Novel Therapies for Prosthetic Joint Infections Caused by Methicillin-Resistant Staphylococcus aureus
Abstract
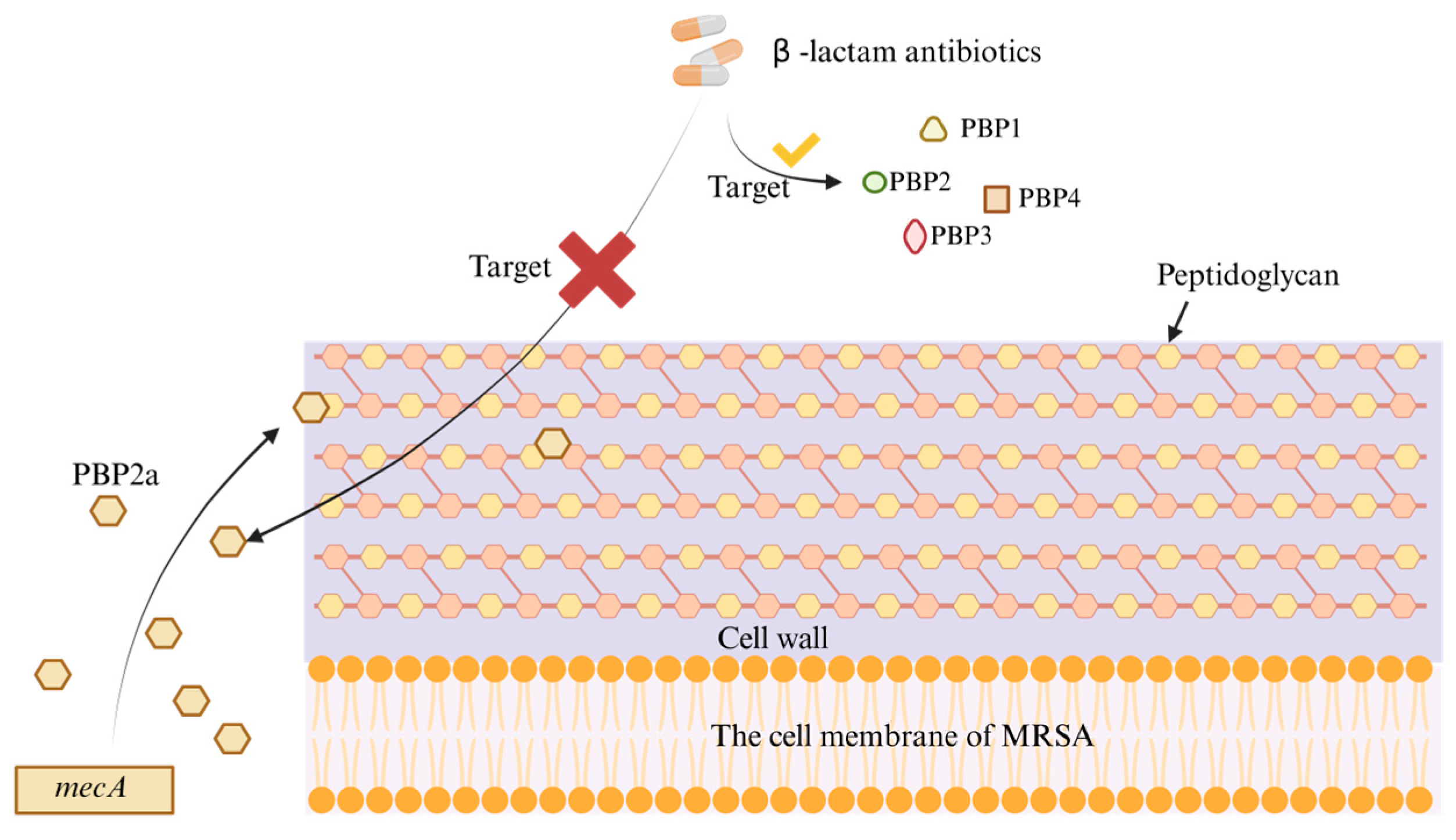
1. Introduction
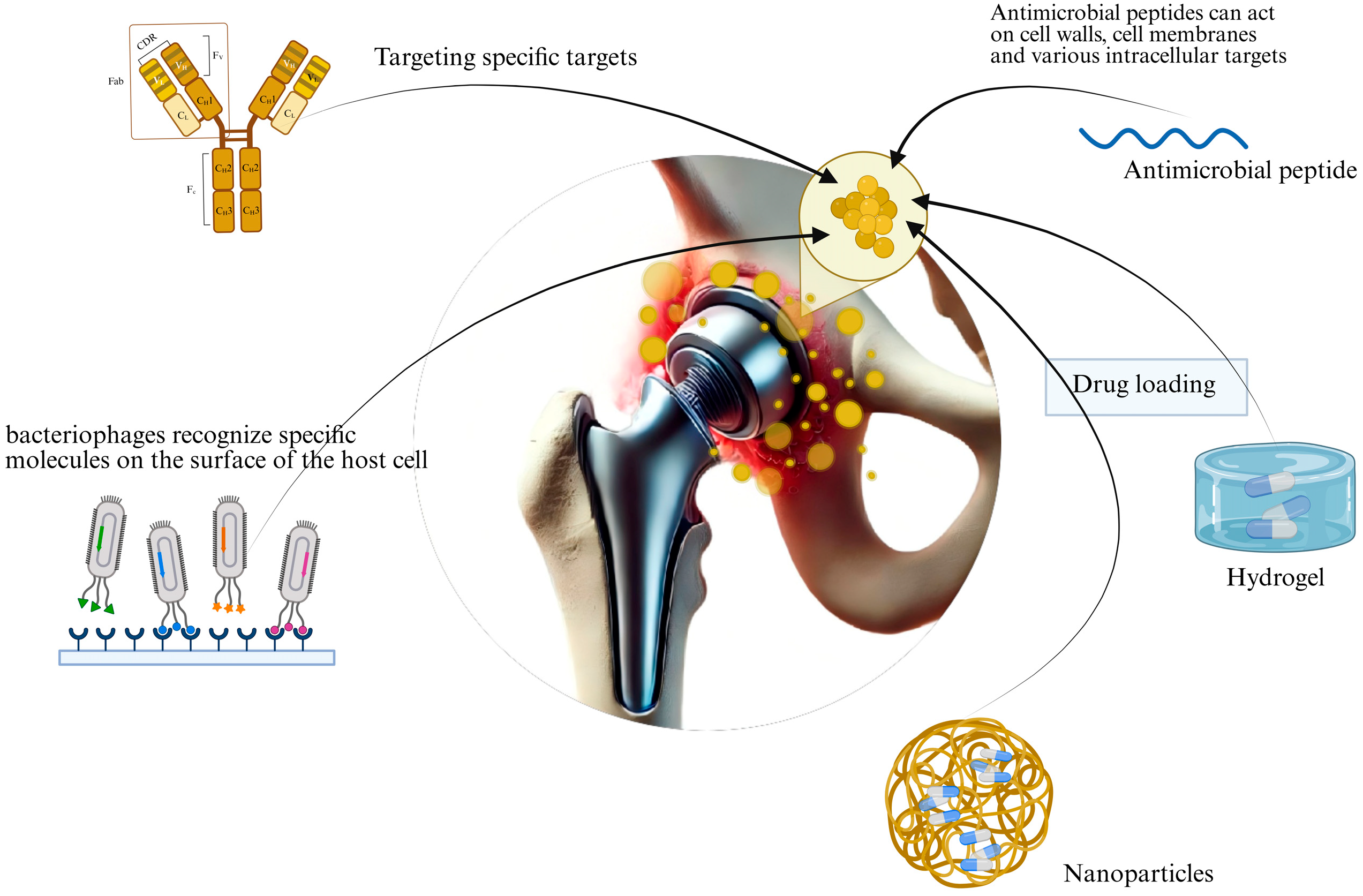
2. Novel Therapeutics
2.1. Monoclonal Antibody Therapy
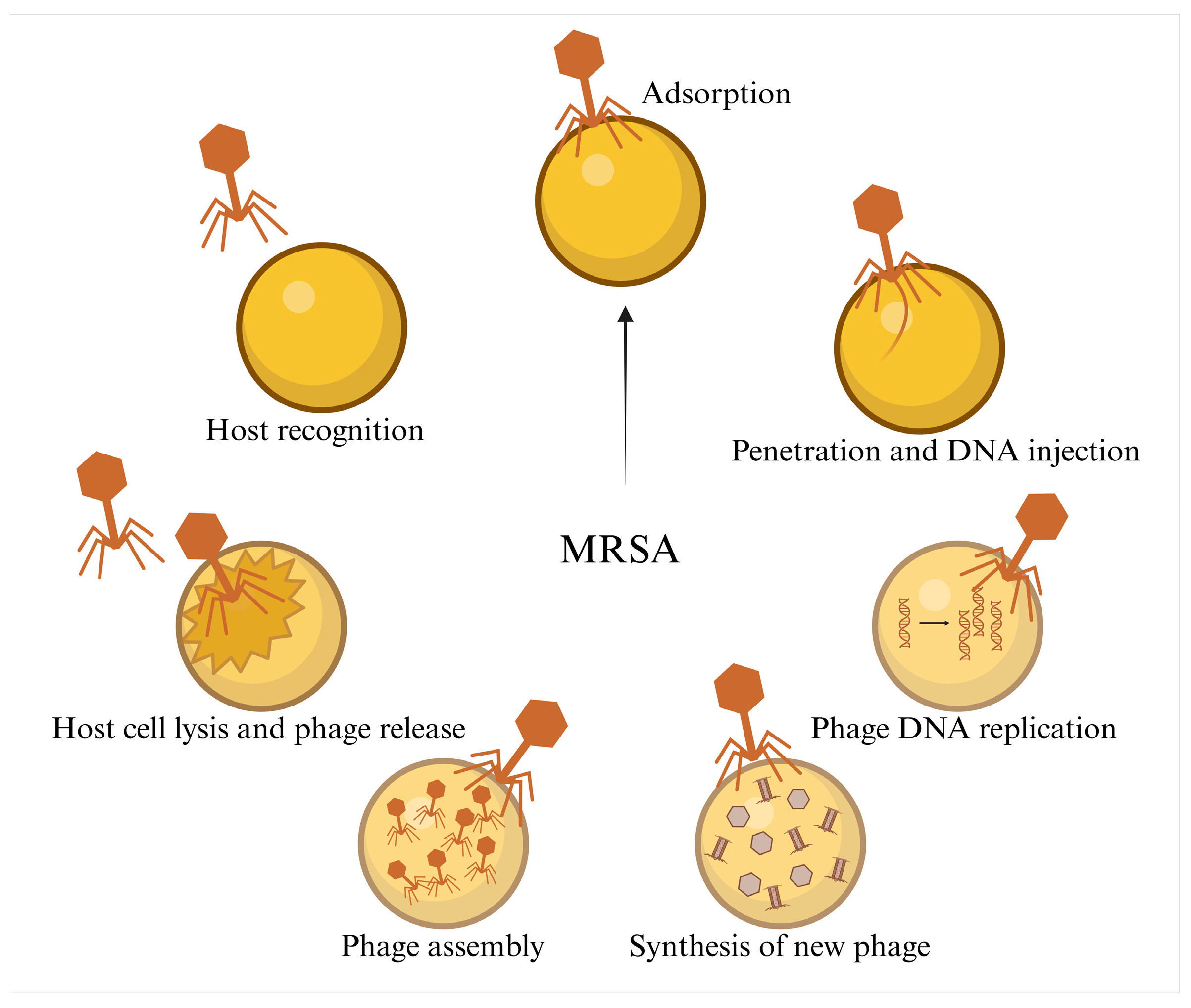
2.2. Phage Therapy
2.3. Antimicrobial Peptide Therapy
3. Emerging Therapeutic Approaches
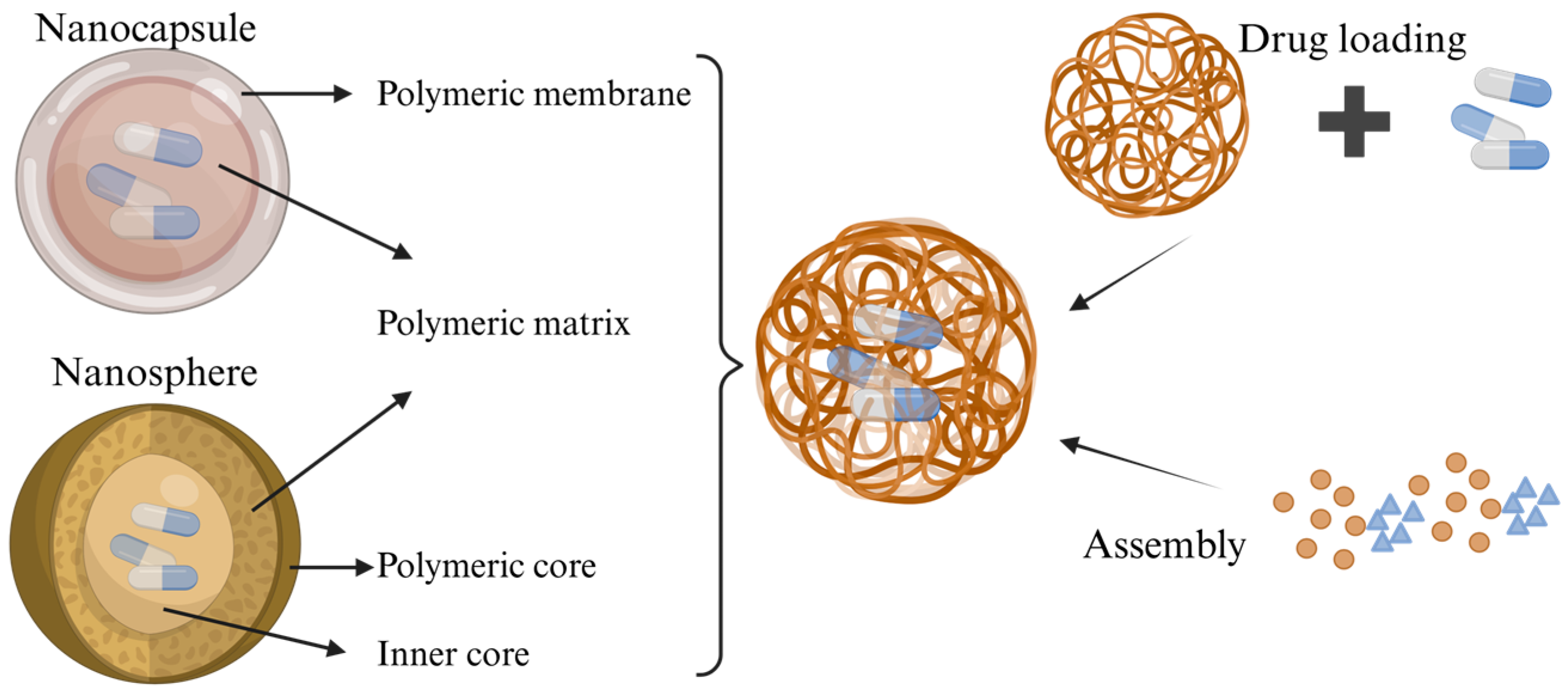
3.1. Nanoparticles
3.2. Hydrogels
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TJR | Total joint replacement |
| PJI | Periprosthetic Joint Infection |
| MRSA | Methicillin-Resistant Staphylococcus Aureus |
| PBP2a | The mec gene on the Staphylococcus chromosome |
| MRSA-PJI | Periprosthetic Joint Infection caused by Methicillin-Resistant Staphylococcus Aureus |
| mAbs | Monoclonal Antibodies |
| IgG | Gamma Immunoglobulin |
| Fab | Antigen-Binding Fragment |
| Fc | Crystallizable Fragment |
| AMP | Antimicrobial Peptides |
| MPS | mononuclear phagocyte system |
| PSA | Poly-sialic acid |
| AI | artificial intelligence |
References
- Baratta, J.L.; Deiling, B.; Hassan, Y.R.; Schwenk, E.S. Total joint replacement in ambulatory surgery. Best Pract. Res. Clin. Anaesthesiol. 2023, 37, 269–284. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Serban, B.; Gheorghe-Barbu, I.; Barbu, I.C.; Cristian, R.E.; Chifiriuc, M.C.; Cirstoiu, C. The Challenge of Periprosthetic Joint Infection Diagnosis: From Current Methods to Emerging Biomarkers. Int. J. Mol. Sci. 2023, 24, 4320. [Google Scholar] [CrossRef]
- Magruder, M.L.; Heckmann, N.D.; Lieberman, J.R.; Scuderi, G.; Lustig, S.; Parvizi, J.; Mont, M.A. Novel Technologies in Periprosthetic Joint Infections: Emerging Therapeutics. J. Arthroplast. 2025. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic Burden of Periprosthetic Joint Infection in the United States. J. Arthroplast. 2012, 27, 61–65.e1. [Google Scholar] [CrossRef]
- Tillander, J.A.N.; Rilby, K.; Malchau, K.S.; Skovbjerg, S.; Lindberg, E.; Rolfson, O.; Trobos, M. Treatment of periprosthetic joint infections guided by minimum biofilm eradication concentration (MBEC) in addition to minimum inhibitory concentration (MIC): Protocol for a prospective randomised clinical trial. BMJ Open 2022, 12, e058168. [Google Scholar] [CrossRef] [PubMed]
- Hieda, Y.; Choe, H.; Maruo, A.; Abe, K.; Shimoda, M.; Ike, H.; Kumagai, K.; Kobayashi, N.; Inaba, Y. Clinical outcomes of continuous local antibiotic perfusion in combination with debridement antibiotics and implant retention for periprosthetic hip joint infection. Sci. Rep. 2025, 15, 26017. [Google Scholar] [CrossRef]
- Hu, L.; Fu, J.; Zhou, Y.; Chai, W.; Zhang, G.; Hao, L.; Chen, J. Microbiological profiles and antibiotic resistance of periprosthetic joint infection after hip replacement in patients with fracture or non-fracture: A comparative study. J. Back Musculoskelet. Rehabil. 2023, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Quan, X.; Zhou, C.; Duan, X.; Nie, M.; Si, H. Risk factors for metachronous periprosthetic joint infection in patients with multiple prosthetic joints: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2025, 20, 293. [Google Scholar] [CrossRef]
