Methodological Quality Assessment with the AGREE II Scale and a Comparison of European and American Guidelines for the Treatment of Lyme Borreliosis: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. Methodological Quality Assessment (cf. Table 2)
2.1.1. Overall Assessment
2.1.2. Scope and Purpose
2.1.3. Stakeholder Involvement
2.1.4. Rigour of Development
2.1.5. Clarity of Presentation
2.1.6. Applicability
2.1.7. Editorial Independence
2.2. Comparison of Recommended LB Treatments
2.2.1. Treatment of Skin Manifestations (cf. Table 3)
Erythema Migrans (EM)
Borrelial Lymphocytoma (BL)
Acrodermatitis Chronica Atrophicans (ACA)
Children and Pregnant Women
2.2.2. Treatment of Lyme Neuroborreliosis (LNB) (cf. Table 4)
Children and Pregnant Women
2.2.3. Treatment of Lyme Arthritis (cf. Table 4)
Children and Pregnant Women
2.2.4. Treatment of Other Manifestations (cf. Table 5)
LB Carditis (LC)
Ophthalmological LB (OLB)
2.2.5. Management of Post-Treatment Lyme Disease Syndrome (PTLDS) (cf. Table 5)
3. Materials and Methods
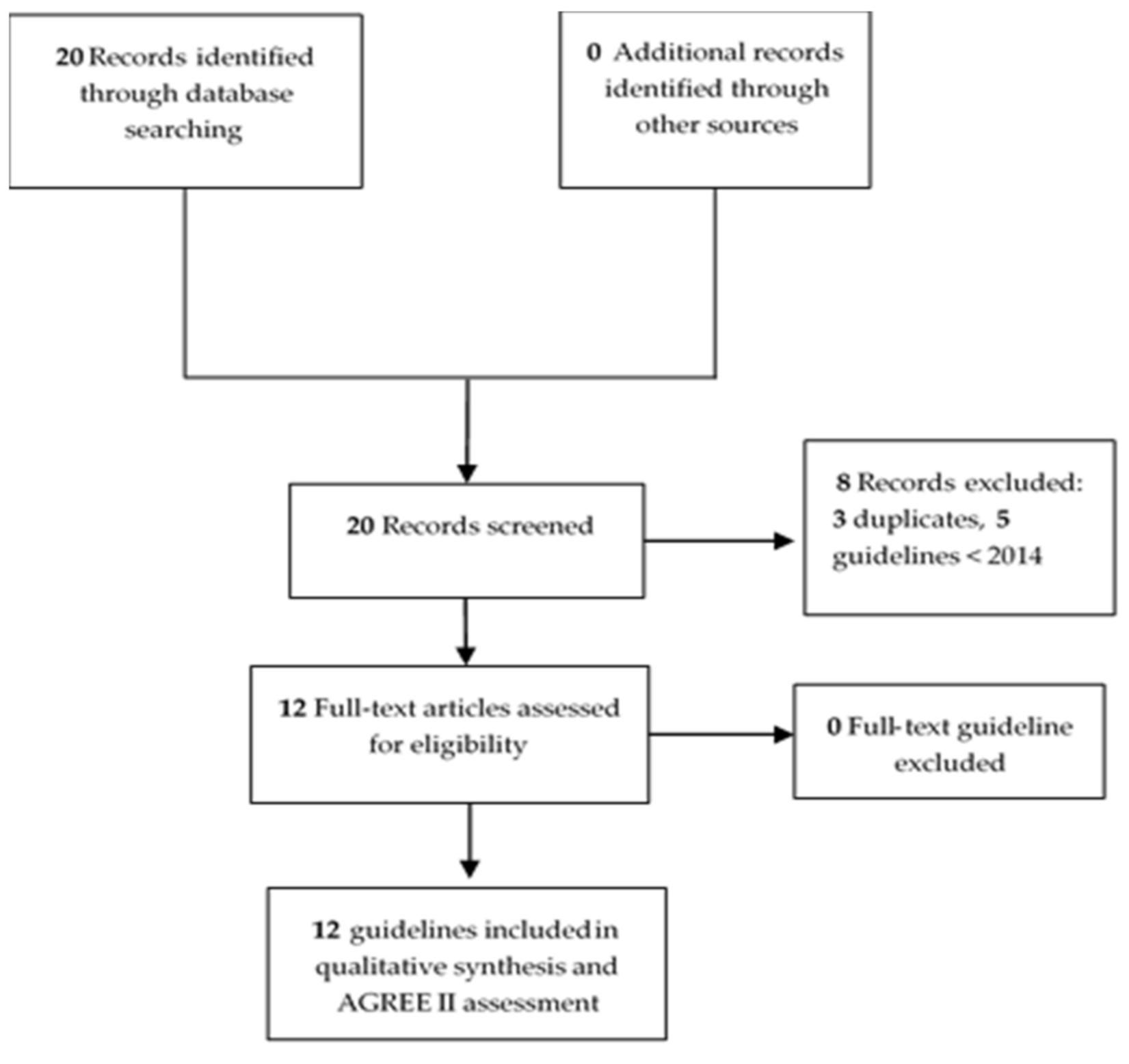
3.1. Data Sources and Search Strategy
3.2. Assessment of the Quality of the Guidelines
3.3. Data Extraction of Guidelines and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD. 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Schwartz, A.M.; Hinckley, A.F.; Mead, P.S.; Hook, S.A.; Kugeler, K.J. Surveillance for Lyme Disease—United States, 2008–2015. MMWR Surveill. Summ. 2017, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Fingerle, V.; Hunfeld, K.-P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef]
- Figoni, J.; Chirouze, C.; Hansmann, Y.; Lemogne, C.; Hentgen, V.; Saunier, A.; Bouiller, K.; Gehanno, J.F.; Rabaud, C.; Perrot, S.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French Scientific Societies (I): Prevention, epidemiology, diagnosis. Méd. Mal. Infect. 2019, 49, 318–334. [Google Scholar] [CrossRef]
- Leeflang, M.M.G.; Ang, C.W.; Berkhout, J.; Bijlmer, H.A.; Van Bortel, W.; Brandenburg, A.H.; Van Burgel, N.D.; Van Dam, A.P.; Dessau, R.B.; Fingerle, V.; et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta-analysis. BMC Infect. Dis. 2016, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Raffetin, A.; Bouiller, K.; Hansmann, Y.; Roblot, F.; Raoult, D.; Parola, P. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med. Mal. Infect. 2019, 49, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Jaulhac, B.; Saunier, A.; Caumes, E.; Bouiller, K.; Gehanno, J.F.; Rabaud, C.; Perrot, S.; Eldin, C.; De Broucker, T.; Roblot, F.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies (II). Biological diagnosis, treatment, persistent symptoms after documented or suspected Lyme borreliosis. Med. Mal. Infect. 2019, 49, 335–346. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Raffetin, A.; Zachary, P.; Jaulhac, B.; Eldin, C. Immunoserological Diagnosis of Human Borrelioses: Current Knowledge and Perspectives. Front. Cell Infect. Microbiol. 2020, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Berende, A.; ter Hofstede, H.J.M.; Vos, F.J.; van Middendorp, H.; Vogelaar, M.L.; Tromp, M.; Van den Hoogen, F.H.; Rogier, T.; Donders, A.; Evers, A.W.M.; et al. Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N. Engl. J. Med. 2016, 374, 1209–1220. [Google Scholar] [CrossRef]
