Abstract
Toxoplasma gondii (T. gondii) infection during pregnancy can cause severe placental damage and fetal impairment. Although triggering the receptor expressed on myeloid cells 2 (Trem2) confers protection against T. gondii infection, the precise molecular mechanisms underlying this immunoregulatory role remain incompletely understood. Using a mouse model, this study identifies a novel Trem2-MICAL1-P-ERK axis in macrophages that protects against T. gondii-induced adverse pregnancy outcomes (APO). RNA-seq of Trem2-overexpressing macrophages revealed significant upregulation of 1857 genes, with MICAL1 among the most markedly altered, highlighting its potential role in Trem2-mediated signaling. Mechanistically, correlation analysis, molecular docking, fluorescence co-localization, and immunoprecipitation assays demonstrate that Trem2 directly interacts with MICAL1, which modulates downstream phosphorylated ERK (P-ERK) signaling. In a T. gondii-infected murine pregnancy model, genetic ablation of Trem2 exacerbated pathogen-induced suppression of MICAL1 and P-ERK, whereas macrophage-specific overexpression of Trem2-DAP12 restored this signaling axis. Conversely, MICAL1 overexpression rescued P-ERK activation but failed to regulate Trem2 expression. Further studies in bone marrow-derived macrophages (BMDMs) revealed that Trem2 deficiency potentiated the inhibitory effects of soluble T. gondii antigens (TgAg) on MICAL1 and P-ERK. These findings elucidate how T. gondii disrupts placental immunity through targeted suppression of Trem2-mediated signaling and establish the Trem2-MICAL1-P-ERK cascade as a core regulatory pathway in immune homeostasis during pregnancy.
1. Introduction
T. gondii infection is a globally distributed zoonotic disease [1]. When contracted during pregnancy, T. gondii can cross the placental barrier, causing congenital toxoplasmosis, manifesting as fetal malformations, intellectual disabilities, and various pregnancy complications [2]. Studies demonstrate that T. gondii induces APO mainly by disrupting the homeostasis of the maternal–fetal immune microenvironment [3,4]. The maternal–fetal interface is a highly dynamic and multicellular collaborative system that serves as the critical region for immune interactions between mother and fetus during pregnancy [5,6]. Composed of maternal decidual immune cells and placental trophoblasts, it regulates and maintains pregnancy immune tolerance [7]. T. gondii can manipulate the expression of inhibitory molecules (e.g., LILRB4, B7-H4, and Tim-3) on decidual immune cells, leading to immune dysfunction and APO [8,9,10]. Decidual immune cells primarily include decidual natural killer (dNK) cells, decidual macrophages (dMφ), and decidual dendritic cells [11]. As the second most abundant immune cell population at the maternal–fetal interface, dMφ play essential roles in maintaining immune homeostasis and clearing apoptotic cells—physiological processes crucial for healthy pregnancy [12]. In T. gondii-induced APO, dMφ dysfunction is primarily mediated through the regulation of key signaling pathways and effector molecules [13]. Research shows that T. gondii-secreted effector proteins (e.g., ROP16 and GRA15) interfere with normal dMφ polarization by activating the STAT3/STAT6 signaling pathway [13,14,15,16]. Additionally, RNA-Seq data confirm that T. gondii infection upregulates pro-inflammatory genes while reducing F-actin polymerization and planar cell polarity, thereby destabilizing the host cytoskeleton [17]. Given these findings, this study investigates the key effector molecules and signaling pathways in dMφ that mediate immune imbalance following T. gondii infection, aiming to elucidate the molecular mechanisms by which the pathogen disrupts maternal–fetal interface homeostasis.
Recent studies reveal that Trem2, specifically expressed in dMφ, plays a regulatory role in this process [18]. As a pivotal immune receptor in phagocytes, Trem2 directly mediates clearance of invasive pathogens by modulating reactive oxygen species (ROS) generation [19]. Its overexpression enhances chemokine receptor expression, promotes cellular migration, and augments phagocytic capacity, whereas endogenous Trem2 downregulation not only suppresses phagocytosis but also significantly increases transcription of pro-inflammatory cytokines (e.g., TNF-α and IL-1β) [20]. The execution of phagocytosis fundamentally relies on dynamic cytoskeletal reorganization [21]. MICAL1, an actin oxidase, mediates F-actin depolymerization, a critical step in cytoskeletal remodeling [22]. T. gondii surface antigen TgSAG1 has been shown to disrupt host S100A6–vimentin complexes, thereby inhibiting actin polymerization while simultaneously promoting TNF-α secretion via the S100A6–Vimentin/PKCθ-NF-κB signaling pathway [23,24]. Beyond its impact on cytoskeletal regulation, T. gondii also modulates host transcriptional programs: our recent work revealed that T. gondii suppresses Trem2 promoter activity via the transcription factor ATF3, a mechanism linked to APO [25]. However, the downstream pathways through which Trem2 exerts its protective effects remain to be fully elucidated. Notably, studies demonstrate that MICAL1 depletion suppresses P-ERK expression, while its overexpression enhances P-ERK nuclear translocation, highlighting its crucial role in ERK signaling activation [26].
To validate the protective role of the Trem2-MICAL1-P-ERK pathway, we established T. gondii-infected pregnancy models using both wild-type (WT) and Trem2−/− mice. Our results revealed that T. gondii infection significantly downregulates Trem2 expression and suppresses MICAL1-P-ERK signaling activity. Notably, Trem2 deficiency significantly exacerbated the suppression of MICAL1-P-ERK signaling activity upon T. gondii challenge. These findings collectively indicate that T. gondii disrupts placental immune defense by targeting the Trem2-MICAL1-P-ERK signaling axis. This study aims to elucidate the precise molecular mechanisms underlying this pathogenic process, potentially offering novel therapeutic strategies for T. gondii infection-induced pregnancy complications.
2. Materials and Methods
2.1. Mice
C57BL/6 mice were housed under standard conditions at Nantong University’s animal facility. Trem2-deficient mice (B6/JGpt-Trem2 em1Cd3332in1/Gpt knockout) were created through CRISPR/Cas9-mediated gene editing by Gempharmatech (Nanjing, China). The experimental mice were randomly assigned to either the WT or Trem2−/− group. Each group included 8 male and 16 female mice, all weighing between 25 and 30 g. To establish mating pairs, one male mouse was co-housed with two female mice per cage. An investigator, who was blinded to the experimental groups, performed all allocations using a random number table. Successful mating was confirmed by vaginal plug detection before 8:00 a.m., with plug-positive females designated as gestation day 0.5 (GD0.5) [27]. The pregnant WT or Trem2−/− mice were randomly allocated to either the normal pregnancy (NP) group or the T. gondii infection (TI) group, which received 300 tachyzoites (RH strain) via intraperitoneal injection at GD8.5. On GD17.5, CO2 euthanasia was performed, followed by placental collection for subsequent experiments. All pregnant mice were monitored continuously, and their survival was ensured throughout the study. Accordingly, no unexpected lethal outcomes were observed during the in vivo experiments. All mouse procedures received approval from the Animal Care and Use Committee of Nantong University (protocol #P20230302–013).