- Xu, Z. 1,2,3-Triazole-containing hybrids with potential antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 206, 112686. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 18033. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.R. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, L.; Sankaran, B.; Prasad, B.V.V.; Palzkill, T. Differential active site requirements for NDM-1 β-lactamase hydrolysis of carbapenem versus penicillin and cephalosporin antibiotics. Nat. Commun. 2018, 9, 4524. [Google Scholar] [CrossRef]
- Lai, L.-Y.; Satishkumar, N.; Cardozo, S.; Hemmadi, V.; Marques, L.B.; Huang, L.; Filipe, S.R.; Pinho, M.G.; Chambers, H.F.; Chatterjee, S.S. Altered PBP4 and GdpP functions synergistically mediate MRSA-like high-level, broad-spectrum β-lactam resistance in Staphylococcus aureus. mBio 2024, 15, e0288923. [Google Scholar] [CrossRef]
- Ju, Y.; An, Q.; Zhang, Y.; Sun, K.; Bai, L.; Luo, Y. Recent advances in Clp protease modulation to address virulence, resistance and persistence of MRSA infection. Drug Discov. Today 2021, 26, 2190–2197. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Klika, A.K.; Lu, Q.; Higuera-Rueda, C.A.; Stappenbeck, T.; Visperas, A. Periprosthetic joint infection and immunity: Current understanding of host–microbe interplay. J. Orthop. Res. 2023, 42, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Pan, Y.; Wang, J.; Jiang, Z.; Ma, T.; Chen, S.; Chen, M.; Wu, Y.; Leng, Y.; Hu, Y.; et al. Bone-targeting ZIF-8 based nanoparticles loaded with vancomycin for the treatment of MRSA-induced periprosthetic joint infection. J. Control. Release 2025, 385, 113965. [Google Scholar] [CrossRef] [PubMed]
- Joel, J.; Graham, S.M.; Peckham-Cooper, A.; Korres, N.; Tsouchnica, H.; Tsiridis, E. Clinical results of linezolid in arthroplasty and trauma MRSA related infections. World J. Orthop. 2014, 5, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, W.; Yang, Y.; Zhang, M.; Yang, Y.; Jiang, Q. Organism profiles and empirical treatments for periprosthetic joint infections. J. Orthop. Surg. Res. 2025, 20, 698. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Lee, M.S.; Lee, C.-H.; Lin, P.-C.; Kuo, F.-C. Daptomycin treatment in patients with resistant staphylococcal periprosthetic joint infection. BMC Infect. Dis. 2017, 17, 736. [Google Scholar] [CrossRef]
- Kuo, F.-C.; Yen, S.-H.; Peng, K.-T.; Wang, J.-W.; Lee, M.S. Methicillin-resistant Staphylococcal periprosthetic joint infections can be effectively controlled by systemic and local daptomycin. BMC Infect. Dis. 2015, 16, 48. [Google Scholar] [CrossRef][Green Version]
