Biflavones from Platonia insignis Mart. Flowers Promote In Vitro Antileishmanial and Immunomodulatory Effects against Internalized Amastigote Forms of Leishmania amazonensis
Abstract
:1. Introduction
2. Results
2.1. Effect of EHPi, FAcOet and MB on Promastigotes of L. amazonensis
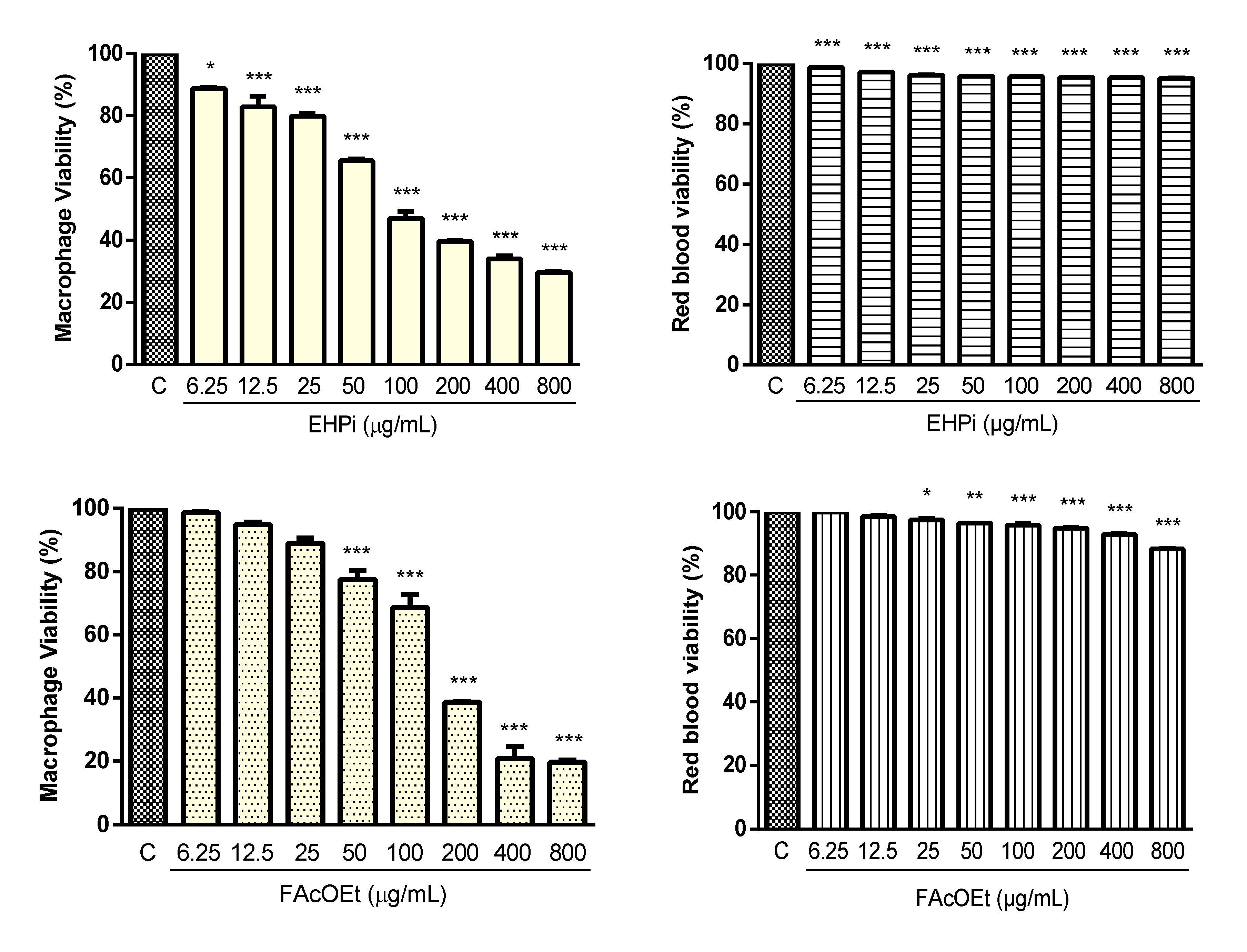
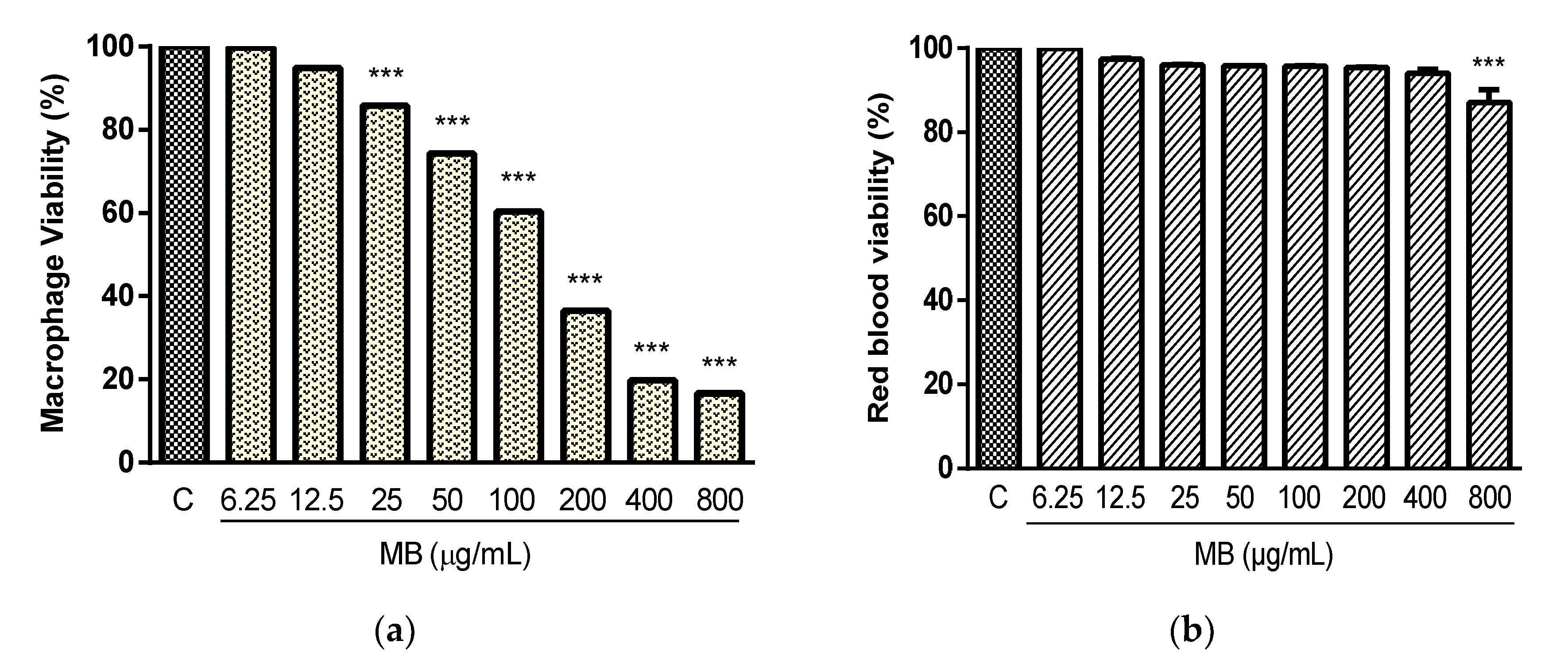
2.2. Cytotoxicity and Hemolysis Assay
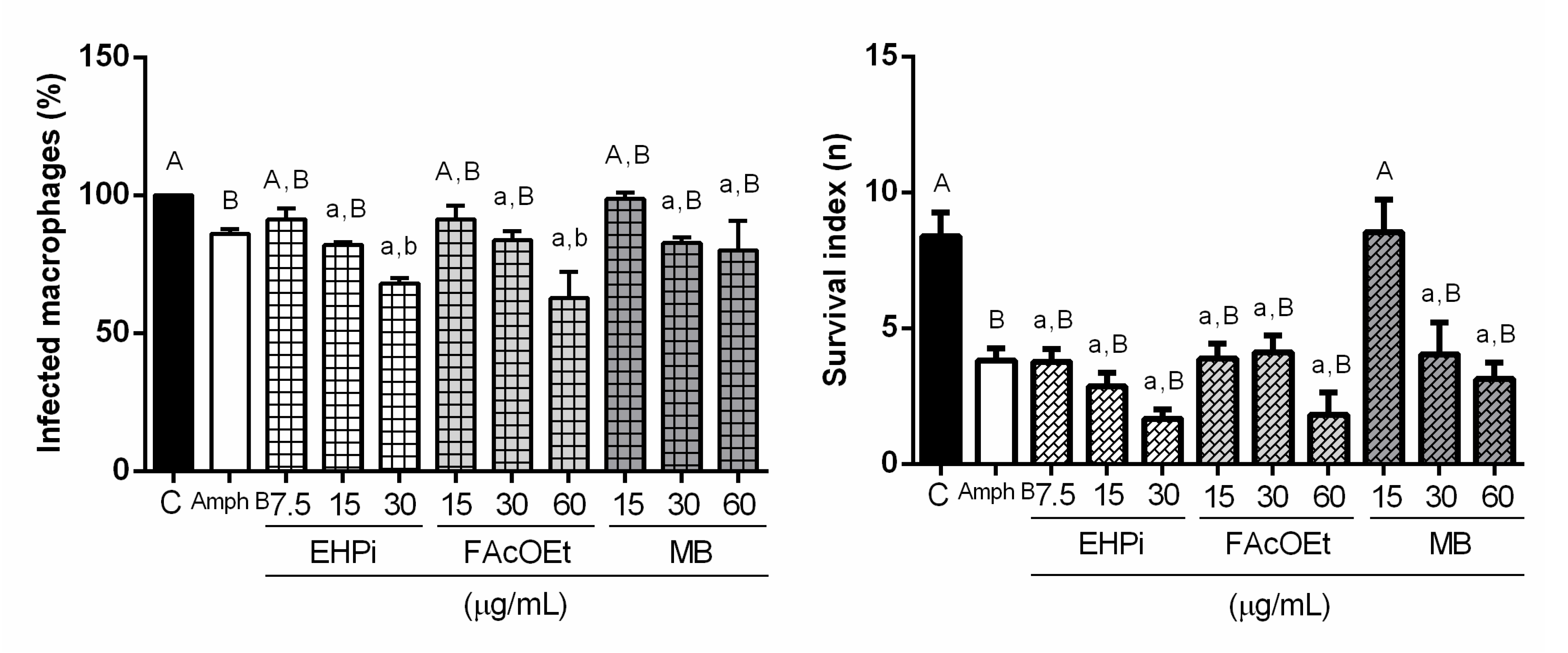
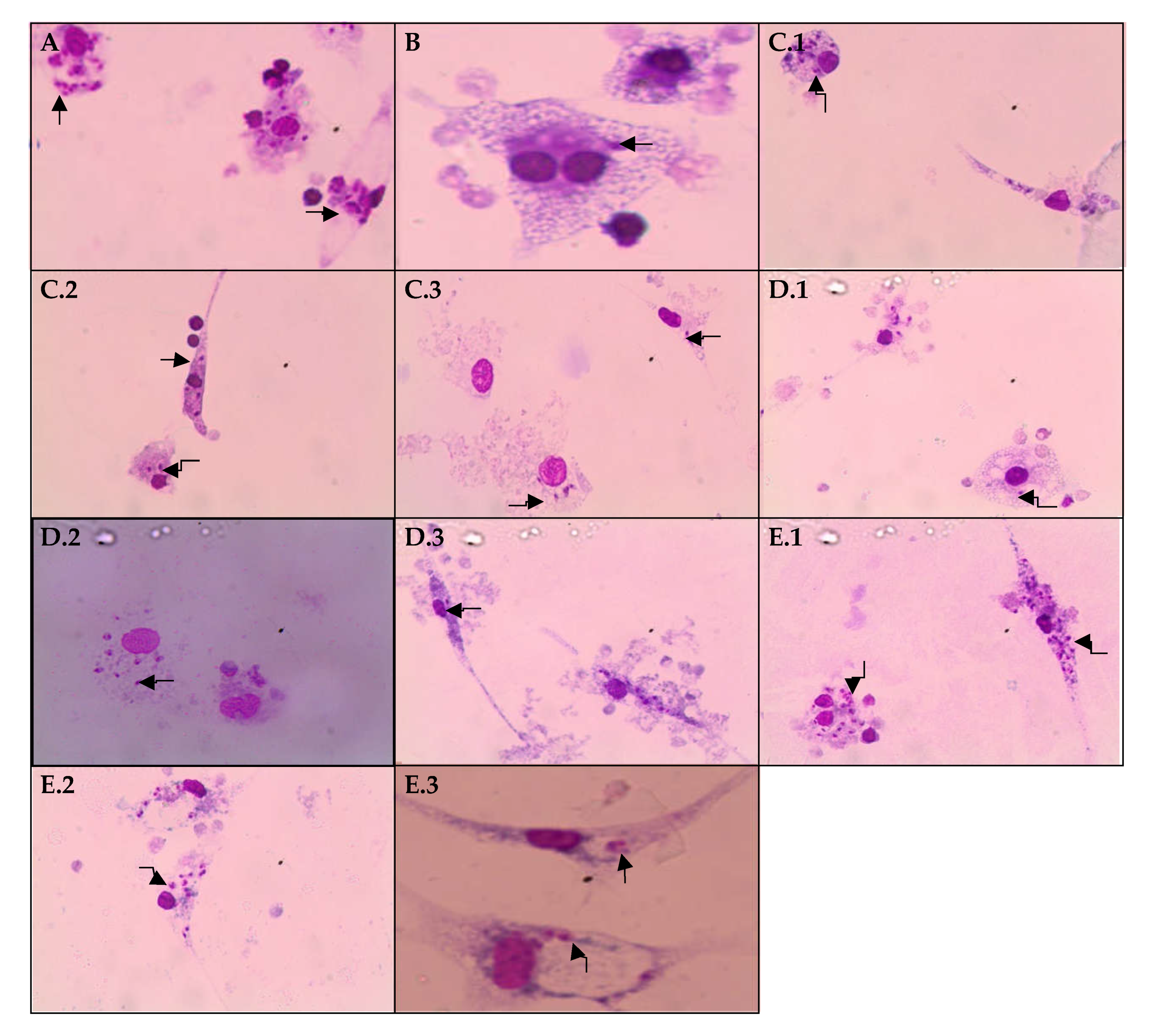
2.3. Effects of EHPi, FAcOEt and MB on L. amazonensis Infection of Macrophages
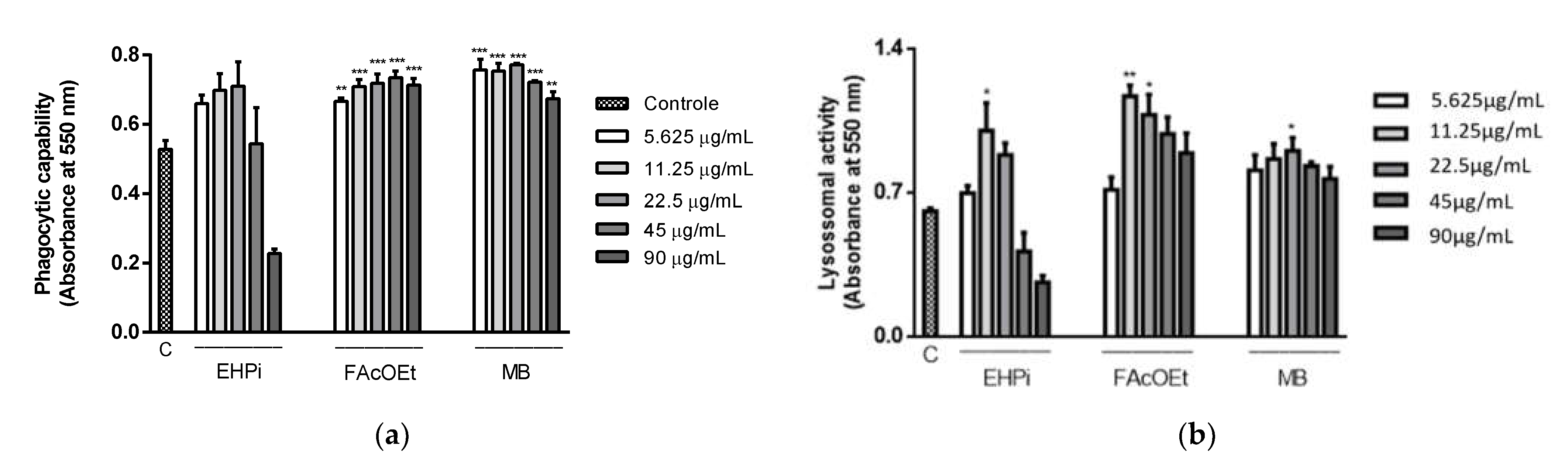
2.4. Determination of Lysosomal Activity and Phagocytic Capacity
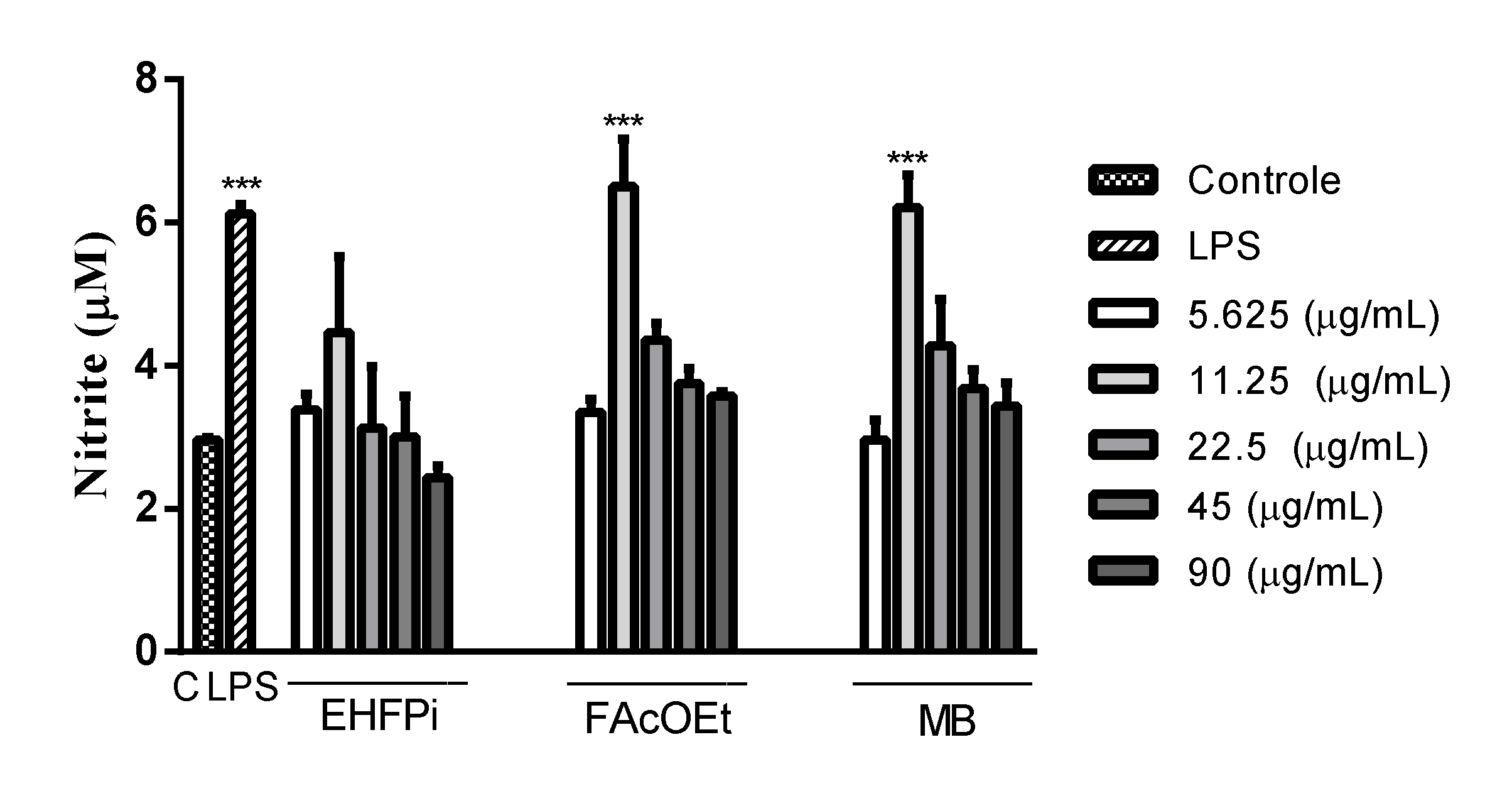
2.5. Determination of Nitrite Production
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Sample Preparation
4.3. Animals
4.4. Parasites and Cells
4.5. Antileishmanial Activity on Promastigotes
4.6. Determination of Cytotoxicity
4.6.1. Cytotoxicity on Murine Macrophages
4.6.2. Hemolytic Activity
4.6.3. Activity of EHPI, FAcOEt and MB on L. amazonensis-Infected Macrophages
4.7. Immunomodulatory Mechanisms of Macrophage Activation Induced by EHPi, FAcOEt and MB Samples
4.7.1. Lysosomal Activity
4.7.2. Evaluation of Phagocytic Capacity
4.7.3. Evaluation of Nitric Oxide Production
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Control of Neglected Tropical Diseases. Available online: https://www.who.int/news/item/27-05-2021-world-health-assembly-adopts-decision-to-recognize-30-january-as-world-ntd-day (accessed on 13 August 2021).
- World Health Organization. Global leishmaniasis Surveillance, 2017–2018, and First Report on 5 Additional Indicators. Control Negl. Trop. Dis. 2020, 95, 265–280. [Google Scholar]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 30 May 2021).