2.2. Preparation of T. gondii Antigens
The antigens were extracted from T. gondii tachyzoites (RH strain) [28]. Briefly, 1 × 108 viable tachyzoites (>95% viability by trypan blue) were incubated in serum-free RPMI-1640 medium for 3 h at 37 °C. The culture supernatant was collected and concentrated with Amicon® Ultra-15 centrifugal filters (Merck Millipore, Darmstadt, Germany). Endotoxins were subsequently removed by an endotoxin removal kit (Thermo Fisher Scientific, Waltham, MA, USA). Aliquots were stored at −80 °C until use.
2.3. Cell Culture
The murine macrophage cell line (Raw264.7 cell) was ordered from the National Collection of Authenticated Cell Cultures (Shanghai, China) and maintained in complete DMEM (Thermo) containing 10% heat-inactivated FBS (ExCell Bio, Suzhou, China) and 1% penicillin-streptomycin (Thermo). Raw264.7 cells were incubated at 37 °C in a 5% CO2 humidified atmosphere and subcultured when they reached 70–80% confluency. For experimental treatments, cells were stimulated with TgAg (5 µg/mL) for 48 h. For primary macrophage isolation, BMDMs were prepared from WT mice (6–8 weeks old) and Trem2−/− mice (6–8 weeks old). Briefly, femurs and tibias were aseptically dissected and flushed with cold PBS, then transferred to complete medium for immersion. Bone ends were opened with sterile scissors, and marrow was flushed using complete medium through a syringe until the bones appeared white. Cells were treated with 5 volumes of red blood cell lysing buffer, centrifuged, and resuspended in DMEM before filtering through a 200-μm mesh. Isolated bone marrow cells were cultured in complete medium containing murine M-CSF (20 ng/mL). After 6 days of differentiation, mature macrophages were harvested on day 8 for subsequent experiments.
2.4. RNA Quantification and Sequencing
Total RNA was extracted from murine macrophage Raw264.7 cells, using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA integrity and concentration were verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and agarose gel electrophoresis. RNA library preparation and sequencing of Raw264.7 cells were performed at Guangzhou GeneDenovo Biotechnology Co., Ltd. (Guangzhou, China). Briefly, poly(A) + mRNA was enriched from total RNA using Oligo (dT) magnetic beads. The purified mRNA was fragmented into short fragments (200–700 nucleotides), followed by first-and second-strand cDNA synthesis using random hexamer primers. The double-stranded cDNA was end-repaired, adenylated, and ligated with Illumina (San Diego, CA, USA) sequencing adapters. The adapter-ligated fragments were then purified and enriched by PCR amplification to generate the final sequencing library. Library quality was assessed using an Agilent High Sensitivity DNA Kit, and qualified libraries were subjected to paired-end sequencing (PE150) on an Illumina NovaSeq 6000 platform.
2.5. Immunoblotting
For protein analysis, cells or tissues were lysed in RIPA buffer containing protease inhibitors as previously described [29]. After quantifying protein concentration, equal amounts were separated by SDS-PAGE (8–12%) and transferred into PVDF membranes (Merck Millipore). PVDF membranes were blocked with 5% non-fat milk buffer for 1 h at room temperature (RT) and then incubated overnight at 4 °C with primary antibodies: Trem2 mouse antibody (R&D Systems, 1: 3000, #AF1729, Minneapolis, MN, USA); MICAL1 rabbit polyclonal antibody (Proteintech, 1: 3000, #14818–1-AP, Rosemont, IL, USA), ERK1/2 rabbit polyAb (Proteintech, 1: 3000, #11257–1-AP), P-ERK1/2 rabbit mAb (Cell Signaling Technology (CST), 1: 3000, #4370, Danvers, MA, USA), GAPDH mouse monoclonal antibody (CST, 1:50,000, 1E6D9, #60004–1-Ig). Following TBST washes, membranes were probed with HRP-conjugated goat anti-mouse IgG (H + L) (Proteintech, 1: 5000, #SA00001–1) or HRP-conjugated goat anti-rabbit IgG (H + L) (Biosharp, 1: 5000, #BL003A, Nantong, China) secondary antibodies for 1 h at RT. Protein band was visualized using ECL substrate (Meilunbio, Dalian, China) and analyzed with ImageJ software (version 4.0.1), with GAPDH serving as loading controls.
2.6. Immunofluorescence Staining
Cells on coverslips were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 5 min, and blocked with 5% BSA for 10–30 min. Primary antibodies were incubated for 1 h at RT: recombinant anti-Trem2 rabbit antibody (Abcam, 1:500, EPR26210–1, #ab305103, Cambridge, UK), MICAL1 rabbit Polyclonal antibody (Proteintech, 1:200, #14818–1-AP), ERK1/2 rabbit polyAb (Proteintech, 1: 10, #11257–1-AP), P-ERK1/2 rabbit mAb (CST, 1:200, #4370), followed by incubation with fluorophore-conjugated secondary antibodies for 10 min. Signal amplification solution was applied for 10 min, and nuclei were counterstained with DapI for 5 min (Absin, Shanghai, China). Coverslips were mounted and imaged using confocal microscopy.
2.7. Co-Immunoprecipitation
The cell lysates were prepared using RIPA buffer supplemented with PMSF. Following the manufacturer’s protocol, 2 μg of primary antibody was introduced to the supernatant and incubated at 4 °C for 2 h. Subsequently, Protein A/G Plus-Agarose (SC-2003, Santa Cruz Biotechnology, Dallas, TX, USA) was added, and the mixture was rotated overnight at 4 °C. After centrifugation, the immunoprecipitated complexes were collected and washed repeatedly with lysis buffer. Finally, both the precipitated samples and input controls were subjected to a Western blot for the detection of co-precipitated proteins.
2.8. Overexpression Constructs
The Trem2-DAP12 fusion was designed by linking the extracellular domain of mouse Trem2 (aa19–171) to mouse DAP12 (aa28–114). A D52A mutation was introduced in DAP12 to prevent binding with other receptors, enabling stable cell surface expression of the Trem2-DAP12 complex. The native signal peptide was replaced with the mouse immunoglobulin κ light chain leader sequence, and a FLAG tag was added at the N-terminus of Trem2 for detection [30]. The lentiviral vectors overexpressing Trem2/MICAL1 (pcSLenti-EF1-EGFP-F2A-Puro-CMV-Igκ leader-3xFLAG-Trem2 (19–171aa)-Tyrobp (28–114aa, G52A)-WPRE/pcSLenti-EF1-EGFP-P2A-Puro-CMV-MICAL1-3xFLAG-WPRE) and control empty vectors (pcSLenti-EF1-EGFP-F2A-Puro-CMV-MCS-WPRE/pcSLenti-EF1-EGFP-P2A-Puro-CMV-MCS-3xFLAG-WPRE) were packaged by OBiO Technology (Shanghai, China). Raw 264.7 cells at 30–40% confluency were infected with lentivirus at MOI = 40. After 12–16 h, the medium was replaced with fresh complete DMEM. Selection began at 72 h post-infection using 2 µg/mL puromycin (Beyotime, Shanghai, China) for 7 days. Overexpression efficiency was validated by Western blot.
2.9. Statistical Analysis
All data represent at least three independent experimental replicates and are presented as mean ± standard deviation (SD). Statistical comparisons were performed using GraphPad Prism 9.0 (La Jolla, CA, USA). For two-group comparisons, we applied an unpaired Student’s t-test. Multiple group analyses employed either one-way ANOVA followed by Tukey’s post hoc test (for single-variable experiments) or two-way ANOVA with Sidak’s multiple comparisons test (for factorial designs). In all analyses, probability values (p) below 0.05 were considered statistically significant.
