Effect of Female Sex Hormones on the Immune Response against Chlamydia abortus and on Protection Conferred by an Inactivated Experimental Vaccine in a Mouse Model
Abstract
1. Introduction
2. Results
2.1. Effect of Sex Hormone Treatment during C. abortus Infection
2.1.1. Morbidity following Infection
2.1.2. Bacterial Isolation
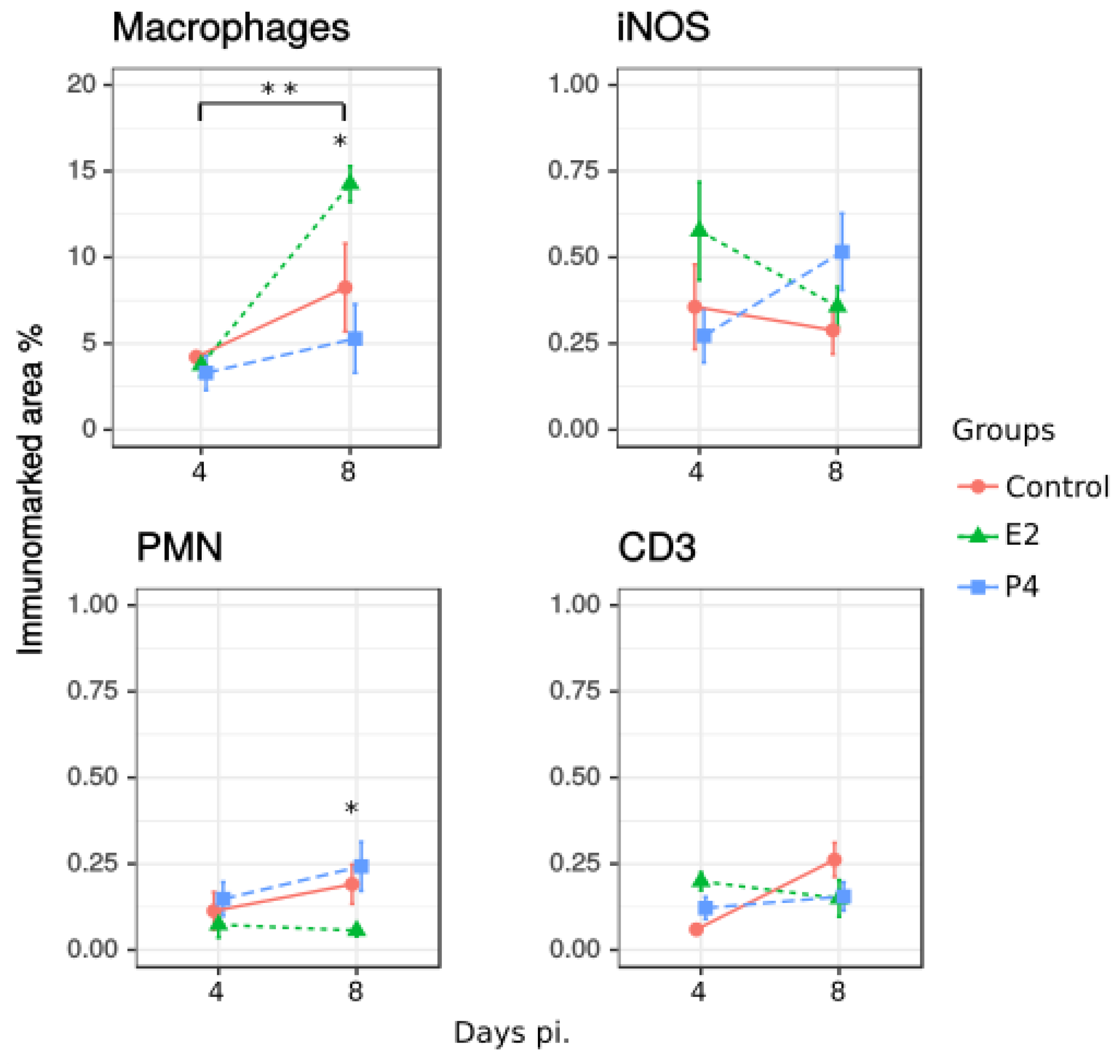
2.1.3. Recruitment of Inflammatory Cells to the Liver after Infection
2.2. Effect of Hormone Treatment on Protection Conferred by the Vaccine
2.2.1. Morbidity in Vaccinated Mice after Treatment with Hormones
2.2.2. Bacterial Isolation and Seroconversion after Vaccination
2.2.3. Immunohistopathology
2.2.4. Effect of Hormone Treatment on Cell Recruitment after Vaccination
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Microorganisms
4.3. Experimental Design
4.3.1. Phase 1. Hormone Treatment and Infection of Mice
4.3.2. Phase 2. Vaccination, Hormone Treatment and Infection of Mice
4.4. Morbidity and Course of Infection
4.5. Detection of Anti-C. abortus Antibodies in Sera
4.6. Histopathology and Immunohistochemistry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OEA | Ovine enzootic abortion |
| E2 | Oestradiol |
| P4 | Progesterone |
| LPS | Bacterial lipopolysaccharides |
| PMNs | Polymorphonuclear neutrophils |
| IFUs | Inclusion forming units |
| iNOS | Inducible nitric oxide synthase |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
References
- Longbottom, D.; Coulter, L.J. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 2003, 128, 217–244. [Google Scholar] [CrossRef]
- Aitken, I.; Longbottom, D. Chlamydial abortion. In Diseases of Sheep; Blackwell Publishing: Oxford, UK, 2007; Volume 4, Chapter 16; pp. 105–112. [Google Scholar]
- Buxton, D.; Barlow, R.M.; Finlayson, J.; Anderson, I.E.; Mackellar, A. Observations on the pathogenesis of Chlamydia psittaci infection of pregnant sheep. J. Comp. Pathol. 1990, 102, 222–237. [Google Scholar] [CrossRef]
- Rodolakis, A.; Salinas, J.; Papp, J. Recent advances on ovine Chlamydial abortion. Vet. Res. 1998, 29, 275–288. [Google Scholar]
- Papp, J.R.; Shewen, P.E.; Gartley, C.J. Abortion and subsequent excretion of chlamydiae from the reproductive tract of sheep during estrus. Infect. Immun. 1994, 62, 3786–3792. [Google Scholar] [CrossRef]
- Livingstone, M.; Wheelhouse, N.; Maley, S.W.; Longbottom, D. Molecular detection of Chlamydophila abortus in post-abortion sheep at oestrus and subsequent lambing. Vet. Microbiol. 2009, 135, 134–141. [Google Scholar] [CrossRef]