- Roatt, B.M.; de Oliveira Aguiar-Soares, R.D.; Coura-Vital, W.; Ker, H.G.; das Dores Moreira, N.; Vitoriano-Souza, J.; Giunchetti, R.C.; Carneiro, C.M.; Reis, A.B. Immunotherapy and immunochemotherapy in visceral leishmaniasis: Promising treatments for this neglected disease. Front. Immunol. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, I.M.; Carvalho, I.P.S.; Neto, J.A.T.; Lopes, G.L.N.; de Sousa Coêlho, E.; Sobrinho-Júnior, E.P.C.; de Moraes Alves, M.M.; de Amorim Carvalho, F.A.; Carvalho, A.L.M. Amphotericin B-loaded emulgel: Effect of chemical enhancers on the release profile and antileishmanial activity in vitro. AAPS PharmSciTech 2019, 20, 122. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Gilbert, B.; Villas-Bôas, G. Oportunidades para inovação no tratamento da leishmaniose usando o potencial das plantas e produtos naturais como fontes de novos fármacos. Rev. FITOS 2013, 8, 33–42. [Google Scholar] [CrossRef]
- Filho, V.C.; Yunes, R.A. Strategies for obtaining pharmacologically active compounds from medicinal plants. Concepts about structural modification for improve the activity. Quim. Nova 1998, 21, 99–105. [Google Scholar] [CrossRef]
- Lovkova, M.Y.; Buzuk, G.N.; Sokolova, S.M.; Kliment’eva, N.I. Chemical features of medicinal plants (review). Appl. Biochem. Microbiol. 2001, 37, 229–237. [Google Scholar] [CrossRef]
- Costa Júnior, J.S.; Ferraz, A.B.F.; Sousa, T.O.; Silva, R.A.C.; De Lima, S.G.; Feitosa, C.M.; Citó, A.M.G.L.; Melo Cavalcante, A.A.C.; Freitas, R.M.; Moura Sperotto, A.R.; et al. Investigation of biological activities of dichloromethane and ethyl acetate fractions of Platonia insignis Mart. seed. Basic Clin. Pharmacol. Toxicol. 2013, 112, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Costa Júnior, J.S.; Ferraz, A.B.F.; Filho, B.A.B.; Feitosa, C.M.; Citó, A.M.G.L.; Freitas, R.M.; Saffi, J. Evaluation of antioxidant effects in vitro of garcinielliptone FC (GFC) isolated from Platonia insignis mart. J. Med. Plants Res. 2011, 5, 293–299. [Google Scholar]
