The Structural Biology of Eastern Equine Encephalitis Virus, an Emerging Viral Threat
Abstract
:1. Introduction
2. Overview of Alphavirus Infection and Assembly Cycles
3. SINV-EEEV Chimera and EEEV Virus-Like Particles (VLPs) for Structural Analyses
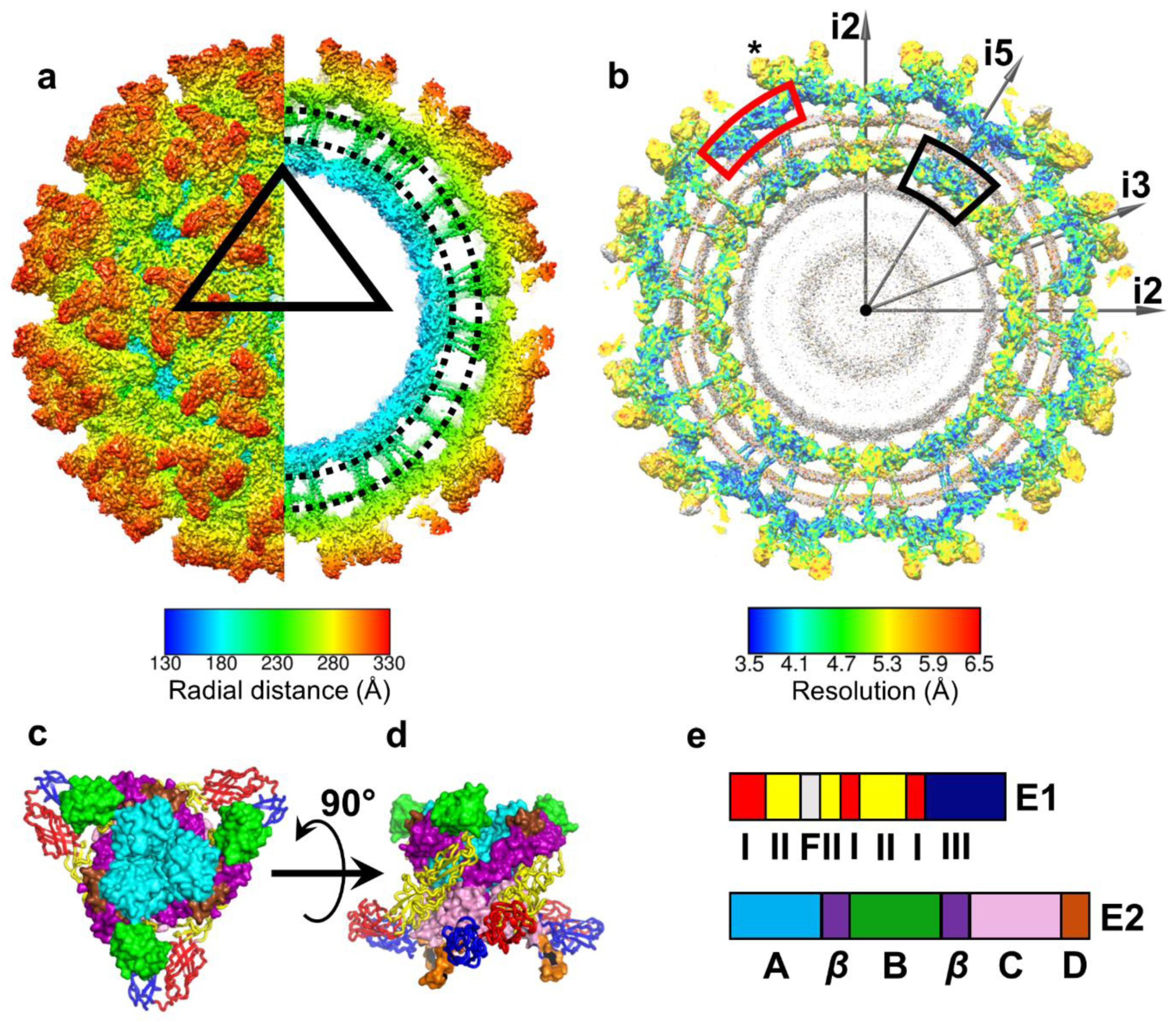
4. E1 and E2 Glycoproteins: Tools to Penetrate Host Membranes
5. A Multifunctional Capsid Protein
6. Structural Basis of Heparan Sulfate (HS) Binding in EEEV
7. Glycosylation of E1 and E2 Proteins in EEEV
8. Structural Basis of EEEV Neutralization by Monoclonal Antibodies
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Suhrbier, A.; Jaffar-Bandjee, M.C.; Gasque, P. Arthritogenic alphaviruses—An overview. Nat. Rev. Rheumatol. 2012, 8, 420–429. [Google Scholar] [CrossRef]
- Levi, L.I.; Vignuzzi, M. Arthritogenic alphaviruses: A worldwide emerging threat? Microorganisms 2019, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Lwande, O.W.; Obanda, V.; Bucht, G.; Mosomtai, G.; Otieno, V.; Ahlm, C.; Evander, M. Global emergence of alphaviruses that cause arthritis in humans. Infect. Ecol. Epidemiol. 2015, 5, 29853. [Google Scholar] [CrossRef] [Green Version]
- Rulli, N.E.; Melton, J.; Wilmes, A.; Ewart, G.; Mahalingam, S. The molecular and cellular aspects of arthritis due to alphavirus infections: Lesson learned from Ross River virus. Ann. N. Y. Acad. Sci. 2007, 1102, 96–108. [Google Scholar] [CrossRef]
- Zacks, M.A.; Paessler, S. Encephalitic alphaviruses. Vet. Microbiol. 2010, 140, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giltner, L.T.; Shahan, M.S. The immunological relationship of Eastern and Western strains of Equine encephalomyelitis virus. Science 1933, 78, 587–588. [Google Scholar] [CrossRef] [PubMed]
- WHO. Outbreak news. Chikungunya and dengue, south-west Indian Ocean. Wkly. Epidemiol. Rec. 2006, 81, 106–108. [Google Scholar]
- Armstrong, P.M.; Andreadis, T.G. Eastern equine encephalitis virus-Old enemy, new threat. N. Engl. J. Med. 2013, 368, 1670–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayres, J.C.; Feemster, R.F. The sequelae of Eastern equine encephalomyelitis. N. Engl. J. Med. 1949, 240, 960–962. [Google Scholar] [CrossRef]
- Lindsey, N.P.; Staples, J.E.; Fischer, M. Eastern equine encephalitis virus in the United States, 2003–2016. Am. J. Trop. Med. Hyg. 2018, 98, 1472–1477. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, N.P.; Martin, S.W.; Staples, J.E.; Fischer, M. Notes from the field: Multistate outbreak of Eastern equine encephalitis virus-United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 50–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreadis, T.G.; Anderson, J.F.; Tirrell-Peck, S.J. Multiple isolations of Eastern equine encephalitis and highlands J viruses from mosquitoes (Diptera: Culicidae) during a 1996 epizootic in southeastern Connecticut. J. Med. Entomol. 