- Caro, M.R.; Buendía, A.J.; Del Rio, L.; Ortega, N.; Gallego, M.C.; Cuello, F.; Navarro, J.A.; Sanchez, J.; Salinas, J. Chlamydophila abortus infection in the mouse: A useful model of the ovine disease. Vet. Microbiol. 2009, 135, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Essig, A.; Longbottom, D. Chlamydia abortus: New aspects of infectious abortion in sheep and potential risk for pregnant women. Curr. Clin. Microbiol. Rep. 2015, 2, 22–34. [Google Scholar] [CrossRef]
- Kintner, J.; Schoborg, R.V.; Wyrick, P.B.; Hall, J.V. Progesterone antagonizes the positive influence of estrogen on Chlamydia trachomatis serovar E in an Ishikawa/SHT-290 co-culture model. Pathog. Dis. 2015, 73, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.V.; Schell, M.; Dessus-Babus, S.; Moore, C.G.; Whittimore, J.D.; Sal, M.; Dill, B.D.; Wyrick, P.B. The multifaceted role of oestrogen in enhancing Chlamydia trachomatis infection in polarized human endometrial epithelial cells. Cell. Microbiol. 2011, 13, 1183–1199. [Google Scholar] [CrossRef] [PubMed]
- Guseva, N.V.; Knight, S.T.; Whittimore, J.D.; Wyrick, P.B. Primary cultures of female swine genital epithelial cells in vitro: A new approach for the study of hormonal modulation of Chlamydia infection. Infect. Immun. 2003, 71, 4700–4710. [Google Scholar] [CrossRef] [PubMed]
- Amirshahi, A.; Wan, C.; Beagley, K.; Latter, J.; Symonds, I.; Timms, P. Modulation of the Chlamydia trachomatis in vitro transcriptome response by the sex hormones estradiol and progesterone. BMC Microbiol. 2011, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Caro, M.; Buendía, A.; Schnee, C.; Ortega, N.; Murcia-Belmonte, A.; Salinas, J. Effect of female sex hormones on the developmental cycle of Chlamydia abortus compared to a penicillin-induced model of persistent infection. BMC Vet. Res. 2019, 15, 259. [Google Scholar] [CrossRef]
- Wan, C.; Latter, J.L.; Amirshahi, A.; Symonds, I.; Finnie, J.; Bowden, N.; Scott, R.J.; Cunningham, K.A.; Timms, P.; Beagley, K.W. Progesterone Activates Multiple Innate Immune Pathways in Chlamydia trachomatis-Infected Endocervical Cells. Am. J. Reprod. Immunol. 2014, 71, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Ravel, J.; Bavoil, P.M.; Gravitt, P.E.; Ghanem, K.G. Microbiome, sex hormones, and immune responses in the reproductive tract: Challenges for vaccine development against sexually transmitted infections. Vaccine 2014, 32, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Kaushic, C.; Zhou, F.; Murdin, A.D.; Wira, C.R. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect. Immun. 2000, 68, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Potluri, T.; Fink, A.L.; Sylvia, K.E.; Dhakal, S.; Vermillion, M.S.; Vom Steeg, L.; Deshpande, S.; Narasimhan, H.; Klein, S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. npj Vaccines 2019, 4, 1–12. [Google Scholar] [CrossRef]