3. Results
3.1. T. gondii-Induced Downregulation of Trem2 Expression
As Trem2 regulates key immunological processes at the maternal–fetal interface and modulates phagocyte activity [31], we investigated its expression changes during T. gondii infection. Pregnant mice, intraperitoneally injected with T. gondii tachyzoites at GD8.5, displayed a significant downregulation of Trem2 in placental tissues collected at GD17.5 compared to non-infected controls, as evidenced by Western blot analysis (Figure 1A). Trem2 expression was primarily observed in macrophages [20]. Therefore, in our in vitro experiments, we employed Raw264.7 cells (the murine monocyte macrophage leukemia cell line) to observe the role of TgAg on Trem2 expression. We found that the treatment of Raw264.7 cells with 5 µg/mL TgAg substantially decreased Trem2 level relative to untreated cells (CON) (Figure 1B), which was consistently observed in in vivo experiments. Immunofluorescence analysis confirmed these findings, demonstrating markedly reduced Trem2 signal intensity (green) in TgAg-exposed cells versus controls, with DapI (blue) counterstaining identifying nuclear localization (Figure 1C,D). These data collectively establish that T. gondii mediates Trem2 suppression in both placental tissues and macrophages.
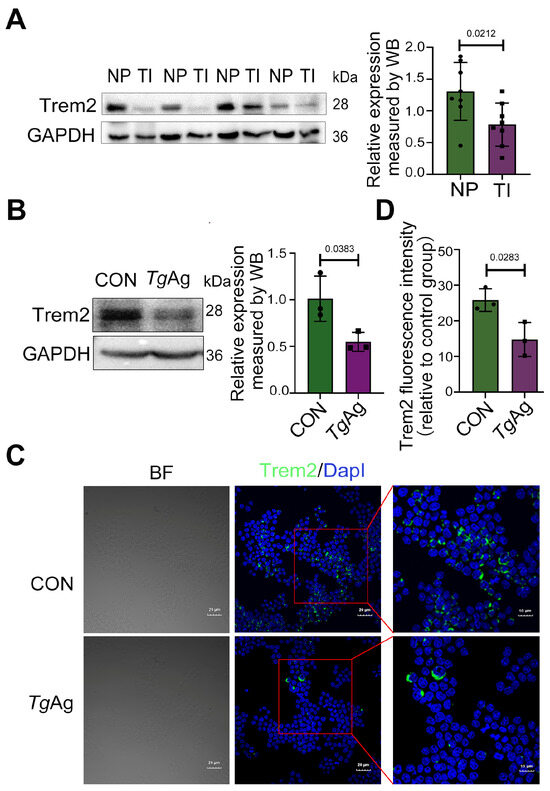
Figure 1.
T. gondii-induced downregulation of Trem2 expression. (A) Placental tissues were collected from WT mice with NP or TI at GD 17.5. Protein levels were analyzed by WB (n = 8 mice per group). (B) WB analysis of Raw264.7 cells after 48 h exposure to TgAg (5 μg/mL). (C) Immunofluorescence staining of Raw264.7 cells showing Trem2 (green) and DapI (blue) following 48 h treatment with TgAg (5 μg/mL). (D) Quantitative analysis of Trem2 fluorescence intensity from panel C. Scale bars, 20 µm. NP: Normal pregnancy; TI: T. gondii infection; CON: Untreated control group; TgAg: Soluble T. gondii antigens; n = 3 per group; A two-tailed unpaired Student’s t-test was used (A,B,D). Western blot bands were quantified using ImageJ, normalized to GAPDH.
3.2. T. gondii Disrupts Trem2-MICAL1 Molecular Interplay in Macrophages
Existing research has established that T. gondii infection induces macrophage dysfunction and compromises trophoblast function as well as placental development, whereas Trem2 upregulation can counteract these detrimental effects [18]. To elucidate the molecular mechanisms underlying Trem2-mediated protection, we aimed to identify potential Trem2-interacting molecules in macrophages involved in the host response to T. gondii infection.
Through RNA-seq analysis of Trem2-overexpressing Raw264.7 macrophages, we identified a total of 1857 significantly up-regulated differentially expressed genes (screening criteria: |log2 (fold-change) (FC)| > 1.5, p Value < 0.05) (Figure 2A). Among these, MICAL1 was ranked among the top 50 up-regulated genes. To further elucidate the functional implications of Trem2 overexpression, Gene Ontology (GO) enrichment analysis revealed that the upregulated genes were significantly associated with autophagy-related processes (Figure 2B). Previous studies demonstrated that Trem2 expression can be upregulated through downregulation of ANGPTL2, leading to effective enhancement of autophagy [32,33]. Correlation analysis of differentially expressed genes further highlighted MICAL1 as one of the most strongly positively correlated genes with the hub gene Trem2 (Pearson R ≥ 0.9) (Figure 2C). Given that MICAL1 is a known regulator of cytoskeletal dynamics, and its knockdown has been shown to suppress autophagy (reducing Beclin1 and LC3B) and promote apoptosis (increasing Bax and C-caspase 3), we propose that Trem2 may enhance macrophage phagocytosis and autophagy by upregulating MICAL1 to facilitate cytoskeletal remodeling [34,35,36].
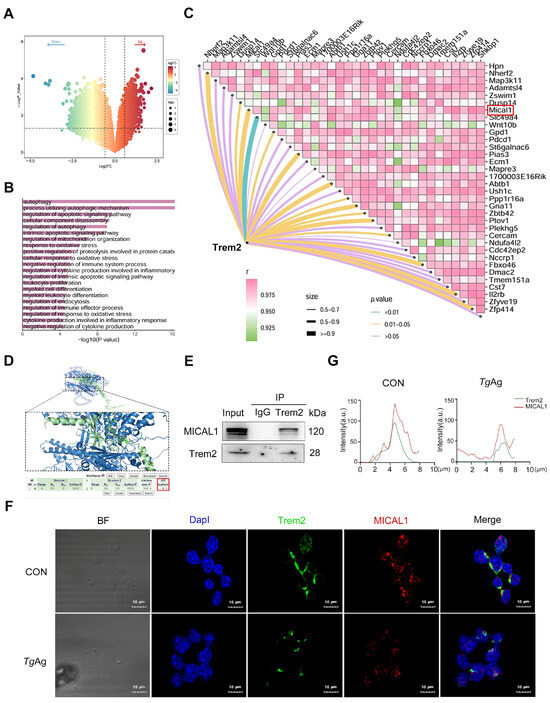
Figure 2.
T. gondii disrupts Trem2-MICAL1 molecular interplay in macrophages. (A) Volcano plots of differentially expressed genes, screening criteria: |log2 (fold-change) (FC)| > 1.5, p Value < 0.05, Right-side genes are upregulated in Trem2-overexpressing macrophages, while left-side genes are downregulated. (B) GO biological process enrichment analysis of differentially expressed genes in Trem2-overexpressing macrophages. (C) Correlation matrix analysis between the hub gene Trem2 and various differentially expressed genes. (D) Protein-protein docking was performed using PDBePISA, which predicted a high-affinity binding between Trem2 and MICAL1 with a binding energy of −36.2 kcal/mol. Structural visualization of the Trem2-MICAL1 complex was generated using Pymol. (E) Cell lysates were immunoprecipitated with anti-Trem2 or control IgG antibody, followed by immunoblotting with antibodies against MICAL1 and Trem2. (F) Immunofluorescence analysis demonstrating double co-localization of Trem2 (green) and MICAL1 (red) in Raw264.7 cells. Compared to the CON, TgAg-stimulated (5 μg/mL, 48 h) groups exhibit reduced co-localization. Nuclei were counterstained with DapI (blue). (G) Fluorescence intensity and co-localization analysis of Trem2 and MICAL1 in TgAg-stimulated Raw264.7 macrophages were performed using ImageJ. Data are presented in arbitrary units (a.u.). CON: Untreated control group; TgAg: Soluble T. gondii antigens.