1998, 35, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.; Tan, Y.; Haight, J.D.; Tober, K.J.; Gall, W.K.; Zink, S.D.; Kramer, L.D.; Campbell, S.R.; Howard, J.J.; Das, S.R.; et al. Spatial and temporal expansions of Eastern equine encephalitis virus and phylogenetic groups isolated from mosquitoes and mammalian cases in New York State from 2013 to 2019. Emerg. Microbes Infect. 2020, 9, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.A.; Misasi, J.; Smole, S.; Feldman, H.A.; Cohen, A.B.; Santagata, S.; McManus, M.; Ahmed, A.A. Eastern equine encephalitis in children, Massachusetts and New Hampshire, USA, 1970–2010. Emerg. Infect. Dis. 2013, 19, 194–201. [Google Scholar] [CrossRef] [PubMed]
- CDC. Eastern Equine Encephalitis (EEE). Available online: https://www.cdc.gov/easternequineencephalitis/tech/epi.html (accessed on 1 June 2021).
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. Eastern equine encephalitis virus-Another emergent arbovirus in the United States. N. Engl. J. Med. 2019, 381, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.A.; Stehman, S.V.; Howard, J.J.; Oliver, J. Cases of Eastern equine encephalitis in humans associated with Aedes canadensis, Coquillettidia perturbans and Culiseta melanura mosquitoes with the virus in New York State from 1971 to 2012 by analysis of aggregated published data. Epidemiol. Infect. 2020, 148, e72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, S.C.; Scott, T.W.; Lorenz, L.H.; Repik, P.M. Detection of Eastern equine encephalomyelitis virus deposition in Culiseta melanura following ingestion of radiolabeled virus in blood meals. Am. J. Trop. Med. Hyg. 1991, 44, 250–259. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Niebylski, M.L.; Smith, G.C.; Karabatsos, N.; Martin, D.; Mutebi, J.P.; Craig, G.B., Jr.; Mahler, M.J. Isolation of Eastern equine encephalitis virus from Aedes albopictus in Florida. Science 1992, 257, 526–527. [Google Scholar] [CrossRef]
- Ronca, S.E.; Dineley, K.T.; Paessler, S. Neurological sequelae resulting from encephalitic alphavirus infection. Front. Microbiol. 2016, 7, 959. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.J.; Woods, C.W.; Welty-Wolf, K.E. Eastern equine encephalitis leading to multi-organ failure and sepsis. J. Clin. Virol. 2008, 42, 418–421. [Google Scholar] [CrossRef]
- Carrera, J.P.; Forrester, N.; Wang, E.; Vittor, A.Y.; Haddow, A.D.; Lopez-Verges, S.; Abadia, I.; Castano, E.; Sosa, N.; Baez, C.; et al. Eastern equine encephalitis in Latin America. N. Engl. J. Med. 2013, 369, 732–744. [Google Scholar] [CrossRef] [Green Version]
- Sidwell, R.W.; Smee, D.F. Viruses of the Bunya- and Togaviridae families: Potential as bioterrorism agents and means of control. Antiviral Res. 2003, 57, 101–111. [Google Scholar] [CrossRef]
- CDC-USDA. HHS and USDA Select Agents and Toxins. Available online: https://www.selectagents.gov/sat/list.htm (accessed on 1 June 2021).