- Buss, N.A.; Henderson, S.J.; McFarlane, M.; Shenton, J.M.; de Haan, L. Monoclonal antibody therapeutics: History and future. Curr. Opin. Pharmacol. 2012, 12, 615–622. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Hua, L.; Varkey, R.; Shi, Y.; Dettinger, L.; Woods, R.; Barnes, A.; MacGill, R.S.; Wilson, S.; Chowdhury, P.; et al. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin. Vaccine Immunol. 2012, 19, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Ramanan, K.; Tobin, J.M.; Nickolich, K.L.; Pilewski, M.J.; Kallewaard, N.L.; Sellman, B.R.; Cohen, T.S.; Alcorn, J.F. Survival during influenza-associated bacterial superinfection improves following viral- and bacterial-specific monoclonal antibody treatment. J. Clin. Investig. 2019, 4, e125554. [Google Scholar] [CrossRef] [PubMed]
- Di Carluccio, C.; Soriano-Maldonado, P.; Berni, F.; de Haas, C.J.C.; Temming, A.R.; Hendriks, A.; Ali, S.; Molinaro, A.; Silipo, A.; van Sorge, N.M.; et al. Antibody Recognition of Different Staphylococcus aureus Wall Teichoic Acid Glycoforms. ACS Cent. Sci. 2022, 8, 1383–1392. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Zhang, Q.; Lu, H.; Li, A.; Huang, Y.; Zhang, W.; Li, H.; Lu, X.; Ming, X.; et al. Inducing bacterial calcification for systematic treatment and immunomodulation against methicillin-resistant Staphylococcus aureus. Nat. Biotechnol. 2025. [Google Scholar] [CrossRef]
- van Dijk, B.; van Duyvenbode, J.F.F.H.; de Vor, L.; Nurmohamed, F.R.H.A.; Lam, M.G.E.H.; Poot, A.J.; Ramakers, R.M.; Koustoulidou, S.; Beekman, F.J.; van Strijp, J.; et al. Evaluating the Targeting of a Staphylococcus-aureus-Infected Implant with a Radiolabeled Antibody In Vivo. Int. J. Mol. Sci. 2023, 24, 4374. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Hu, N.; Zhang, Y.; Yang, J.; Zhao, L.; Zhang, X.; Yang, Y.; Zhang, J.; Zou, Y.; Wei, K.; et al. Antibody-antibiotic conjugate targeted therapy for orthopedic implant-associated intracellular S. aureus infections. J. Adv. Res. 2023, 65, 239–255. [Google Scholar] [CrossRef]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Daugherty, A.L.; Mrsny, R.J. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliv. Rev. 2006, 58, 686–706. [Google Scholar] [CrossRef]
- Karimi, M.; Aslanabadi, A.; Atkinson, B.; Hojabri, M.; Munawwar, A.; Zareidoodeji, R.; Ray, K.; Habibzadeh, P.; Parlayan, H.N.K.; DeVico, A.; et al. Subcutaneous liposomal delivery improves monoclonal antibody pharmacokinetics in vivo. Acta Biomater. 2025, 195, 522–535. [Google Scholar] [CrossRef]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Onsea, J.; Wagemans, J.; Pirnay, J.; Di Luca, M.; Gonzalez-Moreno, M.; Lavigne, R.; Trampuz, A.; Moriarty, T.; Metsemakers, W.-J. Bacteriophage therapy as a treatment strategy for orthopaedic-device-related infections: Where do we stand? Eur. Cells Mater. 2020, 39, 193–210. [Google Scholar] [CrossRef]