- Klempner, M.S.; Hu, L.T.; Evans, J.; Schmid, C.H.; Johnson, G.M.; Trevino, R.P.; Norton, D.; Levy, L.; Wall, D.; McCall, J.; et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N. Engl. J. Med. 2001, 345, 85–92. [Google Scholar] [CrossRef]
- Krupp, L.B.; Hyman, L.G.; Grimson, R.; Coyle, P.K.; Melville, P.; Ahnn, S.; Dattwyler, R.; Chandler, B. Study and treatment of post Lyme disease (STOP-LD): A randomized double masked clinical trial. Neurology 2003, 60, 1923–1930. [Google Scholar] [CrossRef]
- Kaplan, R.F.; Trevino, R.P.; Johnson, G.M.; Levy, L.; Dornbush, R.; Hu, L.T.; Evans, J.; Weinstein, A.; Schmid, C.H.; Klempner, M.S. Cognitive function in post-treatment Lyme disease: Do additional antibiotics help? Neurology 2003, 60, 1916–1922. [Google Scholar] [CrossRef]
- Fallon, B.A.; Keilp, J.G.; Corbera, K.M.; Petkova, E.; Britton, C.B.; Dwyer, E.; Slavov, I.; Cheng, J.; Dobkin, J.; Nelson, D.R.; et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008, 70, 992–1003. [Google Scholar] [CrossRef]
- Ates, L.; Hanssen-Hübner, C.; Norris, D.E.; Richter, D.; Kraiczy, P.; Hunfeld, K.-P. Comparison of in vitro activities of tigecycline, doxycycline, and tetracycline against the spirochete Borrelia burgdorferi. Ticks Tick Borne Dis. 2010, 1, 30–34. [Google Scholar] [CrossRef]
- Ružić-Sabljić, E.; Podreka, T.; Maraspin, V.; Strle, F. Susceptibility of Borrelia afzelii strains to antimicrobial agents. Int. J. Antimicrob. Agents 2005, 25, 474–478. [Google Scholar] [CrossRef]
- Veinović, G.; Cerar, T.; Strle, F.; Lotrič-Furlan, S.; Maraspin, V.; Cimperman, J.; Ružić-Sabljić, E. In vitro susceptibility of European human Borrelia burgdorferi sensu stricto strains to antimicrobial agents. Int. J. Antimicrob. Agents 2013, 41, 288–291. [Google Scholar] [CrossRef]
- Nemeth, J.; Bernasconi, E.; Heininger, U.; Abbas, M.; Nadal, D.; Strahm, C.; Erb, S.; Zimmerli, S.; Furrer, H.; Delaloye, J.; et al. Update of the Swiss guidelines on post-treatment Lyme disease syndrome. Swiss Med Wkly. 2016, 146, w14353. [Google Scholar] [PubMed]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America, American Academy of Neurology, and American College of Rheumatology: 2020 Guidelines for the Prevention, Diagnosis, and Treatment of Lyme Disease. Neurology 2021, 96, 262–273. [Google Scholar]
- Pancewicz, S.A.; Garlicki, A.M.; Moniuszko-Malinowska, A.; Zajkowska, J.; Kondrusik, M.; Grygorczuk, S.; Czupryna, P.; Duna, J. Diagnosis and treatment of tick-borne diseases recommendations of the Polish Society of Epidemiology and Infectious Diseases. Przegl. Epidemiol. 2015, 69, 309–316. [Google Scholar] [PubMed]