- Reichert, E.; Clase, A.; Bacetty, A.; Larsen, J. Alphavirus antiviral drug development: Scientific gap analysis and prospective research areas. Biosecur. Bioterror 2009, 7, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh, D.W.; Sun, C.; Dunn, M.D.; Reed, D.S.; Klimstra, W.B. Rational design of a live-attenuated Eastern equine encephalitis virus vaccine through informed mutation of virulence determinants. PLoS Pathog. 2019, 15, e1007584. [Google Scholar] [CrossRef]
- Strauss, E.G.; Rice, C.M.; Strauss, J.H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology 1984, 133, 92–110. [Google Scholar] [CrossRef]
- Faragher, S.G.; Meek, A.D.; Rice, C.M.; Dalgarno, L. Genome sequences of a mouse-avirulent and a mouse-virulent strain of Ross River virus. Virology 1988, 163, 509–526. [Google Scholar] [CrossRef]
- Garoff, H.; Frischauf, A.M.; Simons, K.; Lehrach, H.; Delius, H. Nucleotide sequence of cDNA coding for Semliki Forest virus membrane glycoproteins. Nature 1980, 288, 236–241. [Google Scholar] [CrossRef]
- Chang, G.J.; Trent, D.W. Nucleotide sequence of the genome region encoding the 26S mRNA of Eastern equine encephalomyelitis virus and the deduced amino acid sequence of the viral structural proteins. J. Gen. Virol. 1987, 68 Pt 8, 2129–2142. [Google Scholar] [CrossRef]
- J H Strauss, A.; Strauss, E.G. Evolution of RNA viruses. Annu. Rev. Microbiol. 1988, 42, 657–683. [Google Scholar] [CrossRef]
- Hasan, S.S.; Sun, C.; Kim, A.S.; Watanabe, Y.; Chen, C.L.; Klose, T.; Buda, G.; Crispin, M.; Diamond, M.S.; Klimstra, W.B.; et al. Cryo-EM structures of Eastern equine encephalitis virus reveal mechanisms of virus disassembly and antibody neutralization. Cell Rep. 2018, 25, 3136–3147.e3135. [Google Scholar] [CrossRef] [Green Version]
- Leung, J.Y.; Ng, M.M.; Chu, J.J. Replication of alphaviruses: A review on the entry process of alphaviruses into cells. Adv. Virol. 2011, 2011, 249640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, J.; Snyder, J.E.; Kuhn, R.J. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.S.; Austin, S.K.; Gardner, C.L.; Zuiani, A.; Reed, D.S.; Trobaugh, D.W.; Sun, C.; Basore, K.; Williamson, L.E.; Crowe, J.E., Jr.; et al. Protective antibodies against Eastern equine encephalitis virus bind to epitopes in domains A and B of the E2 glycoprotein. Nat. Microbiol. 2019, 4, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Akahata, W.; Yang, E.S.; Kong, W.P.; Burke, C.W.; Honnold, S.P.; Nichols, D.K.; Huang, Y.S.; Schieber, G.L.; Carlton, K.; et al. A virus-like particle vaccine prevents equine encephalitis virus infection in nonhuman primates. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Williamson, L.E.; Gilliland, T., Jr.; Yadav, P.K.; Binshtein, E.; Bombardi, R.; Kose, N.; Nargi, R.S.; Sutton, R.E.; Durie, C.L.; Armstrong, E.; et al. Human antibodies protect against aerosolized Eastern equine encephalitis virus infection. Cell 2020, 183, 1884–1900.e1823. [Google Scholar] [CrossRef]
- Kostyuchenko, V.A.; Jakana, J.; Liu, X.; Haddow, A.D.; Aung, M.; Weaver, S.C.; Chiu, W.; Lok, S.M. The structure of Barmah Forest virus as revealed by cryo-electron microscopy at a 6Å resolution has detailed transmembrane protein architecture and interactions. J. Virol. 2011, 85, 9327–9333. [Google Scholar] [CrossRef] [Green Version]
- Mancini, E.J.; Clarke, M.; Gowen, B.E.; Rutten, T.; Fuller, S.D. Cryo-electron microscopy reveals the functional organization of an enveloped virus, Semliki Forest virus. Mol. Cell 2000, 5, 255–266. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Zhang, W.; Gabler, S.; Chipman, P.R.; Strauss, E.G.; Strauss, J.H.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Mapping the structure and function of the E1 and E2 glycoproteins in alphaviruses. Structure 2006, 14, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Sherman, M.B.; Weaver, S.C. Structure of the recombinant alphavirus Western equine encephalitis virus revealed by cryoelectron microscopy. J. Virol. 2010, 84, 9775–9782. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.J.; Cheng, R.H.; Olson, N.H.; Peterson, P.; Chase, E.; Kuhn, R.J.; Baker, T.S. Putative receptor binding sites on alphaviruses as visualized by cryoelectron microscopy. Proc. Natl. Acad. Sci. USA 1995, 92, 10648–10652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Xiang, Y.; Akahata, W.; Holdaway, H.; Pal, P.; Zhang, X.; Diamond, M.S.; Nabel, G.J.; Rossmann, M.G. Structural analyses at pseudo atomic resolution of Chikungunya virus and antibodies show mechanisms of neutralization. eLife 2013, 2, e00435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mukhopadhyay, S.; Pletnev, S.V.; Baker, T.S.; Kuhn, R.J.; Rossmann, M.G. Placement of the structural proteins in Sindbis virus. J. Virol. 2002, 76, 11645–11658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Heil, M.; Kuhn, R.J.; Baker, T.S. Heparin binding sites on Ross River virus revealed by electron cryo-microscopy. Virology 2005, 332, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Hryc, C.F.; Cong, Y.; Liu, X.; Jakana, J.; Gorchakov, R.; Baker, M.L.; Weaver, S.C.; Chiu, W. 4.4 Å cryo-EM structure of an enveloped alphavirus Venezuelan equine encephalitis virus. EMBO J. 2011, 30, 3854–3863. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-Filho, H.V.; Coimbra, L.D.; Cassago, A.; Rocha, R.P.F.; Guerra, J.; de Felicio, R.; Carnieli, C.M.; Leme, L.; Padilha, A.C.; Paes Leme, A.F.; et al. Cryo-EM structure of the mature and infective Mayaro virus at 4.4 Å resolution reveals features of arthritogenic alphaviruses. Nat. Commun. 2021, 12, 3038. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.H.; Kuhn, R.J.; Olson, N.H.; Rossmann, M.G.; Choi, H.K.; Smith, T.J.; Baker, T.S. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 1995, 80, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Forsell, K.; Xing, L.; Kozlovska, T.; Cheng, R.H.; Garoff, H. Membrane proteins organize a symmetrical virus. EMBO J. 2000, 19, 5081–5091. [Google Scholar] [CrossRef]