- Da Costa, J.S.; De Almeida, A.A.C.; da Rocha Tomé, A.; das Graças Lopes Citó, A.M.; Saffi, J.; De Freitas, R.M. Evaluation of possible antioxidant and anticonvulsant effects of the ethyl acetate fraction from Platonia insignis Mart. (Bacuri) on epilepsy models. Epilepsy Behav. 2011, 22, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Costa Júnior, J.S.; de Almeida, A.A.C.; Ferraz, A.D.B.F.; Rossatto, R.R.; Silva, T.G.; Silva, P.B.N.; Militão, G.C.G.; Citó, A.M.D.G.L.; Santana, L.C.L.R.; Carvalho, F.A.D.A.; et al. Cytotoxic and leishmanicidal properties of garcinielliptone FC, a prenylated benzophenone from Platonia insignis. Nat. Prod. Res. 2013, 27, 470–474. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; Arcanjo, D.D.R.; Ribeiro, R.G.; Rodrigues, K.A.F.; Passos, F.F.B.; Piauilino, C.A.; Silva-Filho, J.C.; Araújo, B.Q.; Lima-Neto, J.S.; Costa-Júnior, J.S.; et al. Immunomodulatory and toxicological evaluation of the fruit seeds from Platonia insignis, a native species from Brazilian Amazon rainforest. Rev. Bras. Farmacogn. 2016, 26, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Rufino, S.M.; Alves, R.E.; De Brito, E.S.; Pérez-jiménez, J.; Saura-calixto, F.; Mancini-filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). Fruit Trees and Useful Plants in Amazonian Life: Non-Wood Forest Products, 20; Center for International Forestry Research (CIFOR) and People and Plants International (PPI): Rome, Italy, 2011; Volume 53, ISBN 9788578110796. Available online: http://www.fao.org/3/i2360e/i2360e.pdf (accessed on 30 August 2021).
- e Silva, A.K.F.; dos Reis, A.C.; Pinheiro, E.E.A.; de Sousa, J.N.; de Alcântara Oliveira, F.A.; Moura, A.K.S.; de Sousa, L.; Neto, J.; das Graças, L.; Citó, A.M.; et al. Modulation of the drug resistance by platonia insignis mart. extract, ethyl acetate fraction and morelloflavone/volkensiflavone (Biflavonoids) in staphylococcus aureus strains overexpressing efflux pump genes. Curr. Drug Metab. 2021, 22, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Anderson, H.; Flavin, M.T.; Pai, Y.H.S.; Mata-Greenwood, E.; Pengsuparp, T.; Pezzuto, J.M.; Schinazi, R.F.; Hughes, S.H.; Chen, F.C. In Vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997, 60, 884–888. [Google Scholar] [CrossRef]