- Young, J.; Lee, S.W.; Shariyate, M.J.; Cronin, A.; Wixted, J.J.; Nazarian, A.; Rowley, C.F.; Rodriguez, E.K. Bacteriophage therapy and current delivery strategies for orthopedic infections: A SCOPING review. J. Infect. 2024, 88, 106125. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.; Sillankorva, S.; Azeredo, J. Bacteriophage-encoded depolymerases: Their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Giri, S.S.; Yun, S.; Kim, H.J.; Kang, J.W.; Han, S.J.; Kwon, J.; Oh, W.T.; Jun, J.W.; Park, S.C. Synergistic phage–surfactant combination clears IgE-promoted Staphylococcus aureus aggregation in vitro and enhances the effect in vivo. Int. J. Antimicrob. Agents 2020, 56, 105997. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.; Li, B.; Liu, J.; Wang, L.; Zhang, L.; Li, Y.; Zhong, Q. StAP1 phage: An effective tool for treating methicillin-resistant Staphylococcus aureus infections. Front. Microbiol. 2023, 14, 1267786. [Google Scholar] [CrossRef] [PubMed]
- Arens, D.K.; Rodriguez, A.R.; Huh, E.Y.; Wang, H.-C.H.; Burdette, A.J.; Hwang, Y.Y. Enhancing orthopedic infection control: Carbon scaffold-mediated phage therapy for methicillin-resistant staphylococcus aureus in fracture-related infections. Biomed. Phys. Eng. Express 2025, 11, 017005. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Liu, X.; Wang, C.-Y.; Wan, Q.-Q.; Tian, Y.-F.; Liu, S.-L.; Pang, D.-W.; Wang, Z.-G. Inhalable Polymeric Microparticles for Phage and Photothermal Synergistic Therapy of Methicillin-Resistant Staphylococcus aureus Pneumonia. Nano Lett. 2024, 24, 8752–8762. [Google Scholar] [CrossRef]
- Dedrick, R.M.; E Smith, B.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Brown, A.W.; A Cohen, K.; Davidson, R.M.; van Duin, D.; et al. Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients With Drug-Resistant Mycobacterial Disease. Clin. Infect. Dis. 2022, 76, 103–112. [Google Scholar] [CrossRef]
- Ooi, M.L.; Drilling, A.J.; Morales, S.; Fong, S.; Moraitis, S.; Macias-Valle, L.; Vreugde, S.; Psaltis, A.J.; Wormald, P.-J. Safety and Tolerability of Bacteriophage Therapy for Chronic Rhinosinusitis Due to Staphylococcus aureus. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. mBio 2020, 11, e01462-20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E.; et al. A combination therapy of Phages and Antibiotics: Two is better than one. Int. J. Biol. Sci. 2021, 17, 3573–3582. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Coyne, A.J.K.; Stamper, K.; Bleick, C.; Kebriaei, R.; Lehman, S.M.; Rybak, M.J. Synergistic bactericidal effects of phage-enhanced antibiotic therapy against MRSA biofilms. Microbiol. Spectr. 2024, 12, e0321223. [Google Scholar] [CrossRef]
- Kaur, S.; Harjai, K.; Chhibber, S. In Vivo Assessment of Phage and Linezolid Based Implant Coatings for Treatment of Methicillin Resistant S. aureus (MRSA) Mediated Orthopaedic Device Related Infections. PLoS ONE 2016, 11, e0157626. [Google Scholar] [CrossRef]