- Choi, H.K.; Tong, L.; Minor, W.; Dumas, P.; Boege, U.; Rossmann, M.G.; Wengler, G. Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature 1991, 354, 37–43. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Vaney, M.C.; Roussel, A.; Vigouroux, A.; Reilly, B.; Lepault, J.; Kielian, M.; Rey, F.A. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature 2004, 427, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Lescar, J.; Roussel, A.; Wien, M.W.; Navaza, J.; Fuller, S.D.; Wengler, G.; Wengler, G.; Rey, F.A. The fusion glycoprotein shell of Semliki Forest virus: An icosahedral assembly primed for fusogenic activation at endosomal pH. Cell 2001, 105, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jose, J.; Xiang, Y.; Kuhn, R.J.; Rossmann, M.G. Structural changes of envelope proteins during alphavirus fusion. Nature 2010, 468, 705–708. [Google Scholar] [CrossRef]
- Voss, J.E.; Vaney, M.C.; Duquerroy, S.; Vonrhein, C.; Girard-Blanc, C.; Crublet, E.; Thompson, A.; Bricogne, G.; Rey, F.A. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 2010, 468, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Haag, L.; Garoff, H.; Xing, L.; Hammar, L.; Kan, S.T.; Cheng, R.H. Acid-induced movements in the glycoprotein shell of an alphavirus turn the spikes into membrane fusion mode. EMBO J. 2002, 21, 4402–4410. [Google Scholar] [CrossRef] [Green Version]
- Wahlberg, J.M.; Bron, R.; Wilschut, J.; Garoff, H. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 1992, 66, 7309–7318. [Google Scholar] [CrossRef] [Green Version]
- Byrnes, A.P.; Griffin, D.E. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 1998, 72, 7349–7356. [Google Scholar] [CrossRef] [Green Version]
- Klimstra, W.B.; Ryman, K.D.; Johnston, R.E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 1998, 72, 7357–7366. [Google Scholar] [CrossRef] [Green Version]
- Omar, A.; Koblet, H. Semliki Forest virus particles containing only the E1 envelope glycoprotein are infectious and can induce cell-cell fusion. Virology 1988, 166, 17–23. [Google Scholar] [CrossRef]
- Wahlberg, J.M.; Garoff, H. Membrane fusion process of Semliki Forest virus. I: Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J. Cell Biol. 1992, 116, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjoberg, M.; Lindqvist, B.; Garoff, H. Activation of the alphavirus spike protein is suppressed by bound E3. J. Virol. 2011, 85, 5644–5650. [Google Scholar] [CrossRef] [Green Version]
- Bonatti, S.; Blobel, G. Absence of a cleavable signal sequence in Sindbis virus glycoprotein PE2. J. Biol. Chem. 1979, 254, 12261–12264. [Google Scholar] [CrossRef]
- Bonatti, S.; Migliaccio, G.; Blobel, G.; Walter, P. Role of signal recognition particle in the membrane assembly of Sindbis viral glycoproteins. Eur. J. Biochem. 1984, 140, 499–502. [Google Scholar] [CrossRef]
- Firth, A.E.; Chung, B.Y.; Fleeton, M.N.; Atkins, J.F. Discovery of frameshifting in alphavirus 6K resolves a 20-year enigma. Virol. J. 2008, 5, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liljestrom, P.; Garoff, H. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J. Virol. 1991, 65, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.R.; Haag, L.; Hammar, L.; Wu, B.; Garoff, H.; Xing, L.; Murata, K.; Cheng, R.H. The dynamic envelope of a fusion class II virus. Prefusion stages of Semliki Forest virus revealed by electron cryomicroscopy. J. Biol. Chem. 2007, 282, 6752–6762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Zhang, W. Characterization of an early-stage fusion intermediate of Sindbis virus using cryoelectron microscopy. Proc. Natl. Acad. Sci. USA 2013, 110, 13362–13367. [Google Scholar] [CrossRef] [Green Version]
- Paredes, A.M.; Ferreira, D.; Horton, M.; Saad, A.; Tsuruta, H.; Johnston, R.; Klimstra, W.; Ryman, K.; Hernandez, R.; Chiu, W.; et al. Conformational changes in Sindbis virions resulting from exposure to low pH and interactions with cells suggest that cell penetration may occur at the cell surface in the absence of membrane fusion. Virology 2004, 324, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Klimjack, M.R.; Jeffrey, S.; Kielian, M. Membrane and protein interactions of a soluble form of the Semliki Forest virus fusion protein. J. Virol. 1994, 68, 6940–6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, D.L.; Erk, I.; Reilly, B.; Navaza, J.; Kielian, M.; Rey, F.A.; Lepault, J. Visualization of the target-membrane-inserted fusion protein of Semliki Forest virus by combined electron microscopy and crystallography. Cell 2003, 114, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Fields, W.; Kielian, M. A key interaction between the alphavirus envelope proteins responsible for initial dimer dissociation during fusion. J. Virol. 2013, 87, 3774–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.L.; Zheng, Y.; Kielian, M. Role of conserved histidine residues in the low-pH dependence of the Semliki Forest virus fusion protein. J. Virol. 