Molecular docking analysis using PDBePISA and Pymol predicted that Trem2 and MICAL1 may potentially bind to each other (Figure 2D). The docking results revealed a strong binding affinity with a binding energy of −36.2 kcal/mol. To experimentally validate this interaction, co-immunoprecipitation (co-IP) assays were conducted. The results showed that Trem2 specifically immunoprecipitated with MICAL1 in macrophages, confirming their direct molecular association (Figure 2E). The immunofluorescence co-localization experiments confirmed significant basal co-localization of Trem2 and MICAL1 in unstimulated macrophages. Following stimulation with TgAg, we observed significant downregulation in the expression levels of both Trem2 and MICAL1, along with a marked reduction in their co-localization, indicating disruption of their interaction (Figure 2F,G). These findings collectively suggest that the disruption of the Trem2-MICAL1 interaction may represent a key molecular mechanism by which T. gondii impairs macrophage phagocytic function and immune evasion.
3.3. T. gondii Suppresses Both MICAL1 and P-ERK Expression
Extending Trem2’s established functions in phagocyte regulation and signal transduction, we provide the first evidence whereby T. gondii coordinately downregulates the expression of the Trem2, MICAL1, and P-ERK axis in mouse placentas (Figure 3A). This suggests that the suppression of P-ERK may be a consequence of MICAL1 downregulation, consistent with the known functional link between MICAL1 and ERK activation [26]. This suppression was recapitulated in vitro, where TgAg-stimulated Raw264.7 macrophages exhibited reduced protein levels of Trem2, MICAL1, and P-ERK (Figure 3B). Similarly, immunofluorescence analysis further revealed that TgAg stimulation markedly decreased the fluorescence intensity of MICAL1 and P-ERK in macrophages (Figure 3C), while total ERK levels remained unchanged. To establish that Trem2 is essential for MICAL1 and P-ERK signal transduction during T. gondii challenge, we performed immunoblotting to quantify MICAL1, P-ERK, and ERK levels in Trem2−/− mice. Quantitative comparisons revealed comparable MICAL1 and P-ERK expression between uninfected Trem2-deficient and WT placental tissues. However, infected Trem2 knockout mice showed substantially lower MICAL1 and P-ERK levels in placental tissue relative to infected WT controls (Figure 3D). We hypothesize that compensatory upregulation of other receptors or signaling pathways may maintain P-ERK and MICAL1 expression under basal conditions. Nevertheless, the enhanced suppression of MICAL1 and P-ERK upon the infection in Trem2−/− mice indicates that Trem2 likely plays a critical protective role during the infection, alleviating the parasite’s ability to over-suppress MICAL1/P-ERK signaling.
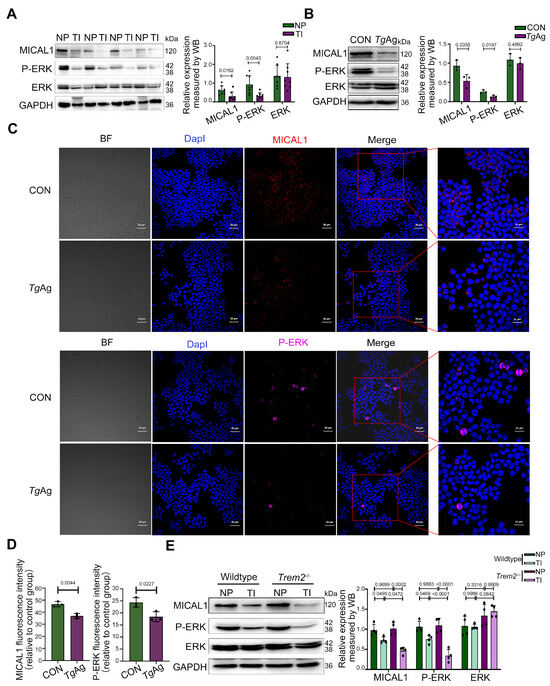
Figure 3.
T. gondii suppresses both MICAL1 and P-ERK expression. (A) WB analysis of MICAL1, P-ERK, and total ERK expression in placental tissues from NP and TI groups in wildtype mice at GD17.5 (n = 8 mice per group). (B) WB analysis of MICAL1, P-ERK, and total ERK in cells stimulated with or without TgAg (5 μg/mL, 48 h). n = 3 per group; (C) Immunofluorescence staining showing MICAL1 (red) and P-ERK (magenta) localization in Raw264.7 cells under CON and TgAg (5 μg/mL, 48 h)-stimulated conditions. Nuclei were counterstained with DapI (blue). (D) Quantitative analysis of fluorescence intensity for MICAL1 and P-ERK in TgAg-treated Raw264.7 cells. n = 3 per group; (E) WB analysis of MICAL1, P-ERK, and total ERK expression in placental tissues from WT and Trem2−/− mice (NP and TI groups) at GD17.5. n = 4 mice per group; NP: Normal pregnancy; TI: T. gondii infection; CON: Untreated control group; TgAg: Soluble T. gondii antigens; Performed statistical analysis using a two-tailed unpaired Student’s t-test (A,B,D) and two-way ANOVA with Sidak′s multiple comparisons test (E). Western blot bands were quantified using ImageJ, normalized to GAPDH.
3.4. Functional Rescue of MICAL1/P-ERK Pathway by Trem2 in T. gondii-Infected Macrophage
To confirm the role of Trem2 in regulating the MICAL1/P-ERK signaling pathway during T. gondii infection, we introduced a Trem2-DAP12 fusion construct into Raw264.7 cells that maintains native Trem2 signaling through DAP12. The chimeric protein was generated by fusing the extracellular and transmembrane domains of mouse Trem2 (aa19–171) with the transmembrane and cytoplasmic regions of mouse DAP12 (aa28–114), incorporating a G52A mutation in DAP12 to prevent interactions with other receptors and ensure proper formation of the Trem2-DAP12 complex [30]. Four experimental groups were established: pcSLenti (empty vector control), pcSLenti + TgAg, pcSLenti-Trem2-Tyrobp (Trem2-DAP12 complex overexpression), and pcSLenti-Trem2-Tyrobp + TgAg. Significantly elevated Trem2 expression in the pcSLenti-Trem2-Tyrobp group confirmed successful establishment of the Trem2-DAP12 overexpression model. Compared to control macrophages carrying the pcSLenti, TgAg stimulation caused significant downregulation of MICAL1 and P-ERK expression (Figure 4A). Crucially, this suppression was completely reversed in cells overexpressing the Trem2-Tyrobp complex, with protein levels restored to baseline. These results demonstrate that Trem2-DAP12 signaling is sufficient to protect the MICAL1/P-ERK pathway from pathogen-mediated disruption. We next stably overexpressed MICAL1 in Raw264.7 macrophages, generating four groups: pcSLenti, pcSLenti + TgAg, pcSLenti-MICAL1 (MICAL1 overexpression), and pcSLenti-MICAL1 + TgAg. Elevated MICAL1 expression in pcSLenti-MICAL1 macrophages confirmed successful model generation. Compared to the control, TgAg induced significant downregulation of Trem2 and P-ERK. While MICAL1 overexpression significantly attenuated the suppression of P-ERK, it notably did not reverse the TgAg-induced downregulation of Trem2 (Figure 4B). These findings indicate that although MICAL1 functions downstream to modulate ERK activity, it was unable to compensate for the Trem2 deficiency during infection. Consistent with our observations in placental tissue, studies in BMDMs from Trem2−/− mice revealed no difference in MICAL1 or P-ERK expression compared to WT BMDMs under uninfected conditions. However, following TgAg stimulation, the suppression of MICAL1 and P-ERK was significantly exacerbated in Trem2−/− BMDMs compared to stimulated WT controls (Figure 4C). The concordance between placental tissue protein data and BMDM results solidifies Trem2’s status as a master regulator of anti-T. gondii defense in macrophages, highlighting its potential as a therapeutic target for protecting MICAL1/P-ERK signaling from pathogen assault.