- Antonelli, B.; Chen, A.F. Reducing the risk of infection after total joint arthroplasty: Preoperative optimization. Arthroplasty 2019, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Deo, S.; Turton, K.L.; Kainth, T.; Kumar, A.; Wieden, H.-J. Strategies for improving antimicrobial peptide production. Biotechnol. Adv. 2022, 59, 107968. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Shi, J.; Chen, C.; Kong, P.; Yu, F.; Lv, Q.; Wang, Z.; Liu, Y. Non-Membrane Active Peptide Resensitizes MRSA to β-Lactam Antibiotics and Inhibits S. aureus Virulence. Adv. Sci. 2025, 12, e2416260. [Google Scholar] [CrossRef]
- Songnaka, N.; Lertcanawanichakul, M.; Hutapea, A.M.; Krobthong, S.; Yingchutrakul, Y.; Atipairin, A. Purification and Characterization of Novel Anti-MRSA Peptides Produced by Brevibacillus sp. SPR-20. Molecules 2022, 27, 8452. [Google Scholar] [CrossRef] [PubMed]
- Melicherčík, P.; Kotaška, K.; Jahoda, D.; Landor, I.; Čeřovský, V. Antimicrobial peptide in polymethylmethacrylate bone cement as a prophylaxis of infectious complications in orthopedics–an experiment in a murine model. Folia Microbiol. 2022, 67, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Weng, X.; Zhang, J.; Mao, J. Protective effect of additional cathelicidin antimicrobial peptide PR-39 on prosthetic-joint infections. J. Orthop. Surg. 2023, 31, 10225536231175237. [Google Scholar] [CrossRef] [PubMed]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hsu, C.-Y.; Lai, S.-M.; Syu, W.-J.; Wang, T.-Y.; Lai, P.-S. Metal nanobullets for multidrug resistant bacteria and biofilms. Adv. Drug Deliv. Rev. 2014, 78, 88–104. [Google Scholar] [CrossRef]
- Salatin, S.; Bazmani, A.; Shahi, S.; Naghili, B.; Memar, M.Y.; Dizaj, S.M. Antimicrobial Benefits of Flavonoids and their Nanoformulations. Curr. Pharm. Des. 2022, 28, 1419–1432. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef]
- Fu, S.; Yi, X.; Li, Y.; Li, Y.; Qu, X.; Miao, P.; Xu, Y. Berberine and chlorogenic acid-assembled nanoparticles for highly efficient inhibition of multidrug-resistant Staphylococcus aureus. J. Hazard. Mater. 2024, 473, 134680. [Google Scholar] [CrossRef]
- Hong, Q.; Zhang, W.; Liu, Z.; Li, B.; Liu, X.; Wang, Z.; Wang, R.; Yang, J.; Nie, B.; Yue, B. Infection microenvironment-triggered nanoparticles eradicate MRSA by thermally amplified chemodynamic therapy and M1 macrophage. J. Nanobiotechnol. 2024, 22, 448. [Google Scholar] [CrossRef]
- Foster, A.L.; Boot, W.; Stenger, V.; D’ESte, M.; Jaiprakash, A.; Eglin, D.; Zeiter, S.; Richards, R.G.; Moriarty, T.F. Single-stage revision of MRSA orthopedic device-related infection in sheep with an antibiotic-loaded hydrogel. J. Orthop. Res. 2020, 39, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Bédouet, L.; Beilvert, A.; Servais, E.; Pascale, F.; Ghegediban, S.H.; Namur, J.; Moine, L. Degradable Hydrophilic Poly(ethylene glycol) Microspheres for the Sustained Delivery of Peptide-Based Antibiotics for Local Anti-infective Therapies. ACS Infect. Dis. 2025, 11, 1673–1685. [Google Scholar] [CrossRef]