2009, 83, 4670–4677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, B.; Gudigamolla, N.K.; Chowdary, T.K. Acidic pH-induced conformational changes in Chikungunya virus fusion protein E1: A spring-twisted region in the domain I–III linker acts as a hinge point for swiveling motion of domains. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Zheng, Y.; Sanchez-San Martin, C.; Qin, Z.L.; Kielian, M. The domain I-domain III linker plays an important role in the fusogenic conformational change of the alphavirus membrane fusion protein. J. Virol. 2011, 85, 6334–6342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Duijl-Richter, M.K.; Hoornweg, T.E.; Rodenhuis-Zybert, I.A.; Smit, J.M. Early events in Chikungunya virus infection-From virus cell binding to membrane fusion. Viruses 2015, 7, 3647–3674. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Moore, A.C.; Kolokoltsov, A.A.; Davey, R.A. Venezuelan equine encephalitis virus infection of mosquito cells requires acidification as well as mosquito homologs of the endocytic proteins Rab5 and Rab7. Virology 2007, 369, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Marsh, M.; Bolzau, E.; Helenius, A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell 1983, 32, 931–940. [Google Scholar] [CrossRef]
- Kolokoltsov, A.A.; Fleming, E.H.; Davey, R.A. Venezuelan equine encephalitis virus entry mechanism requires late endosome formation and resists cell membrane cholesterol depletion. Virology 2006, 347, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Rey, F.A.; Heinz, F.X.; Mandl, C.; Kunz, C.; Harrison, S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 1995, 375, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Roussel, A.; Lescar, J.; Vaney, M.C.; Wengler, G.; Wengler, G.; Rey, F.A. Structure and interactions at the viral surface of the envelope protein E1 of Semliki Forest virus. Structure 2006, 14, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Linger, B.R.; Kunovska, L.; Kuhn, R.J.; Golden, B.L. Sindbis virus nucleocapsid assembly: RNA folding promotes capsid protein dimerization. RNA 2004, 10, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Owen, K.E.; Choi, H.K.; Lee, H.; Lu, G.; Wengler, G.; Brown, D.T.; Rossmann, M.G.; Kuhn, R.J. Identification of a protein binding site on the surface of the alphavirus nucleocapsid and its implication in virus assembly. Structure 1996, 4, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Owen, K.E.; Kuhn, R.J. Identification of a region in the Sindbis virus nucleocapsid protein that is involved in specificity of RNA encapsidation. J. Virol. 1996, 70, 2757–2763. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.S.; Anastasakis, D.G.; Hafner, M.; Kielian, M. Multiple capsid protein binding sites mediate selective packaging of the alphavirus genomic RNA. Nat. Commun. 2020, 11, 4693. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. A decade and a half of protein intrinsic disorder: Biology still waits for physics. Protein. Sci. 2013, 22, 693–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Jose, J.; Chipman, P.; Zhang, W.; Kuhn, R.J.; Baker, T.S. Molecular links between the E2 envelope glycoprotein and nucleocapsid core in Sindbis virus. J. Mol. Biol. 2011, 414, 442–459. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, M.; Zhu, D.; Sun, Z.; Ma, J.; Wang, J.; Kong, L.; Wang, S.; Liu, Z.; Wei, L.; et al. Implication for alphavirus host-cell entry and assembly indicated by a 3.5Å resolution cryo-EM structure. Nat. Commun. 2018, 9, 5326. [Google Scholar] [CrossRef] [Green Version]
- Pletnev, S.V.; Zhang, W.; Mukhopadhyay, S.; Fisher, B.R.; Hernandez, R.; Brown, D.T.; Baker, T.S.; Rossmann, M.G.; Kuhn, R.J. Locations of carbohydrate sites on alphavirus glycoproteins show that E1 forms an icosahedral scaffold. Cell 2001, 105, 127–136. [Google Scholar] [CrossRef]
- Wengler, G.; Wurkner, D.; Wengler, G. Identification of a sequence element in the alphavirus core protein which mediates interaction of cores with ribosomes and the disassembly of cores. Virology 1992, 191, 880–888. [Google Scholar] [CrossRef]
- Wengler, G.; Gros, C.; Wengler, G. Analyses of the role of structural changes in the regulation of uncoating and assembly of alphavirus cores. Virology 1996, 222, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Wengler, G. The regulation of disassembly of alphavirus cores. Arch. Virol. 2009, 154, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Wengler, G.; Wengler, G. Identification of a transfer of viral core protein to cellular ribosomes during the early stages of alphavirus infection. Virology 1984, 134, 435–442. [Google Scholar] [CrossRef]
- Tellinghuisen, T.L.; Hamburger, A.E.; Fisher, B.R.; Ostendorp, R.; Kuhn, R.J. In Vitro assembly of alphavirus cores by using nucleocapsid protein expressed in Escherichia coli. J. Virol. 1999, 73, 5309–5319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.C.; Chen, C.; Rayaprolu, V.; Mukhopadhyay, S.; Zlotnick, A. Self-assembly of an alphavirus core-like particle is distinguished by strong inter-subunit association energy and structural defects. ACS Nano 2015, 9, 8898–8906. [Google Scholar] [CrossRef] [Green Version]