- Island, P.E. Guidelines for the Management and Control of Lyme Disease; Chief Public Health Office: Charlottetown, PE, Canada, 2019. [Google Scholar]
- Borréliose de Lyme et Autres Maladies Vectorielles à Tiques (MVT). 2018, pp. 1–26. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2018-06/reco266_rbp_borreliose_de_lyme_cd_2018_06_13__recommandations.pdf (accessed on 30 April 2021).
- Lyme Disease. Available online: https://www.nice.org.uk/guidance/ng95/resources/lyme-disease-pdf-1837756839877 (accessed on 30 April 2021).
- Hofmann, H.; Fingerle, V.; Hunfeld, K.-P.; Huppertz, H.-I.; Krause, A.; Rauer, S.; Ruf, B.; Consensus Group. Cutaneous Lyme borreliosis: Guideline of the German Dermatology Society. GMS Ger. Med. Sci. 2017, 15. [Google Scholar] [CrossRef]
- Rauer, S.; Kastenbauer, S.; Fingerle, V.; Hunfeld, K.-P.; Huppertz, H.-I.; Dersch, R. Lyme Neuroborreliosis. Dtsch. Arztebl. Int. 2018, 115, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, L.; Delaere, B.; De Loof, G.; De Munter, P.; Frippiat, F.; Jacobs, F. Borréliose de Lyme (Infection à Borrelia); The Belgian Antibiotic Policy Coordination Committee: Brussels, Belgium, 2016; p. 28. [Google Scholar]
- Cameron, D.J.; Johnson, L.B.; Maloney, E.L. Evidence assessments and guideline recommendations in Lyme disease: The clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti-Infect. Ther. 2014, 12, 1103–1135. [Google Scholar] [CrossRef]
- Barsic, B.; Maretic, T.; Majerus, L.; Strugar, J. Comparison of azithromycin and doxycycline in the treatment of erythema migrans. Infection 2000, 28, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Cerar, D.; Cerar, T.; Ruzić-Sabljić, E.; Wormser, G.P.; Strle, F. Subjective symptoms after treatment of early Lyme disease. Am. J. Med. 2010, 123, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nadelman, R.B.; Luger, S.W.; Frank, E.; Wisniewski, M.; Collins, J.J.; Wormser, G.P. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann. Intern. Med. 1992, 117, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, K.E.; Reiso, H.; Berild, D.; Lindbæk, M. Comparison of phenoxymethylpenicillin, amoxicillin, and doxycycline for erythema migrans in general practice. A randomized controlled trial with a 1-year follow-up. Clin. Microbiol. Infect. 2018, 24, 1290–1296. [Google Scholar] [CrossRef]
- Luft, B.J.; Dattwyler, R.J.; Johnson, R.C.; Luger, S.W.; Bosler, E.M.; Rahn, D.W.; Masters, E.J.; Grunwaldt, E.; Gadgil, S.D. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double-blind, randomized, controlled trial. Ann. Intern. Med. 1996, 124, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Eppes, S.C.; Childs, J.A. Comparative study of cefuroxime axetil versus amoxicillin in children with early Lyme disease. Pediatrics 2002, 109, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Stupica, D.; Maraspin, V.; Bogovic, P.; Ogrinc, K.; Blagus, R.; Cerar, T.; Strle, F. Comparison of Clinical Course and Treatment Outcome for Patients with Early Disseminated or Early Localized Lyme Borreliosis. JAMA Dermatol. 2018, 154, 1050–1056. [Google Scholar] [CrossRef]