- Yang, Q.; Xiang, X.; Wang, H.; Liao, Y.; Li, X. Oral natural material hydrogels: A new strategy for enhancing oral drug delivery efficiency. J. Biomater. Sci. Polym. Ed. 2025, 2025. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, X.; Li, W.; Lin, Z.; Wang, W.; Chen, L.; Li, Q.; Xie, X.; Xu, X.; Lu, Y. Copper-containing chitosan-based hydrogels enabled 3D-printed scaffolds to accelerate bone repair and eliminate MRSA-related infection. Int. J. Biol. Macromol. 2023, 240, 124463. [Google Scholar] [CrossRef]
- Liu, W.; Gao, R.; Yang, C.; Feng, Z.; Ou-Yang, W.; Pan, X.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. ECM-mimetic immunomodulatory hydrogel for methicillin-resistant Staphylococcus aureus—Infected chronic skin wound healing. Sci. Adv. 2022, 8, eabn7006. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Lu, H.; Zhou, T.; Zhang, W.; Deng, L.; Cao, W.; Yang, Z.; Wang, Z.; Wu, X.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, W.; Lin, Y.; Ma, J.; Leng, T.; Cheng, W.; Wang, Y.; Wang, M.; Ning, J.; Yang, S.; et al. Multifunctional antibacterial bioactive nanoglass hydrogel for normal and MRSA infected wound repair. J. Nanobiotechnol. 2023, 21, 162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, H.; Gao, J.; Liu, X.; Li, M.; Wu, D.; Liu, J.; Wang, X.; Wang, Z.; Tang, P. First-Aid Hydrogel Wound Dressing with Reliable Hemostatic and Antibac terial Capability for Traumatic Injuries. Adv. Healthc. Mater. 2023, 12, e2300312. [Google Scholar] [CrossRef]
- Indelli, P.F.; Iannotti, F.; Ferretti, A.; Valtanen, R.; Prati, P.; Prieto, D.P.; Kort, N.P.; Violante, B.; Tandogan, N.R.; Panni, A.S.; et al. Recommendations for periprosthetic joint infections (PJI) prevention: The European Knee Associates (EKA)–International Committee American Association of Hip and Knee Surgeons (AAHKS)–Arthroplasty Society in Asia (ASIA) survey of members. Knee Surg. Sports Traumatol. Arthrosc. 2021, 30, 3932–3943. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.R.; Dusane, D.H.; Moore, K.; Gupta, T.; Delury, C.; Aiken, S.S.; Laycock, P.A.; Sullivan, A.C.; Granger, J.F.; Dipane, M.V.; et al. Pseudomonas aeruginosa biofilm killing beyond the spacer by antibiotic-loaded calcium sulfate beads: An in vitro study. J. Bone Jt. Infect. 2021, 6, 119–129. [Google Scholar] [CrossRef]
- Chan, A.C.; Martyn, G.D.; Carter, P.J. Fifty years of monoclonals: The past, present and future of antibody therapeutics. Nat. Rev. Immunol. 2025, 25, 745–765. [Google Scholar] [CrossRef]
- Zhang, Y. Evolution of phage display libraries for therapeutic antibody discovery. mAbs 2023, 15, 2213793. [Google Scholar] [CrossRef]
- Kim, J.W.; Min, S.W.; Lee, J.; Shin, H.G.; Choi, H.L.; Yang, H.R.; Lee, J.H.; Bin Cho, Y.; Shim, H.; Lee, S. Development and Characterization of Phage-Display-Derived Novel Human Monoclonal Antibodies against the Receptor Binding Domain of SARS-CoV-2. Biomedicines 2022, 10, 3274. [Google Scholar] [CrossRef]
