | 73% |
| N | 417 | 65% |
| O | 111 | 70% |
| P | 1015 | 54% |
| Q | 642 | 23% |
| R | 346 | 96% |
| S | 444 | 46% |
| T | 235 | 46% |
| U | 17 | 29% |
| Total | 12,014 | 35% |
| PLCC Status | SCC | DSCC | Proportion of Samples | ||
|---|---|---|---|---|---|
| Mean | Std. Dev. | Mean | Std. Dev. | ||
| Below 5000 cells/mL | 4.0 | 0.16 | 35.4 | 11.3 | 6.5% |
| Over 5000 cells/mL | 4.9 | 0.61 | 63.8 | 16.9 | 93.5% |
| Model | Coefficient | p | 95.0% Confidence Interval | ||
|---|---|---|---|---|---|
| B | Std. Err | Lower | Higher | ||
| Constant | 0.089 | 0.010 | <0.001 | 0.068 | 0.109 |
| PLCC < 5000/mL freq. | 0.807 | 0.122 | <0.001 | 0.566 | 1.048 |
| Calculated PLCC Threshold | 2.0% | 4.4% |
|---|---|---|
| Parameter | AMT/CH > 6% | AMT/CH > 10% |
| Sensitivity | 85.0% | 64.9% |
| Lower bound (95%) | 78.0% | 54.8% |
| Upper bound (95%) | 90.0% | 73.8% |
| Specificity | 56.8% | 68.9% |
| Lower bound (95%) | 42.2% | 58.7% |
| Upper bound (95%) | 70.3% | 77.5% |
| Positive predictive value | 86.2% | 68.5% |
| Negative predictive value | 54.3% | 65.3% |
| Positive likelihood ratio | 1.97 | 2.09 |
| Negative likelihood ratio | 0.26 | 0.51 |
| Accuracy | 78.3% | 66.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zecconi, A.; Sora, V.; Invernizzi, E.; Zaghen, F.; Chierici Guido, V. Low Neutrophil Counts in Milk Are Associated with an Increased Frequency of Antimicrobial Treatments. Pathogens 2025, 14, 1104. https://doi.org/10.3390/pathogens14111104
Zecconi A, Sora V, Invernizzi E, Zaghen F, Chierici Guido V. Low Neutrophil Counts in Milk Are Associated with an Increased Frequency of Antimicrobial Treatments. Pathogens. 2025; 14(11):1104. https://doi.org/10.3390/pathogens14111104
Chicago/Turabian StyleZecconi, Alfonso, Valerio Sora, Emanuele Invernizzi, Francesca Zaghen, and Viviana Chierici Guido. 2025. "Low Neutrophil Counts in Milk Are Associated with an Increased Frequency of Antimicrobial Treatments" Pathogens 14, no. 11: 1104. https://doi.org/10.3390/pathogens14111104
APA StyleZecconi, A., Sora, V., Invernizzi, E., Zaghen, F., & Chierici Guido, V. (2025). Low Neutrophil Counts in Milk Are Associated with an Increased Frequency of Antimicrobial Treatments. Pathogens, 14(11), 1104. https://doi.org/10.3390/pathogens14111104