- Lamb, K.; Lokesh, G.L.; Sherman, M.; Watowich, S. Structure of a Venezuelan equine encephalitis virus assembly intermediate isolated from infected cells. Virology 2010, 406, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Paredes, A.; Alwell-Warda, K.; Weaver, S.C.; Chiu, W.; Watowich, S.J. Structure of isolated nucleocapsids from Venezuelan equine encephalitis virus and implications for assembly and disassembly of enveloped virus. J. Virol. 2003, 77, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Chipman, P.R.; Hong, E.M.; Kuhn, R.J.; Rossmann, M.G. In Vitro-assembled alphavirus core-like particles maintain a structure similar to that of nucleocapsid cores in mature virus. J. Virol. 2002, 76, 11128–11132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enzmann, P.J.; Weiland, F. Separation of alphavirus nucleocapsids from envelope fragments. Z. Naturforsch C Biosci. 1980, 35, 145–149. [Google Scholar] [CrossRef]
- Button, J.M.; Mukhopadhyay, S. Removing the polyanionic cargo requirement for assembly of alphavirus core-like particles to make an empty alphavirus core. Viruses 2020, 12, 846. [Google Scholar] [CrossRef]
- Rayaprolu, V.; Moore, A.; Wang, J.C.; Goh, B.C.; Perilla, J.R.; Zlotnick, A.; Mukhopadhyay, S. Length of encapsidated cargo impacts stability and structure of in vitro assembled alphavirus core-like particles. J. Phys. Condens. Matter. 2017, 29, 484003. [Google Scholar] [CrossRef]
- Singh, I.; Helenius, A. Role of ribosomes in Semliki Forest virus nucleocapsid uncoating. J. Virol. 1992, 66, 7049–7058. [Google Scholar] [CrossRef] [Green Version]
- Esko, J.D.; Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Investig. 2001, 108, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.L.; Busch, S.J.; Cardin, A.D. Glycosaminoglycans: Molecular properties, protein interactions, and role in physiological processes. Physiol. Rev. 1991, 71, 481–539. [Google Scholar] [CrossRef] [PubMed]
- Ryman, K.D.; Gardner, C.L.; Burke, C.W.; Meier, K.C.; Thompson, J.M.; Klimstra, W.B. Heparan sulfate binding can contribute to the neurovirulence of neuroadapted and nonneuroadapted Sindbis viruses. J. Virol. 2007, 81, 3563–3573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, K.A.; Klimstra, W.B.; Johnston, R.E. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 2000, 276, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Heil, M.L.; Albee, A.; Strauss, J.H.; Kuhn, R.J. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J. Virol. 2001, 75, 6303–6309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, J.M.; Waarts, B.L.; Kimata, K.; Klimstra, W.B.; Bittman, R.; Wilschut, J. Adaptation of alphaviruses to heparan sulfate: Interaction of Sindbis and Semliki forest viruses with liposomes containing lipid-conjugated heparin. J. Virol. 2002, 76, 10128–10137. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.L.; Ebel, G.D.; Ryman, K.D.; Klimstra, W.B. Heparan sulfate binding by natural Eastern equine encephalitis viruses promotes neurovirulence. Proc. Natl. Acad. Sci. USA 2011, 108, 16026–16031. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.L.; Choi-Nurvitadhi, J.; Sun, C.; Bayer, A.; Hritz, J.; Ryman, K.D.; Klimstra, W.B. Natural variation in the heparan sulfate binding domain of the Eastern equine encephalitis virus E2 glycoprotein alters interactions with cell surfaces and virulence in mice. J. Virol. 2013, 87, 8582–8590. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.L.; Hasan, S.S.; Klose, T.; Sun, Y.; Buda, G.; Sun, C.; Klimstra, W.B.; Rossmann, M.G. Cryo-EM structure of Eastern equine encephalitis virus in complex with heparan sulfate analogues. Proc. Natl. Acad. Sci. USA 2020, 117, 8890–8899. [Google Scholar] [CrossRef]
- Mulloy, B.; Gray, E.; Barrowcliffe, T.W. Characterization of unfractionated heparin: Comparison of materials from the last 50 years. Thromb. Haemost 2000, 84, 1052–1056. [Google Scholar] [PubMed]
- Aksnes, I.; Markussen, T.; Braaen, S.; Rimstad, E. Mutation of N-glycosylation sites in salmonid alphavirus (SAV) envelope proteins attenuate the virus in cell culture. Viruses 2020, 12, 1071. [Google Scholar] [CrossRef]
- Hikke, M.C.; Braaen, S.; Villoing, S.; Hodneland, K.; Geertsema, C.; Verhagen, L.; Frost, P.; Vlak, J.M.; Rimstad, E.; Pijlman, G.P. Salmonid alphavirus glycoprotein E2 requires low temperature and E1 for virion formation and induction of protective immunity. Vaccine 2014, 32, 6206–6212. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.A.; Herrero, L.J.; Jeffery, J.A.L.; Hoehn, M.; Rudd, P.A.; Supramaniam, A.; Kay, B.H.; Ryan, P.A.; Mahalingam, S. Role of envelope N-linked glycosylation in Ross River virus virulence and transmission. J. Gen. Virol. 2016, 97, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.L.; Schultz, K.L.; Kent, R.J.; Venkatesan, M.; Griffin, D.E. Role of N-linked glycosylation for sindbis virus infection and replication in vertebrate and invertebrate systems. J. Virol. 2009, 83, 5640–5647. [Google Scholar] [CrossRef] [Green Version]
- Naim, H.Y.; Koblet, H. Investigation of the role of glycans for the biological activity of Semliki Forest virus grown in Aedes albopictus cells using inhibitors of asparagine-linked oligosaccharides trimming. Arch. Virol. 1988, 102, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, W.B.; Nangle, E.M.; Smith, M.S.; Yurochko, A.D.; Ryman, K.D. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 2003, 77, 12022–12032. [Google Scholar] [CrossRef] [Green Version]
- Engering, A.; Geijtenbeek, T.B.; van Vliet, S.J.; Wijers, M.; van Liempt, E.; Demaurex, N.; Lanzavecchia, A.; Fransen, J.; Figdor, C.G.; Piguet, V.; et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 2002, 168, 2118–2126. [Google Scholar] [CrossRef] [Green Version]
- Bashirova, A.A.; Geijtenbeek, T.B.; van Duijnhoven, G.C.; van Vliet, S.J.; Eilering, J.B.; Martin, M.P.; Wu, L.; Martin, T.D.; Viebig, N.; Knolle, P.A.; et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 2001, 193, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Soilleux, E.J.; Morris, L.S.; Lee, B.; Pohlmann, S.; Trowsdale, J.; Doms, R.W.; Coleman, N. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 2001, 195, 586–592. [Google Scholar] [CrossRef]