- Gontijo, V.S.; De Souza, T.C.; Rosa, I.A.; Soares, M.G.; Da Silva, M.A.; Vilegas, W.; Viegas, C.; Dos Santos, M.H. Isolation and evaluation of the antioxidant activity of phenolic constituents of the Garcinia brasiliensis epicarp. Food Chem. 2012, 132, 1230–1235. [Google Scholar] [CrossRef] [Green Version]
- Otuki, M.F.; Bernardi, C.A.; Prudente, A.S.; Laskoski, K.; Gomig, F.; Horinouchi, C.D.S.; Guimarães, C.L.; Ferreira, J.; Delle-Monache, F.; Cechinel-Filho, V.; et al. Garcinia gardneriana (Planchon & Triana) Zappi. (Clusiaceae) as a topical anti-inflammatory alternative for cutaneous inflammation. Basic Clin. Pharmacol. Toxicol. 2011, 109, 56–62. [Google Scholar] [CrossRef]
- Verdi, L.G.; Pizzolatti, M.G.; Montanher, A.B.P.; Brighente, I.M.C.; Smânia Júnior, A.; Smânia, E.D.F.A.; Simionatto, E.L.; Monache, F.D. Antibacterial and brine shrimp lethality tests of biflavonoids and derivatives of Rheedia gardneriana. Fitoterapia 2004, 75, 360–363. [Google Scholar] [CrossRef]
- Tuansulong, K.A.; Hutadilok-Towatana, N.; Mahabusarakam, W.; Pinkaew, D.; Fujise, K. Morelloflavone from Garcinia dulcis as a novel biflavonoid inhibitor of HMG-CoA reductase. Phyther. Res. 2011, 25, 424–428. [Google Scholar] [CrossRef]
- Alves, M.M.M.; Brito, L.M.; Souza, A.C.; Queiroz, B.C.S.H.; de Carvalho, T.P.; Batista, J.F.; Oliveira, J.S.S.M.; de Mendonça, I.L.; Lira, S.R.S.; Chaves, M.H.; et al. Gallic and ellagic acids: Two natural immunomodulator compounds solve infection of macrophages by Leishmania major. Naunyn. Schmiedebergs. Arch. Pharmacol. 2017, 390, 893–903. [Google Scholar] [CrossRef]