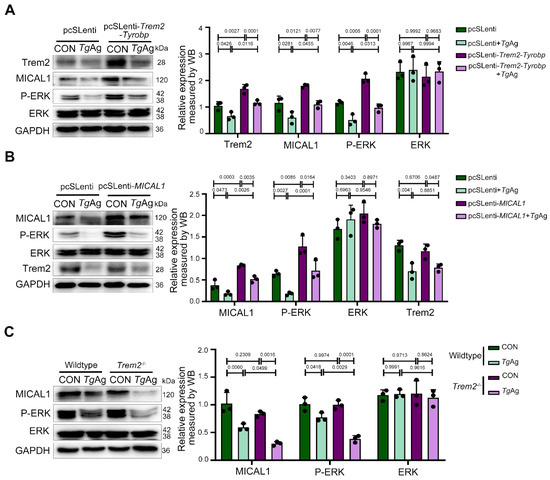
Figure 4.
Functional rescue of MICAL1/P-ERK pathway by Trem2 in T. gondii antigen-stimulated macrophage. (A) Overexpression of Trem2-Tyrobp in Raw264.7 cells: WB analysis of Trem2, MICAL1, P-ERK, and total ERK expression in pcSLenti-empty vector (control) and pcSLenti-Trem2-Tyrobp transfected cells, with or without TgAg (5 μg/mL, 48 h) treatment. (B) Overexpression of MICAL1 in Raw264.7 cells: WB analysis of MICAL1, P-ERK, total ERK, and Trem2 expression in pcSLenti-empty vector and pcSLenti-MICAL1-transfected Raw264.7 cells, with or without TgAg stimulation (5 μg/mL, 48 h). (C) WB analysis of MICAL1, P-ERK, and total ERK expression in BMDMs from wildtype and Trem2−/− mice (CON and TgAg). CON: Untreated control group; TgAg: Soluble T. gondii antigens; n = 3 per group; Statistical analysis was performed using two-way ANOVA with Sidak′s multiple comparisons test (A–C). Western blot bands were quantified using ImageJ, normalized to GAPDH.
4. Discussion
The effective control of toxoplasmosis, a globally distributed zoonosis caused by T. gondii, necessitates a One Health approach [37]. Central to this control is the strengthening of coordination, collaboration, and communication at the human–animal–environment interface, a measure fundamental to ensuring global health security [38]. Within the host (including humans and animals), T. gondii promotes its own survival and replication by modulating host cell function, signal transduction, and immune responses [39]. The immunosuppressive conditions during pregnancy make pregnant women more susceptible to T. gondii, and this susceptibility is closely linked to the immune characteristics of the maternal–fetal interface [40]. At this interface, dMφ serves as a crucial immune barrier, playing a central protective role in maintaining pregnancy homeostasis [41]. However, T. gondii infection disrupts this balance via strongly inducing the polarization of dMφ towards the pro-inflammatory M1 phenotype, thereby triggering a severe inflammatory immune response. Studies have shown that in BMDMs, the matrix antigen protein 1 (MAG1) from T. gondii (ME49 strain) is secreted into the host cytosol and suppresses IL-1β transcription [42]. In contrast, BMDMs infected with MAG1-deficient parasites exhibit increased IL-1β release mediated by GRA15 [42]. This suggests that while T. gondii disrupts host immune balance by secreting MAG1 to limit excessive inflammation, the presence of GRA15 suppresses key anti-inflammatory molecules [43]. Interestingly, Trem2 is a critical anti-inflammatory regulator expressed on dMφ, and its dysregulation exacerbates inflammation [44]. Our research has also determined that in T. gondii-infected placental tissue and TgAg-stimulated macrophages, T. gondii targets and suppresses the immune regulatory molecule Trem2, expressed on placental decidual macrophages, to disrupt placental defense. Single-cell sequencing demonstrated that Trem2 knockout enhances pro-inflammatory macrophages in pancreatic ductal adenocarcinoma and exacerbates pathogenic inflammation [45]. Our previous findings establish Trem2 as a key defender of placental immune homeostasis against T. gondii, as its deficiency exacerbates APO [18]. Meanwhile, Trem2-deficient pregnant mouse models showed heightened susceptibility to T. gondii (RH strain) [18]. Therefore, Trem2 may regulate placental immunity through specific signaling pathways, and its deficiency exacerbates immune damage by promoting excessive inflammatory responses.
Trem2, which is a receptor for multiple ligands and suppresses inflammation, can also enhance the phagocytic activity of macrophages and autophagic-lysosomal activation [46,47,48]. Macrophage responses to pathogenic organisms and their molecular components (e.g., LPS) require actin cytoskeletal rearrangement [49]. Upon ligand binding to Trem2, phosphorylation of DAP12 initiates the Syk-PLCγ2 signaling axis. This induces calcium release and activates the PKC/mTOR pathway, ultimately driving actin cytoskeleton rearrangement [50]. However, the T. gondii surface antigen TgSAG1 can target the host protein S100A6, interfering with the interaction between S100A6 and vimentin [24]. This interference directly disrupts the dynamic reorganization of the cytoskeleton, potentially inhibiting phagocytosis-related structures. Notably, T. gondii can further suppress phagocytosis through autophagy-dependent lysosomal degradation or vacuolar disruption (e.g., mediated by GRA effector proteins) [51]. Therefore, we hypothesize that T. gondii disrupts autophagy-dependent lysosomal degradation and synergizes with other immunosuppressive mechanisms to antagonize the Trem2-mediated phagocytic clearance pathway, creating favorable conditions for its intracellular survival. In our study, through RNA-seq analysis of Trem2-overexpressing Raw264.7 macrophages, we found that MICAL1, a key contributor of autophagy, was ranked among the top 50 most significantly up-regulated genes. The correlation analysis further indicates a strong correlation between MICAL1 and Trem2. It is well known that the MICAL protein family has emerged as important redox enzymes regulating actin, influencing the properties of actin in vitro and in vivo [52]. Importantly, MICAL1 knockdown in oligodendrocyte cells could significantly reduce the levels of pro-autophagy factors (Beclin1 and LC3B), suggesting that MICAL1 can protect oligodendrocytes from oxidative injury via regulating autophagy [36]. RNA-seq analysis, molecular docking, fluorescence co-localization, and immunoprecipitation assays demonstrate that Trem2 directly interacts with MICAL1. Therefore, we identified MICAL1 as a critical target molecule within the Trem2-DAP12 signaling pathway, which conferred protection against T. gondii infection.