- Figdor, C.G.; van Kooyk, Y.; Adema, G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2002, 2, 77–84. [Google Scholar] [CrossRef]
- Curtis, B.M.; Scharnowske, S.; Watson, A.J. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 1992, 89, 8356–8360. [Google Scholar] [CrossRef] [Green Version]
- Feinberg, H.; Mitchell, D.A.; Drickamer, K.; Weis, W.I. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 2001, 294, 2163–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.A.; Fadden, A.J.; Drickamer, K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 2001, 276, 28939–28945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, C.M.; Strauss, J.H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc. Natl. Acad. Sci. USA 1981, 78, 2062–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naim, H.Y.; Koblet, H. Asparagine-linked oligosaccharides of Semliki Forest virus grown in mosquito cells. Arch. Virol. 1992, 122, 45–60. [Google Scholar] [CrossRef]
- Gardner, C.L.; Burke, C.W.; Tesfay, M.Z.; Glass, P.J.; Klimstra, W.B.; Ryman, K.D. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: Impact of altered cell tropism on pathogenesis. J. Virol. 2008, 82, 10634–10646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispin, M.; Harvey, D.J.; Bitto, D.; Bonomelli, C.; Edgeworth, M.; Scrivens, J.H.; Huiskonen, J.T.; Bowden, T.A. Structural plasticity of the Semliki Forest virus glycome upon interspecies transmission. J. Proteome Res. 2014, 13, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.A.; Fox, J.M.; Kose, N.; Kim, A.S.; Majedi, M.; Bombardi, R.; Carnahan, R.H.; Slaughter, J.C.; Morrison, T.E.; Diamond, M.S.; et al. Human monoclonal antibodies against Ross River virus target epitopes within the E2 protein and protect against disease. PLoS Pathog. 2020, 16, e1008517. [Google Scholar] [CrossRef]
- Earnest, J.T.; Basore, K.; Roy, V.; Bailey, A.L.; Wang, D.; Alter, G.; Fremont, D.H.; Diamond, M.S. Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity. J. Exp. Med. 2019, 216, 2282–2301. [Google Scholar] [CrossRef]
- Burke, C.W.; Froude, J.W.; Miethe, S.; Hulseweh, B.; Hust, M.; Glass, P.J. Human-like neutralizing antibodies protect mice from aerosol exposure with Western equine encephalitis virus. Viruses 2018, 10, 147. [Google Scholar] [CrossRef] [Green Version]
- Hulseweh, B.; Rulker, T.; Pelat, T.; Langermann, C.; Frenzel, A.; Schirrmann, T.; Dubel, S.; Thullier, P.; Hust, M. Human-like antibodies neutralizing Western equine encephalitis virus. MAbs 2014, 6, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Hunt, A.R.; Roehrig, J.T. Biochemical and biological characteristics of epitopes on the E1 glycoprotein of Western equine encephalitis virus. Virology 1985, 142, 334–346. [Google Scholar] [CrossRef]
- Broeckel, R.; Fox, J.M.; Haese, N.; Kreklywich, C.N.; Sukulpovi-Petty, S.; Legasse, A.; Smith, P.P.; Denton, M.; Corvey, C.; Krishnan, S.; et al. Therapeutic administration of a recombinant human monoclonal antibody reduces the severity of Chikungunya virus disease in rhesus macaques. PLoS Negl. Trop. Dis. 2017, 11, e0005637. [Google Scholar] [CrossRef]
- Fong, R.H.; Banik, S.S.; Mattia, K.; Barnes, T.; Tucker, D.; Liss, N.; Lu, K.; Selvarajah, S.; Srinivasan, S.; Mabila, M.; et al. Exposure of epitope residues on the outer face of the Chikungunya virus envelope trimer determines antibody neutralizing efficacy. J. Virol. 2014, 88, 14364–14379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, J.M.; Roy, V.; Gunn, B.M.; Huang, L.; Edeling, M.A.; Mack, M.; Fremont, D.H.; Doranz, B.J.; Johnson, S.; Alter, G.; et al. Optimal therapeutic activity of monoclonal antibodies against Chikungunya virus requires Fc-FcγR interaction on monocytes. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Jin, J.; Liss, N.M.; Chen, D.H.; Liao, M.; Fox, J.M.; Shimak, R.M.; Fong, R.H.; Chafets, D.; Bakkour, S.; Keating, S.; et al. Neutralizing monoclonal antibodies block Chikungunya virus entry and release by targeting an epitope critical to viral pathogenesis. Cell Rep. 2015, 13, 2553–2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kose, N.; Fox, J.M.; Sapparapu, G.; Bombardi, R.; Tennekoon, R.N.; de Silva, A.D.; Elbashir, S.M.; Theisen, M.A.; Humphris-Narayanan, E.; Ciaramella, G.; et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against Chikungunya infection. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Fong, R.H.; Austin, S.K.; Chen, Z.; Klose, T.; Fokine, A.; Liu, Y.; Porta, J.; Sapparapu, G.; Akahata, W.; et al. Cryo-EM structures elucidate neutralizing mechanisms of anti-chikungunya human monoclonal antibodies with therapeutic activity. Proc. Natl. Acad. Sci. USA 2015, 112, 13898–13903. [Google Scholar] [CrossRef] [Green Version]
- Pal, P.; Dowd, K.A.; Brien, J.D.; Edeling, M.A.; Gorlatov, S.; Johnson, S.; Lee, I.; Akahata, W.; Nabel, G.J.; Richter, M.K.; et al. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog. 2013, 9, e1003312. [Google Scholar] [CrossRef] [Green Version]