- Vom Steeg, L.G.; Flores-Garcia, Y.; Zavala, F.; Klein, S.L. Irradiated sporozoite vaccination induces sex-specific immune responses and protection against malaria in mice. Vaccine 2019, 37, 4468–4476. [Google Scholar] [CrossRef]
- Murcia-Belmonte, A.; Álvarez, D.; Ortega, N.; Navarro, J.; Gómez-Lucia, E.; Buendía, A.; Sánchez, J.; Del Río, L.; Salinas, J.; Caro, M. Effect of progesterone on the vaccination and immune response against Chlamydia abortus in sheep. Vet. Immunol. Immunopathol. 2019, 213, 109887. [Google Scholar] [CrossRef]
- Nadkarni, S.; McArthur, S. Oestrogen and immunomodulation: New mechanisms that impact on peripheral and central immunity. Curr. Opin. Pharmacol. 2013, 13, 576–581. [Google Scholar] [CrossRef]
- Karpuzoglu, E.; Fenaux, J.B.; Phillips, R.A.; Lengi, A.J.; Elvinger, F.; Ansar Ahmed, S. Estrogen Up-Regulates Inducible Nitric Oxide Synthase, Nitric Oxide, and Cyclooxygenase-2 in Splenocytes Activated with T Cell Stimulants: Role of Interferon-γ. Endocrinology 2006, 147, 662–671. [Google Scholar] [CrossRef]
- Dai, R.; Phillips, R.A.; Karpuzoglu, E.; Khan, D.; Ahmed, S.A. Estrogen Regulates Transcription Factors STAT-1 and NF-κβ to Promote Inducible Nitric Oxide Synthase and Inflammatory Responses. J. Immunol. 2009, 183, 6998–7005. [Google Scholar] [CrossRef] [PubMed]
- Maret, A.; Coudert, J.D.; Garidou, L.; Foucras, G.; Gourdy, P.; Krust, A.; Dupont, S.; Chambon, P.; Druet, P.; Bayard, F.; et al. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor α expression in hematopoietic cells. Eur. J. Immunol. 2003, 33, 512–521. [Google Scholar] [CrossRef]
- Priyanka, H.P.; Krishnan, H.C.; Singh, R.V.; Hima, L.; ThyagaRajan, S. Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: Effects on intracellular molecular targets and antioxidant enzymes. Mol. Immunol. 2013, 56, 328–339. [Google Scholar] [CrossRef]
- Stubelius, A.; Andersson, A.; Islander, U.; Carlsten, H. Ovarian hormones in innate inflammation. Immunobiology 2017, 222, 878–883. [Google Scholar] [CrossRef]
- Bini, E.I.; Mata Espinosa, D.; Marquina Castillo, B.; Barrios Payán, J.; Colucci, D.; Cruz, A.F.; Zatarain, Z.L.; Alfonseca, E.; Pardo, M.R.; Bottasso, O.; et al. The Influence of Sex Steroid Hormones in the Immunopathology of Experimental Pulmonary Tuberculosis. PLoS ONE 2014, 9, e93831. [Google Scholar] [CrossRef]