MICAL1 directly regulates cytoskeletal dynamics by disassembling F-actin and mediates ROS-dependent inflammatory signaling [52]. Based on the knowledge that the Trem2-DAP12 pathway activates PKC to promote actin reorganization, we preliminarily explored the potential association between Trem2 and MICAL1 using molecular docking simulations and fluorescence co-localization analysis. These studies revealed a high-affinity binding interaction between them. Recent studies have proposed that MICAL1 activation leads to upregulated ROS in HeLa cells and promotes autophagy [26,36]. Concurrently, MICAL1 depletion suppresses P-ERK expression, while MICAL1 overexpression increases P-ERK nuclear translocation [26]. These findings suggest that MICAL1 may participate in regulating the immune response of macrophages through the ERK signaling pathway, and that T. gondii infection may disrupt this balance by interfering with the Trem2-MICAL1-P-ERK pathway.
In macrophages, P-ERK, as a downstream effector molecule of the Trem2-MICAL1 pathway, dynamically regulates the balance of immune responses through its phosphorylation levels [53,54]. MICAL1-induced breast cancer cell invasion relies on the ROS/PI3K/Akt signaling pathway, and inhibition of ROS activation can downregulate the MAPK/ERK pathway [26,55,56]. Both our animal and cell culture models revealed that T. gondii infection suppresses ERK phosphorylation, while overexpression of Trem2/MICAL1 preserves P-ERK levels. Notably, in Trem2-knockout mice, T. gondii infection resulted in a more severe downregulation of P-ERK and enhanced APO, indicating that the Trem2-MICAL1 axis acts as a positive upstream regulator of P-ERK. These findings suggest that T. gondii may target and inhibit the Trem2-MICAL1 signaling pathway. This inhibition consequently suppresses the activity of its key downstream effector molecule, P-ERK, ultimately blocking the ROS activation pathway [57]. This suppression of the Trem2-MICAL1-P-ERK axis represents a key mechanism by which T. gondii weakens host immune defenses and induces APO.
In summary, we identified MICAL1 as a critical target molecule within the Trem2 signaling pathway and unraveled a critical protective role of the Trem2-MICAL1-P-ERK immunoregulatory signaling pathway in T. gondii-mediated APO. T. gondii disrupts the integrity of this signaling pathway by targeting Trem2 and impairing its interaction with MICAL1. This disruption leads to the loss of downstream P-ERK activity, ultimately compromising macrophage phagocytic clearance capacity and immunoregulatory functions.
5. Limitations of the Study
While this study establishes that Trem2 deficiency exacerbates APO after T. gondii infection, the functional impact of Trem2 reconstitution or knock-in remains undetermined. Moreover, the relevance of these findings to humans is unclear, as evidence is primarily derived from animal models, and a lack of clinical data hampers the translation of Trem2’s role in human pregnancy.
Author Contributions
X.G.: Conceptualization, Investigation, Methodology, Formal analysis, Writing—original draft. H.Y.: Conceptualization, Investigation, Data curation, Formal analysis. Z.W.: Methodology, Formal analysis. Z.C.: Methodology. J.C.: Writing—review and editing, Writing—original draft, Resources. M.Y.: Writing—original draft, Supervision, Resources, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81401683), the Natural Science Foundation of Nantong Municipality (JC2023100 and JC2024029), and the Natural Science Foundation of Jiangsu Province (BK20251836). The funders were not involved in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Nantong University (protocol #P20230302–013 approved 2 March 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The study data are openly accessible in Mendeley Data at https://data.mendeley.com/datasets/pvgdgx59tz/1, accessed on 1 September 2025.
Acknowledgments
All authors sincerely appreciate Y. Wang from Nanjing Medical University for supplying T. gondii.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Reimão, J.Q.; Evangelista, F.F.; Alves, S.O.; Torres, T.; Lobo, J.d.E.S.; Perroni, K.T.R.; Mariante, R.M. Chemotherapy against Toxoplasma Gondii: A Bibliometric Analysis of in Vitro and Mouse Model Studies (2015–2024). Biomed. Pharmacother. 2025, 186, 117956. [Google Scholar] [CrossRef]
- Perdana, T.M.; Dwiputro, A.H.; Kusuma, S.; Simanjuntak, A.M.T.; Wijayanto, F.P.S. Seroprevalence of Anti-ToxoplasmaIgG among the Human Population in Indonesia: A Systematic Review and Meta-Analysis. BMC Public Health 2025, 25, 194. [Google Scholar] [CrossRef]
- Gao, X.; Zhong, Y.; Li, K.; Miao, A.; Chen, N.; Ding, R.; Xu, Y.; Chen, J. Toxoplasma Gondii Promotes microRNA-34a to Inhibit Foxp3 Expression in Adverse Outcomes of Pregnancy in Mice. Int. Immunopharmacol. 2022, 107, 108648. [Google Scholar] [CrossRef] [PubMed]
- Salomão Lopes, C.; Carvalho, R.J.V.; da Silva, T.L.; Barros, H.L.S.; Costa, L.V.S.; Mota, D.C.A.M.; Barbosa, B.F.; Vieira, L.S.; de Araújo, T.M.; Costa, A.R.; et al. Pregnant Women Chronically Infected by Toxoplasma Gondii with Depressive Disorder: Differential Modulation of Pro-Inflammatory and Anti-Inflammatory Cytokines. Pathogens 2025, 14, 330. [Google Scholar] [CrossRef] [PubMed]
- Levenson, D.; Romero, R.; Miller, D.; Galaz, J.; Garcia-Flores, V.; Neshek, B.; Pique-Regi, R.; Gomez-Lopez, N. The Maternal-Fetal Interface at Single-Cell Resolution: Uncovering the Cellular Anatomy of the Placenta and Decidua. Am. J. Obstet. Gynecol. 2025, 232, S55–S79. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, M.; Xie, Y.; Xu, Y.; Du, R.; Wu, B.; Guan, Z.; Wang, W.; Sun, W.; Xu, T.; et al. Bone Marrow-Derived Mesenchymal Stem Cells Accelerate Angiogenesis in Pregnant Experimentally Induced Deep Venous Thrombosis Rat Model via up-Regulation of pro-Angiogenic Secretogranin II. Int. Immunopharmacol. 2023, 118, 110025. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Deng, W.; Kzhyshkowska, J.; Chen, D.; Zhang, S. Macrophage at Maternal-Fetal Interface: Perspective on Pregnancy and Related Disorders. Placenta 2025, S0143-4004(25)00158-4. [Google Scholar] [CrossRef]