- Carvalho, C.E.S.; Sobrinho-Junior, E.P.C.; Brito, L.M.; Nicolau, L.A.D.; Carvalho, T.P.; Moura, A.K.S.; Rodrigues, K.A.F.; Carneiro, S.M.P.; Arcanjo, D.D.R.; Citó, A.M.G.L.; et al. Anti-Leishmania activity of essential oil of Myracrodruon urundeuva (Engl.) Fr. All.: Composition, cytotoxity and possible mechanisms of action. Exp. Parasitol. 2017, 175, 59–67. [Google Scholar] [CrossRef]
- Silva, T.; Oliveira, A. Leishmanicidal plants from Brazilian Amazonia: A review. Rev. Fitos 2016, 10, 339–363. [Google Scholar] [CrossRef]
- Frescura, V.D.S.; Kuhn, A.W.; Laughinghouse IV, H.D.; Paranhos, J.T.; Tedesco, S.B. Post-treatment with plant extracts used in Brazilian folk medicine caused a partial reversal of the antiproliferative effect of glyphosate in the Allium cepa test. Biocell 2013, 37, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Vale, L.; Calou, B.F.; De Deus, M.; Socorro, M.; Pinheiro, P.M.; Paula, A. Flavonoids: Chemical composition, medical actions and toxicity. Acta Toxicológica Argent. 2015, 23, 36–43. [Google Scholar]
- Silva, F.; Sales, M.; Sá, O.; Santana, G.; Deus, M.; Sousa, J.; Ferreira, P.; Peron, A. Potencial citotóxico, genotóxico e citoprotetor de extratos aquosos de Caesalpinia Pyramidalis tul., Caesalpinia Ferrea Mart. e Caesalpinia Pulcherrima Sw. Rev. Bras. Biociências 2015, 101–109. [Google Scholar]
- Wagner, H. Search for potent immunomodulatory agents from plants and other sources. In Bioassay Methods in Natural Product Research and Drug Development; Springer: Dordrecht, The Netherlands, 1999; pp. 113–118. [Google Scholar] [CrossRef]
- Islamuddin, M.; Chouhan, G.; Farooque, A.; Dwarakanath, B.S.; Sahal, D.; Afrin, F. Th1-Biased immunomodulation and therapeutic potential of artemisia annua in murine visceral leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchieri, G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ). Curr. Opin. Immunol. 1997, 9, 17–23. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Wei, W.C.; Su, Y.H.; Chen, S.S.; Sheu, J.H.; Yang, N.S. GM-CSF plays a key role in zymosan-stimulated human dendritic cells for activation of Th1 and Th17 cells. Cytokine 2011, 55, 79–89. [Google Scholar] [CrossRef]