- Ferguson, M.R.; Delgado, K.N.; McBride, S.; Orbe, I.C.; La Vake, C.J.; Caimano, M.J.; Mendez, Q.; Moraes, T.F.; Schryvers, A.B.; Moody, M.A.; et al. Use of Epivolve phage display to generate a monoclonal antibody with opsonic activity directed against a subdominant epitope on extracellular loop 4 of Treponema pallidum BamA (TP0326). Front. Immunol. 2023, 14, 1222267. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, C.; Gonzales, F.; Buckley, M.; Biswas, B.; Henry, M.; Deschenes, M.V.; Horne, B.; Fackler, J.; Brownstein, M.J.; Schooley, R.T.; et al. Successful Treatment of Staphylococcus aureus Prosthetic Joint Infection with Bacteriophage Therapy. Viruses 2021, 13, 1182. [Google Scholar] [CrossRef]
- Lenneman, B.R.; Fernbach, J.; Loessner, M.J.; Lu, T.K.; Kilcher, S. Enhancing phage therapy through synthetic biology and genome engineering. Curr. Opin. Biotechnol. 2021, 68, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Büyükkiraz, M.E.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2021, 132, 1573–1596. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Gao, N.; Sun, J.; Li, X.; Yao, Y.; Hu, Y.; Zhao, J.; Shan, A.; Wang, J. Overcoming delivery challenges of antimicrobial peptides for clinical translation: From nanocarriers to molecular modifications. Drug Resist. Updat. 2025, 83, 101289. [Google Scholar] [CrossRef]
- Sharma, L.; Bisht, G.S. Short Antimicrobial Peptides: Therapeutic Potential and Recent Advancements. Curr. Pharm. Des. 2023, 29, 3005–3017. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Frazar, E.M.; Klaus, M.V.X.; Paul, P.; Hilt, J.Z. Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare through Human and Environmental Treatment. Adv. Healthc. Mater. 2021, 11, 2101820. [Google Scholar] [CrossRef]
- Jacqueline, C.; Caillon, J.; Meyer, O.; Dailly, E.; Simonsson, C.; Lenaerts, V.; Asehnoune, K.; Reghal, A.; Potel, G. Efficacy of Nanoencapsulated Daptomycin in an Experimental Methicillin-Resistant Staphylococcus aureus Bone and Joint Infection Model. Antimicrob. Agents Chemother. 2021, 65, e00768-21. [Google Scholar] [CrossRef]
- Boot, W.; Schmid, T.; D’este, M.; Guillaume, O.; Foster, A.; Decosterd, L.; Richards, R.G.; Eglin, D.; Zeiter, S.; Moriarty, T.F. A Hyaluronic Acid Hydrogel Loaded with Gentamicin and Vancomycin Successfully Eradicates Chronic Methicillin-Resistant Staphylococcus aureus Orthopedic Infection in a Sheep Model. Antimicrob. Agents Chemother. 2021, 65, e01840-20. [Google Scholar] [CrossRef]
- Isler, B.; Welyczko, Z.; Jorgensen, N.; Davis, J.; Paterson, D.L. Advancing the management of prosthetic joint infections: A review of randomized controlled trials and emerging evidence. Antimicrob. Agents Chemother. 2025, 69, e0033825. [Google Scholar] [CrossRef] [PubMed]
- Santos-Júnior, C.D.; Torres, M.D.; Duan, Y.; del Río, Á.R.; Schmidt, T.S.; Chong, H.; Fullam, A.; Kuhn, M.; Zhu, C.; Houseman, A.; et al. Discovery of antimicrobial peptides in the global microbiome with machine learning. Cell 2024, 187, 3761–3778.e16. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gan, L.; Yang, X.; Tan, Z.; Shi, H.; Lai, C.; Zhang, D. Progress of AI assisted synthesis of polysaccharides-based hydrogel and their applications in biomedical field. Int. J. Biol. Macromol. 2024, 287, 138643. [Google Scholar] [CrossRef] [PubMed]