- Zhan, S.; Zheng, J.; Zhang, H.; Zhao, M.; Liu, X.; Jiang, Y.; Yang, C.; Ren, L.; Liu, Z.; Hu, X. LILRB4 Decrease on uDCs Exacerbate Abnormal Pregnancy Outcomes Following Toxoplasma Gondii Infection. Front. Microbiol. 2018, 9, 588. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Ren, L.; Li, Z.; Jiang, Y.; Wang, C.; Liu, X.; Ren, Y.; Hu, X. Effect of B7-H4 Downregulation Induced by Toxoplasma Gondii Infection on Dysfunction of Decidual Macrophages Contributes to Adverse Pregnancy Outcomes. Parasites Vectors 2022, 15, 464. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Xu, X.; Jiang, Y.; Ren, L.; Zhang, H.; Li, Z.; Liu, X.; Hu, X.; Ren, Y. The Effect of Toxoplasma Gondii Infection on Galectin-9 Expression in Decidual Macrophages Contributing to Dysfunction of Decidual NK Cells during Pregnancy. Parasites Vectors 2024, 17, 299. [Google Scholar] [CrossRef]
- Lv, M.; Jia, Y.; Dong, J.; Wu, S.; Ying, H. The Landscape of Decidual Immune Cells at the Maternal–Fetal Interface in Parturition and Preterm Birth. Inflamm. Res. 2025, 74, 44. [Google Scholar] [CrossRef]
- Ma, W.; Sun, C.; Mao, S.; Luo, X.; Li, Q.; Hu, M. Interaction of Decidual Macrophages and Other Maternal-Fetal Interface Cells in Recurrent Pregnancy Loss. Placenta 2025, S0143-4004(25)00179-1. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Wei, L.; Li, S.; Wang, J.; Zhang, Y.; Yang, R.; Zhang, H.; Xu, A.; Jiang, Y.; et al. Toxoplasma Gondii ROP18 Induces Maternal-Fetal Dysfunction by Downregulating CD73 Expression on Decidual Macrophages. Parasites Vectors 2025, 18, 72. [Google Scholar] [CrossRef]
- Butcher, B.A.; Fox, B.A.; Rommereim, L.M.; Kim, S.G.; Maurer, K.J.; Yarovinsky, F.; Herbert, D.R.; Bzik, D.J.; Denkers, E.Y. Toxoplasma Gondii Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control. PLoS Pathog. 2011, 7, e1002236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, J.; Zhang, H.; Li, Z.; Ren, Y.; Jiang, Y.; Liu, X.; Hu, X. LILRB4 Regulates the Function of Decidual MDSCs via the SHP-2/STAT6 Pathway during Toxoplasma Gondii Infection. Parasites Vectors 2023, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.D.C.; Hu, K.; Whitmarsh, R.J.; Hassan, M.A.; Julien, L.; Lu, D.; Chen, L.; Hunter, C.A.; Saeij, J.P.J. Toxoplasma Gondii Rhoptry 16 Kinase Promotes Host Resistance to Oral Infection and Intestinal Inflammation Only in the Context of the Dense Granule Protein GRA15. Infect. Immun. 2013, 81, 2156–2167. [Google Scholar] [CrossRef] [PubMed]
- Franklin-Murray, A.L.; Mallya, S.; Jankeel, A.; Sureshchandra, S.; Messaoudi, I.; Lodoen, M.B. Toxoplasma Gondii Dysregulates Barrier Function and Mechanotransduction Signaling in Human Endothelial Cells. mSphere 2020, 5, e00550-19. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, Y.; Ye, S.; Ding, M.; Ge, W.; Liang, Y.; Chen, J. Trem2/Syk/PI3K Axis Contributes to the Host Protection against Toxoplasma Gondii-Induced Adverse Pregnancy Outcomes via Modulating Decidual Macrophages. PLoS Pathog. 2024, 20, e1012543. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.; Fang, X.; Shu, Q.; Chen, Q. Function and Mechanism of TREM2 in Bacterial Infection. PLoS Pathog. 2024, 20, e1011895. [Google Scholar] [CrossRef]
- Colonna, M. The Biology of TREM Receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef]
- May, R.C.; Machesky, L.M. Phagocytosis and the Actin Cytoskeleton. J. Cell Sci. 2001, 114, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Vanoni, M.A. Structure-Function Studies of MICAL, the Unusual Multidomain Flavoenzyme Involved in Actin Cytoskeleton Dynamics. Arch. Biochem. Biophys. 2017, 632, 118–141. [Google Scholar] [CrossRef] [PubMed]
- Haikazian, S.; Olson, M.F. MICAL1 Monooxygenase in Autosomal Dominant Lateral Temporal Epilepsy: Role in Cytoskeletal Regulation and Relation to Cancer. Genes 2022, 13, 715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Peng, J.; Chen, M.; Yao, L.-J.; Zou, W.H.; He, C.Y.; Peng, H.-J. Toxoplasma Gondii SAG1 Targeting Host Cell S100A6 for Parasite Invasion and Host Immunity. iScience 2021, 24, 103514. [Google Scholar] [CrossRef]
- Geng, X.; Yao, T.; Wang, X.; Wang, J.; Zhang, T.; Qian, T.; Liu, C.; Chen, J. ATF3-Mediated Inhibition of Trem2 by Toxoplasma Gondii Contributes to Adverse Pregnancy Outcomes. Parasites Vectors 2025, 18, 245. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Zhao, S.; Zhang, Y.; Chen, Y.; Zhao, X.; Liu, L.; Sun, S.; Zhang, L.; Ye, B.; et al. MICAL1 Facilitates Breast Cancer Cell Proliferation via ROS-sensitive ERK/Cyclin D Pathway. J. Cell Mol. Med. 2018, 22, 3108–3118. [Google Scholar] [CrossRef]
- Guo, M.; Yan, P.; Zhu, M.; Choi, M.; Li, X.; Huang, J.; Zou, J.; Yuan, J.; Ding, W.; Li, D.; et al. Microcystin-LR Prenatal Exposure Drives Preeclampsia-like Changes in Mice by Inhibiting the Expression of TGF-β and VEGFA. Food Chem. Toxicol. 2023, 182, 114189. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, L.; Zhang, R.; Ge, K.; Guo, H.; Liu, X.; Liu, J.; Kong, D.; Wang, Y. Identification of a TNF-α Inducer MIC3 Originating from the Microneme of Non-Cystogenic, Virulent Toxoplasma Gondii. Sci. Rep. 2016, 6, 39407. [Google Scholar] [CrossRef]
- Wang, C.; Ding, S.; Sun, B.; Shen, L.; Xiao, L.; Han, Z.; Huang, H. Hsa-miR-4271 Downregulates the Expression of Constitutive Androstane Receptor and Enhances in Vivo the Sensitivity of Non-Small Cell Lung Cancer to Gefitinib. Pharmacol. Res. 2020, 161, 105110. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Jiang, L.-L.; Fan, X.; Ku, Z.; Li, L.; Liu, X.; Deng, M.; Arase, H.; Zhu, J.-J.; et al. LILRB2-Mediated TREM2 Signaling Inhibition Suppresses Microglia Functions. Mol. Neurodegener. 2022, 17, 44. [Google Scholar] [CrossRef]
- Gustafsson, C.; Mjösberg, J.; Matussek, A.; Geffers, R.; Matthiesen, L.; Berg, G.; Sharma, S.; Buer, J.; Ernerudh, J. Gene Expression Profiling of Human Decidual Macrophages: Evidence for Immunosuppressive Phenotype. PLoS ONE 2008, 3, e2078. [Google Scholar] [CrossRef] [PubMed]