- Berry, A.; Hall, J.V. The complexity of interactions between female sex hormones and Chlamydia trachomatis infections. Curr. Clin. Microbiol. Rep. 2019, 6, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Peterson, E.M.; de la Maza, L.M. A Murine Model for the Study of Chlamydia trachomatis Genital Infections during Pregnancy. Infect. Immun. 1999, 67, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Rank, R.; Sanders, M.; Kidd, A. Influence of the estrous cycle on the development of upper genital tract pathology as a result of chlamydial infection in the guinea pig model of pelvic inflammatory disease. Am. J. Pathol. 1993, 142, 1291. [Google Scholar]
- Pennock, J.W.; Stegall, R.; Bell, B.; Vargas, G.; Motamedi, M.; Milligan, G.; Bourne, N. Estradiol improves genital herpes vaccine efficacy in mice. Vaccine 2009, 27, 5830–5836. [Google Scholar] [CrossRef]
- Bhavanam, S.; Snider, D.P.; Kaushic, C. Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone. Vaccine 2008, 26, 6165–6172. [Google Scholar] [CrossRef]
- Anipindi, V.C.; Bagri, P.; Roth, K.; Dizzell, S.E.; Nguyen, P.V.; Shaler, C.R.; Chu, D.K.; Jiménez-Saiz, R.; Liang, H.; Swift, S.; et al. Estradiol enhances CD4+ T-cell anti-viral immunity by priming vaginal DCs to induce Th17 responses via an IL-1-dependent pathway. PLoS Pathog. 2016, 12, e1005589. [Google Scholar] [CrossRef]
- Bagri, P.; Ghasemi, R.; McGrath, J.J.; Thayaparan, D.; Yu, E.; Brooks, A.G.; Stämpfli, M.R.; Kaushic, C. Estradiol enhances antiviral CD4+ tissue-resident memory T cell responses following mucosal herpes simplex virus 2 vaccination through an IL-17-mediated pathway. J. Virol. 2020, 95, e01206-20. [Google Scholar] [CrossRef]
- Buendía, A.J.; Sánchez, J.; Del Rio, L.; Garcés, B.; Gallego, M.D.C.; Caro, M.R.; Bernabé, A.; Salinas, J. Differences in the immune response against ruminant chlamydial strains in a murine model. Vet. Res. 1999, 30, 495–507. [Google Scholar] [PubMed]
- Ström, J.O.; Theodorsson, A.; Ingberg, E.; Isaksson, I.M.; Theodorsson, E. Ovariectomy and 17β-estradiol replacement in rats and mice: A visual demonstration. JOVE (J. Vis. Exp.) 2012, 64, e4013. [Google Scholar]
- de la Fuente, J.G.; Gutierrez-Martin, C.; Ortega, N.; Rodriguez-Ferri, E.; Del Rio, M.; Gonzalez, O.; Salinas, J. Efficacy of different commercial and new inactivated vaccines against ovine enzootic abortion. Vet. Microbiol. 2004, 100, 65–76. [Google Scholar] [CrossRef]
- Álvarez, D.; Salinas, J.; Buendía, A.J.; Ortega, N.; del Río, L.; Sánchez, J.; Navarro, J.A.; Gallego, M.C.; Murcia-Belmonte, A.; Cuello, F.; et al. Intratracheal infection as an efficient route for testing vaccines against Chlamydia abortus in sheep. Vet. J. 2015, 205, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Caro, M.R.; Ortega, N.; Buendía, A.J.; Gallego, M.C.; Del Río, L.; Cuello, F.; Salinas, J. Relationship between the immune response and protection conferred by new designed inactivated vaccines against ovine enzootic abortion in a mouse model. Vaccine 2003, 21, 3126–3136. [Google Scholar] [CrossRef]