- Quiroz, J.A.; Malonis, R.J.; Thackray, L.B.; Cohen, C.A.; Pallesen, J.; Jangra, R.K.; Brown, R.S.; Hofmann, D.; Holtsberg, F.W.; Shulenin, S.; et al. Human monoclonal antibodies against Chikungunya virus target multiple distinct epitopes in the E1 and E2 glycoproteins. PLoS Pathog. 2019, 15, e1008061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvarajah, S.; Sexton, N.R.; Kahle, K.M.; Fong, R.H.; Mattia, K.A.; Gardner, J.; Lu, K.; Liss, N.M.; Salvador, B.; Tucker, D.F.; et al. A neutralizing monoclonal antibody targeting the acid-sensitive region in Chikungunya virus E2 protects from disease. PLoS Negl. Trop. Dis. 2013, 7, e2423. [Google Scholar] [CrossRef]
- Smith, S.A.; Silva, L.A.; Fox, J.M.; Flyak, A.I.; Kose, N.; Sapparapu, G.; Khomandiak, S.; Ashbrook, A.W.; Kahle, K.M.; Fong, R.H.; et al. Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against Chikungunya virus. Cell Host Microbe 2015, 18, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agapov, E.V.; Razumov, I.A.; Frolov, I.V.; Kolykhalov, A.A.; Netesov, S.V.; Loktev, V.B. Localization of four antigenic sites involved in Venezuelan equine encephalomyelitis virus protection. Arch. Virol. 1994, 139, 173–181. [Google Scholar] [CrossRef]
- Burke, C.W.; Froude, J.W.; Rossi, F.; White, C.E.; Moyer, C.L.; Ennis, J.; Pitt, M.L.; Streatfield, S.; Jones, R.M.; Musiychuk, K.; et al. Therapeutic monoclonal antibody treatment protects nonhuman primates from severe Venezuelan equine encephalitis virus disease after aerosol exposure. PLoS Pathog. 2019, 15, e1008157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, A.R.; Short, W.A.; Johnson, A.J.; Bolin, R.A.; Roehrig, J.T. Synthetic peptides of the E2 glycoprotein of Venezuelan equine encephalomyelitis virus. II. Antibody to the amino terminus protects animals by limiting viral replication. Virology 1991, 185, 281–290. [Google Scholar] [CrossRef]
- Porta, J.; Jose, J.; Roehrig, J.T.; Blair, C.D.; Kuhn, R.J.; Rossmann, M.G. Locking and blocking the viral landscape of an alphavirus with neutralizing antibodies. J. Virol. 2014, 88, 9616–9623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EnCheng, S.; Jing, Z.; Tao, Y.; QingYuan, X.; Yongli, Q.; WenShi, W.; Peng, W.; Liang, S.; Jing, S.; DongLai, W. Analysis of murine B-cell epitopes on Eastern equine encephalitis virus glycoprotein E2. Appl. Microbiol. Biotechnol. 2013, 97, 6359–6372. [Google Scholar] [CrossRef]
- Pereboev, A.V.; Razumov, I.A.; Svyatchenko, V.A.; Loktev, V.B. Glycoproteins E2 of the Venezuelan and Eastern equine encephalomyelitis viruses contain multiple cross-reactive epitopes. Arch. Virol. 1996, 141, 2191–2205. [Google Scholar] [CrossRef]
- Sun, E.; Zhao, J.; Sun, L.; Xu, Q.; Yang, T.; Qin, Y.; Wang, W.; Wei, P.; Sun, J.; Wu, D. Comprehensive mapping of common immunodominant epitopes in the Eastern equine encephalitis virus E2 protein recognized by avian antibody responses. PLoS ONE 2013, 8, e69349. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, E.C.; Liu, N.H.; Yang, T.; Xu, Q.Y.; Qin, Y.L.; Yang, Y.H.; Wu, D.L. Phage display identifies an Eastern equine encephalitis virus glycoprotein E2-specific B cell epitope. Vet. Immunol. Immunopathol. 2012, 148, 364–368. [Google Scholar] [CrossRef]
- Zhou, Q.F.; Fox, J.M.; Earnest, J.T.; Ng, T.S.; Kim, A.S.; Fibriansah, G.; Kostyuchenko, V.A.; Shi, J.; Shu, B.; Diamond, M.S.; et al. Structural basis of Chikungunya virus inhibition by monoclonal antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 27637–27645. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Kostyuchenko, V.A.; Lim, E.X.; Zhang, S.; Fibriansah, G.; Ng, T.S.; Ooi, J.S.; Shi, J.; Lok, S.M. Structure of the thermally stable Zika virus. Nature 2016, 533, 425–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, S.; Kim, B.S.; Chipman, P.R.; Rossmann, M.G.; Kuhn, R.J. Structure of West Nile virus. Science 2003, 302, 248. [Google Scholar] [CrossRef] [Green Version]
- Sirohi, D.; Chen, Z.; Sun, L.; Klose, T.; Pierson, T.C.; Rossmann, M.G.; Kuhn, R.J. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 2016, 352, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, S.H.; Zhu, L.; Nian, Q.G.; Yuan, S.; Gao, Q.; Hu, Z.; Ye, Q.; Li, X.F.; Xie, D.Y.; et al. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat. Commun. 2017, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Fuzik, T.; Formanova, P.; Ruzek, D.; Yoshii, K.; Niedrig, M.; Plevka, P. Structure of tick-borne encephalitis virus and its neutralization by a monoclonal antibody. Nat. Commun. 2018, 9, 436. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Wang, Q.; Qi, J.; Shi, Y.; Yan, J.; Gao, G.F. Molecular basis of antibody-mediated neutralization and protection against flavivirus. IUBMB Life 2016, 68, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.A.; Lok, S.M. Common features of enveloped viruses and implications for immunogen design for next-generation vaccines. Cell 2018, 172, 1319–1334. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, S.S.; Dey, D.; Singh, S.; Martin, M. The Structural Biology of Eastern Equine Encephalitis Virus, an Emerging Viral Threat. Pathogens 2021, 10, 973. https://doi.org/10.3390/pathogens10080973
Hasan SS, Dey D, Singh S, Martin M. The Structural Biology of Eastern Equine Encephalitis Virus, an Emerging Viral Threat. Pathogens. 2021; 10(8):973. https://doi.org/10.3390/pathogens10080973
Chicago/Turabian StyleHasan, S. Saif, Debajit Dey, Suruchi Singh, and Matthew Martin. 2021. "The Structural Biology of Eastern Equine Encephalitis Virus, an Emerging Viral Threat" Pathogens 10, no. 8: 973. https://doi.org/10.3390/pathogens10080973
APA StyleHasan, S. S., Dey, D., Singh, S., & Martin, M. (2021). The Structural Biology of Eastern Equine Encephalitis Virus, an Emerging Viral Threat. Pathogens, 10(8), 973. https://doi.org/10.3390/pathogens10080973