- Krishnatry, A.S.; Brazeau, D.A.; Fung, H.L. Broad regulation of matrix and adhesion molecules in THP-1 human macrophages by nitroglycerin. Nitric Oxide—Biol. Chem. 2010, 22, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Melo Neto, B.; Leitão, J.M.S.R.; Oliveira, L.G.C.; Santos, S.E.M.; Carneiro, S.M.P.; Rodrigues, K.A.F.; Chaves, M.H.; Arcanjo, D.D.R.; Carvalho, F.A.A. Inhibitory effects of Zanthoxylum rhoifolium lam. (Rutaceae) against the infection and infectivity of macrophages by Leishmania amazonensis. An. Acad. Bras. Ciências 2016, 88, 1851–1861. [Google Scholar] [CrossRef] [Green Version]
- Kayser, O.; Kiderlen, A.F.; Croft, S.L. Natural products as antiparasitic drugs. Parasitol. Res. 2003, 90, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Chatterjee, M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine 2011, 18, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.M.P.; Carvalho, F.A.A.; Santana, L.C.L.R.; Sousa, A.P.L.; Neto, J.M.M.; Chaves, M.H. The cytotoxic and antileishmanial activity of extracts and fractions of leaves and fruits of Azadirachta indica (A Juss.). Biol. Res. 2012, 45, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Valadares, D.G.; Duarte, M.C.; Oliveira, J.S.; Chávez-Fumagalli, M.A.; Martins, V.T.; Costa, L.E.; Leite, J.P.V.; Santoro, M.M.; Régis, W.C.B.; Tavares, C.A.P.; et al. Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitol. Int. 2011, 60, 357–363. [Google Scholar] [CrossRef]
- De Medeiros, M.D.G.F.; da Silva, A.C.; Citó, A.M.D.G.L.; Borges, A.R.; de Lima, S.G.; Lopes, J.A.D.; Figueiredo, R.C.B.Q. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011, 60, 237–241. [Google Scholar] [CrossRef]