- Del Río, L.; Buendía, A.J.; Sánchez, J.; Gallego, M.C.; Caro, M.R.; Ortega, N.; Seva, J.; Pallarés, F.J.; Cuello, F.; Salinas, J. Endogenous interleukin-12 is not required for resolution of Chlamydophila abortus (Chlamydia psittaci serotype 1) infection in mice. Infect. Immun. 2001, 69, 4808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Del Río, L.; Buendía, A.J.; Sánchez, J.; Garcés, B.; Caro, M.R.; Gallego, M.C.; Bernabé, A.; Cuello, F.; Salinas, J. Chlamydophila abortus (Chlamydia psittaci serotype 1) clearance is associated with the early recruitment of neutrophils and CD8+ T cells in a mouse model. J. Comp. Pathol. 2000, 123, 171–181. [Google Scholar] [CrossRef]
- Del Río, L.; Murcia, A.; Buendía, A.J.; Álvarez, D.; Ortega, N.; Navarro, J.A.; Salinas, J.; Caro, M.R. Development of an in vivo model of Chlamydia abortus chronic infection in mice overexpressing IL-10. Vet. Microbiol. 2018, 213, 28–34. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Peng, R.D.; Dominici, F.; Zeger, S.L. Reproducible epidemiologic research. Am. J. Epidemiol. 2006, 163, 783–789. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Rio, L.; Murcia-Belmonte, A.; Buendía, A.J.; Navarro, J.A.; Ortega, N.; Alvarez, D.; Salinas, J.; Caro, M.R. Effect of Female Sex Hormones on the Immune Response against Chlamydia abortus and on Protection Conferred by an Inactivated Experimental Vaccine in a Mouse Model. Pathogens 2022, 11, 93. https://doi.org/10.3390/pathogens11010093
Del Rio L, Murcia-Belmonte A, Buendía AJ, Navarro JA, Ortega N, Alvarez D, Salinas J, Caro MR. Effect of Female Sex Hormones on the Immune Response against Chlamydia abortus and on Protection Conferred by an Inactivated Experimental Vaccine in a Mouse Model. Pathogens. 2022; 11(1):93. https://doi.org/10.3390/pathogens11010093
Chicago/Turabian StyleDel Rio, Laura, Antonio Murcia-Belmonte, Antonio Julián Buendía, Jose Antonio Navarro, Nieves Ortega, Daniel Alvarez, Jesús Salinas, and María Rosa Caro. 2022. "Effect of Female Sex Hormones on the Immune Response against Chlamydia abortus and on Protection Conferred by an Inactivated Experimental Vaccine in a Mouse Model" Pathogens 11, no. 1: 93. https://doi.org/10.3390/pathogens11010093
APA StyleDel Rio, L., Murcia-Belmonte, A., Buendía, A. J., Navarro, J. A., Ortega, N., Alvarez, D., Salinas, J., & Caro, M. R. (2022). Effect of Female Sex Hormones on the Immune Response against Chlamydia abortus and on Protection Conferred by an Inactivated Experimental Vaccine in a Mouse Model. Pathogens, 11(1), 93. https://doi.org/10.3390/pathogens11010093