- Jian, T.; Wang, M.; Hettige, J.; Li, Y.; Wang, L.; Gao, R.; Yang, W.; Zheng, R.; Zhong, S.; Baer, M.D.; et al. Self-Assembling and Pore-Forming Peptoids as Antimicrobial Biomaterials. ACS Nano 2024, 18, 23077–23089. [Google Scholar] [CrossRef]
- Shariati, A.; Moradabadi, A.; Azimi, T.; Ghaznavi-Rad, E. Wound healing properties and antimicrobial activity of platelet-derived biomaterials. Sci. Rep. 2020, 10, 1032. [Google Scholar] [CrossRef]
- Zamora-Mendoza, L.; Guamba, E.; Miño, K.; Romero, M.P.; Levoyer, A.; Alvarez-Barreto, J.F.; Machado, A.; Alexis, F. Antimicrobial Properties of Plant Fibers. Molecules 2022, 27, 7999. [Google Scholar] [CrossRef]
- Kelley, B.; De Moor, P.; Douglas, K.; Renshaw, T.; Traviglia, S. Monoclonal antibody therapies for COVID-19: Lessons learned and implications for the development of future products. Curr. Opin. Biotechnol. 2022, 78, 102798. [Google Scholar] [CrossRef] [PubMed]
- Garfall, A.L. New Biological Therapies for Multiple Myeloma. Annu. Rev. Med. 2024, 75, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef] [PubMed]

| Treatment Strategy | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Monoclonal Antibodies | Specific antibodies targeting resistant bacteria | Strong specificity, low side effects | High production cost, low bioavailability |
| Phage Therapy | Utilizing viruses to directly kill resistant bacteria | Strong targeting, effective against resistant bacteria | Narrow host range, potential for immune rejection |
| Antimicrobial Peptides | Disrupting cell membranes or cell walls leading to cell lysis | Low toxicity, strong thermal stability | Long-term use may induce bacterial resistance, Poor bioavailability, short half-life |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, X.; Jin, X.; Yang, Q.; Zou, L.; Wang, Y.; Wang, T.; Sun, X. Novel Therapies for Prosthetic Joint Infections Caused by Methicillin-Resistant Staphylococcus aureus. Pathogens 2025, 14, 1102. https://doi.org/10.3390/pathogens14111102
Xiang X, Jin X, Yang Q, Zou L, Wang Y, Wang T, Sun X. Novel Therapies for Prosthetic Joint Infections Caused by Methicillin-Resistant Staphylococcus aureus. Pathogens. 2025; 14(11):1102. https://doi.org/10.3390/pathogens14111102
Chicago/Turabian StyleXiang, Xi, Xin Jin, Qi Yang, Lili Zou, Yueqing Wang, Tianxu Wang, and Xun Sun. 2025. "Novel Therapies for Prosthetic Joint Infections Caused by Methicillin-Resistant Staphylococcus aureus" Pathogens 14, no. 11: 1102. https://doi.org/10.3390/pathogens14111102
APA StyleXiang, X., Jin, X., Yang, Q., Zou, L., Wang, Y., Wang, T., & Sun, X. (2025). Novel Therapies for Prosthetic Joint Infections Caused by Methicillin-Resistant Staphylococcus aureus. Pathogens, 14(11), 1102. https://doi.org/10.3390/pathogens14111102