- Gonçalves, J.C.R.; Coulidiati, T.H.; Monteiro, A.L.; de Carvalho-Gonçalves, L.C.T.; de Oliveira Valença, W.; de Oliveira, R.N.; de Amorim Câmara, C.; de Araújo, D.A.M. Antitumoral activity of novel 1,4-naphthoquinone derivative involves L-type calcium channel activation in human colorectal cancer cell line. J. Appl. Biomed. 2016, 14, 229–234. [Google Scholar] [CrossRef]
- Löfgren, S.E.; Miletti, L.C.; Steindel, M.; Bachère, E.; Barracco, M.A. Trypanocidal and leishmanicidal activities of different antimicrobial peptides (AMPs) isolated from aquatic animals. Exp. Parasitol. 2008, 118, 197–202. [Google Scholar] [CrossRef] [PubMed]
- De Castro Oliveira, L.G.; Brito, L.M.; de Moraes Alves, M.M.; Amorim, L.V.; Sobrinho-Júnior, E.P.C.; de Carvalho, C.E.S.; da Franca Rodrigues, K.A.; Arcanjo, D.D.R.; das Graças Lopes Citó, A.M.; de Amorim Carvalho, F.A. In Vitro Effects of the Neolignan 2,3-Dihydrobenzofuran Against Leishmania Amazonensis. Basic Clin. Pharmacol. Toxicol. 2017, 120, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bonatto, S.J.R.; Folador, A.; Aikawa, J.; Yamazaki, R.K.; Pizatto, N.; Oliveira, H.H.P.; Vecchi, R.; Curi, R.; Calder, P.C.; Fernandes, L.C. Lifelong exposure to dietary fish oil alters macrophage responses in Walker 256 tumor-bearing rats. Cell. Immunol. 2004, 231, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Grando, F.C.C.; Felício, C.A.; Twardowschy, A.; Paula, F.M.; Batista, V.G.; Fernandes, L.C.; Curi, R.; Nishiyama, A. Modulation of peritoneal macrophage activity by the saturation state of the fatty acid moiety of phosphatidylcholine. Braz. J. Med. Biol. Res. 2009, 42, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.; Pereira, C.G.; Meireles, M.Â.A.; Saraiva, E.M. Leishmanicidal activity of a supercritical fluid fraction obtained from Tabernaemontana catharinensis. Parasitol. Int. 2007, 56, 135–139. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Macrophages CC50 (μg/mL) | Red Cells CH50 (μg/mL) | Promastigotes EC50 (μg/mL) | Intramacrophage Amastigotes EC50 (μg/mL) SIm | |
|---|---|---|---|---|---|