- Pocock, J.; Vasilopoulou, F.; Svensson, E.; Cosker, K. Microglia and TREM2. Neuropharmacology 2024, 257, 110020. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, M.; Liu, Y.; Hu, Y.; Chen, Y.; Yu, Y.; Deng, L. ANGPTL2 Knockdown Induces Autophagy to Relieve Alveolar Macrophage Pyroptosis by Reducing LILRB2-Mediated Inhibition of TREM2. J. Cell Mol. Med. 2024, 28, e18280. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-B.; Zhang, W.-J.; Zou, L.; Huang, P.-J.; Sun, B.-J. Identification Potential Biomarkers in Pulmonary Tuberculosis and Latent Infection Based on Bioinformatics Analysis. BMC Infect. Dis. 2016, 16, 500. [Google Scholar] [CrossRef][Green Version]
- Vanoni, M.A.; Vitali, T.; Zucchini, D. MICAL, the Flavoenzyme Participating in Cytoskeleton Dynamics. Int. J. Mol. Sci. 2013, 14, 6920–6959. [Google Scholar] [CrossRef]
- Xu, C.; Mao, L.; Tian, H.; Lin, S.; Zhao, X.; Lin, J.; Li, D.; Li, X.; Mei, X. MICAL1 (Molecule Interacting with CasL 1) Protects Oligodendrocyte Cells from Oxidative Injury through Regulating Apoptosis, Autophagy in Spinal Cord Injury. Neurosci. Lett. 2021, 750, 135712. [Google Scholar] [CrossRef]
- Huertas-López, A.; Álvarez-García, G.; Sánchez-Sánchez, R.; Cantos-Barreda, A.; Ibáñez-López, F.J.; Martínez-Subiela, S.; Cerón, J.J.; Martínez-Carrasco, C. A Systematic Review and Meta-Analysis of the Serological Diagnosis of Toxoplasma Gondii Infection Highlight the Lack of a One Health Integrative Research. Res. Vet. Sci. 2023, 155, 137–149. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. Ecohealth 2019, 16, 378–390. [Google Scholar] [CrossRef]
- Fan, S.-J.; Pan, M.; Xia, C.-Y.; Yang, P.; Huang, S.-Y. Global Research Landscape and Trends in Toxoplasma Gondii from 2003 to 2022: A Bibliometric Analysis. Vet. Parasitol. 2025, 335, 110438. [Google Scholar] [CrossRef]
- Abamecha, F.; Awel, H. Seroprevalence and Risk Factors of Toxoplasma Gondii Infection in Pregnant Women Following Antenatal Care at Mizan Aman General Hospital, Bench Maji Zone (BMZ), Ethiopia. BMC Infect. Dis. 2016, 16, 460. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, W.; Yang, G.; Yang, Y.; Wang, Y.; Zheng, L.; Fu, Y.; Cheng, X. Toxoplasma Gondii Rhoptry Protein 7 (ROP7) Interacts with NLRP3 and Promotes Inflammasome Hyperactivation in THP-1-Derived Macrophages. Cells 2022, 11, 1630. [Google Scholar] [CrossRef]
- Tomita, T.; Mukhopadhyay, D.; Han, B.; Yakubu, R.; Tu, V.; Mayoral, J.; Sugi, T.; Ma, Y.; Saeij, J.P.J.; Weiss, L.M. Toxoplasma Gondii Matrix Antigen 1 Is a Secreted Immunomodulatory Effector. mBio 2021, 12, e00603-21. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, S.Y.; Pandori, W.J.; Lodoen, M.B. Capers with Caspases: Toxoplasma Gondii Tales of Inflammation and Survival. Curr. Opin. Microbiol. 2023, 72, 102264. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Gutierrez, R.A.; Bhat, M.A. Microglia, Trem2, and Neurodegeneration. Neuroscientist 2025, 31, 159–176. [Google Scholar] [CrossRef]
- Yang, D.; Sun, X.; Wang, H.; Wistuba, I.I.; Wang, H.; Maitra, A.; Chen, Y. TREM2 Depletion in Pancreatic Cancer Elicits Pathogenic Inflammation and Accelerates Tumor Progression via Enriching IL-1β+ Macrophages. Gastroenterology 2025, 168, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.-W.; Chu, Y.-H.; Zhou, L.-Q.; Chen, M.; You, Y.-F.; Tang, Y.; Yang, S.; Zhang, H.; Xiao, J.; Deng, G.; et al. Trem2 Deficiency Attenuates Microglial Phagocytosis and Autophagic-Lysosomal Activation in White Matter Hypoperfusion. J. Neurochem. 2023, 167, 489–504. [Google Scholar] [CrossRef]
- Walter, J. The Triggering Receptor Expressed on Myeloid Cells 2: A Molecular Link of Neuroinflammation and Neurodegenerative Diseases. J. Biol. Chem. 2016, 291, 4334–4341. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, L.; Xin, W.; Pan, Y.; Tatenhorst, L.; Hao, Z.; Gerner, S.T.; Huber, S.; Juenemann, M.; Butz, M.; et al. TREM2 Regulates Microglial Lipid Droplet Formation and Represses Post-Ischemic Brain Injury. Biomed. Pharmacother. 2024, 170, 115962. [Google Scholar] [CrossRef]
- Underhill, D.M.; Ozinsky, A. Phagocytosis of Microbes: Complexity in Action. Annu. Rev. Immunol. 2002, 20, 825–852. [Google Scholar] [CrossRef]
- Peng, Q.; Malhotra, S.; Torchia, J.A.; Kerr, W.G.; Coggeshall, K.M.; Humphrey, M.B. TREM2- and DAP12-Dependent Activation of PI3K Requires DAP10 and Is Inhibited by SHIP1. Sci. Signal 2010, 3, ra38. [Google Scholar] [CrossRef]
- Subauste, C.S. Interplay Between Toxoplasma Gondii, Autophagy, and Autophagy Proteins. Front. Cell Infect. Microbiol. 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Terman, J.R.; Reisler, E. MICAL-Mediated Oxidation of Actin and Its Effects on Cytoskeletal and Cellular Dynamics. Front. Cell Dev. Biol. 2023, 11, 1124202. [Google Scholar] [CrossRef]
- Hua, R.; Yu, J.; Yan, X.; Ni, Q.; Zhi, X.; Li, X.; Jiang, B.; Zhu, J. Syndecan-2 in Colorectal Cancer Plays Oncogenic Role via Epithelial-Mesenchymal Transition and MAPK Pathway. Biomed. Pharmacother. 2020, 121, 109630. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, M.; Su, H.; Liang, M.; Wu, H.; Zhao, L.; Zhang, J.; He, J.; Yong, Y.; Yu, Z.; et al. Baicalin Decreases the LPS-Induced Intestine Inflammatory Responses by ROS/p-ERK/p-P38 Signal Pathways In Vivo and In Vitro. Biomedicines 2025, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, T.; Niu, C.; Sun, S.; Liu, D. ROS-Activated MAPK/ERK Pathway Regulates Crosstalk between Nrf2 and Hif-1α to Promote IL-17D Expression Protecting the Intestinal Epithelial Barrier under Hyperoxia. Int. Immunopharmacol. 2023, 116, 109763. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Gu, L.; Duan, B.; Cui, J.; Zhang, Y.; Chen, Y.; Sun, S.; Dong, J.; Du, J. MICAL1 Controls Cell Invasive Phenotype via Regulating Oxidative Stress in Breast Cancer Cells. BMC Cancer 2016, 16, 489. [Google Scholar] [CrossRef]
- Fan, J.; Ren, D.; Wang, J.; Liu, X.; Zhang, H.; Wu, M.; Yang, G. Bruceine D Induces Lung Cancer Cell Apoptosis and Autophagy via the ROS/MAPK Signaling Pathway in Vitro and in Vivo. Cell Death Dis. 2020, 11, 126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).