| EHPi | 81.78 | NT | 30.05 | 6.08 | 38.55 |
| FacOEt | 159.67 | NT | 23.05 | 13.44 | 3.55 |
| MB | 134,28 | NT | 45.71 | 16.33 | 0.20 |
| Amph B | 8.75 | NT | 1.74 | 9.81 | 43.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, É.A.; Alves, M.M.d.M.; Lima, S.K.R.; Pinheiro, E.E.A.; Amorim, L.V.; Lima Neto, J.d.S.; Carvalho, F.A.d.A.; Citó, A.M.d.G.L.; Arcanjo, D.D.R. Biflavones from Platonia insignis Mart. Flowers Promote In Vitro Antileishmanial and Immunomodulatory Effects against Internalized Amastigote Forms of Leishmania amazonensis. Pathogens 2021, 10, 1166. https://doi.org/10.3390/pathogens10091166
Bezerra ÉA, Alves MMdM, Lima SKR, Pinheiro EEA, Amorim LV, Lima Neto JdS, Carvalho FAdA, Citó AMdGL, Arcanjo DDR. Biflavones from Platonia insignis Mart. Flowers Promote In Vitro Antileishmanial and Immunomodulatory Effects against Internalized Amastigote Forms of Leishmania amazonensis. Pathogens. 2021; 10(9):1166. https://doi.org/10.3390/pathogens10091166
Chicago/Turabian StyleBezerra, Érika Alves, Michel Mualém de Moraes Alves, Simone Kelly Rodrigues Lima, Emanuelly Elanny Andrade Pinheiro, Layane Valéria Amorim, José de Sousa Lima Neto, Fernando Aécio de Amorim Carvalho, Antônia Maria das Graças Lopes Citó, and Daniel Dias Rufino Arcanjo. 2021. "Biflavones from Platonia insignis Mart. Flowers Promote In Vitro Antileishmanial and Immunomodulatory Effects against Internalized Amastigote Forms of Leishmania amazonensis" Pathogens 10, no. 9: 1166. https://doi.org/10.3390/pathogens10091166
APA StyleBezerra, É. A., Alves, M. M. d. M., Lima, S. K. R., Pinheiro, E. E. A., Amorim, L. V., Lima Neto, J. d. S., Carvalho, F. A. d. A., Citó, A. M. d. G. L., & Arcanjo, D. D. R. (2021). Biflavones from Platonia insignis Mart. Flowers Promote In Vitro Antileishmanial and Immunomodulatory Effects against Internalized Amastigote Forms of Leishmania amazonensis. Pathogens, 10(9), 1166. https://doi.org/10.3390/pathogens10091166