- Strle, F.; Preac-Mursic, V.; Cimperman, J.; Ruzic, E.; Maraspin, V.; Jereb, M. Azithromycin versus doxycycline for treatment of erythema migrans: Clinical and microbiological findings. Infection 1993, 21, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, E.M.; Luger, S.W.; Rahn, D.W.; Messner, R.P.; Wong, J.B.; Johnson, R.C.; Steere, A.C. Treatment of early Lyme disease. Am. J. Med. 1992, 92, 396–403. [Google Scholar] [CrossRef]
- Wormser, G.P.; Ramanathan, R.; Nowakowski, J.; McKenna, D.; Holmgren, D.; Visintainer, P.; Dornbush, R.; Singh, B.; Nadelman, R.B. Duration of antibiotic therapy for early Lyme disease. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2003, 138, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Preac-Mursic, V.; Neubert, U.; Thurmayr, R.; Herzer, P.; Wilske, B.; Schierz, G.; Marget, W. Antibiotic therapy of early European Lyme borreliosis and acrodermatitis chronica atrophicans. Ann. N. Y. Acad. Sci. 1988, 539, 324–345. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, A.; Czupryna, P.; Pancewicz, S.; Kondrusik, M.; Penza, P.; Zajkowska, J. Borrelial lymphocytoma—A case report of a pregnant woman. Ticks Tick Borne Dis. 2012, 3, 257–258. [Google Scholar] [CrossRef]
- Aberer, E.; Breier, F.; Stanek, G.; Schmidt, B. Success and failure in the treatment of acrodermatitis chronica atrophicans. Infection 1996, 24, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Kindstrand, E.; Nilsson, B.Y.; Hovmark, A.; Pirskanen, R.; Asbrink, E. Peripheral neuropathy in acrodermatitis chronica atrophicans—Effect of treatment. Acta Neurol. Scand. 2002, 106, 253–257. [Google Scholar] [CrossRef]
- Lenormand, C.; Jaulhac, B.; Debarbieux, S.; Dupin, N.; Granel-Brocard, F.; Adamski, H.; Barthel, C.; Cribier, B.; Lipsker, D. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans [ACA]): A prospective study of 20 culture- and/or polymerase chain reaction (PCR)-documented cases. J. Am. Acad. Dermatol. 2016, 74, 685–692. [Google Scholar] [CrossRef]
- Asbrink, E.; Olsson, I.; Hovmark, A. Erythema chronicum migrans Afzelius in Sweden. A study on 231 patients. Zent. Bakteriol. Mikrobiol. Hyg. Ser. A 1986, 263, 229–236. [Google Scholar] [CrossRef]
- Asbrink, E.; Hovmark, A. Lyme borreliosis. Clin Dermatol. 1993, 11, 329–330. [Google Scholar]
- Dychter, S.S.; Gold, D.A.; Carson, D.; Haller, M. Intravenous therapy: A review of complications and economic considerations of peripheral access. J. Infus. Nurs. 2012, 35, 84–91. [Google Scholar] [CrossRef]
- Dotevall, L.; Hagberg, L. Penetration of doxycycline into cerebrospinal fluid in patients treated for suspected Lyme neuroborreliosis. Antimicrob. Agents Chemother. 1989, 33, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Hammers, S.; Nilsson-Ehle, I.; Malmborg, A.S.; Wretlind, B. Concentrations of doxycycline and penicillin G in sera and cerebrospinal fluid of patients treated for neuroborreliosis. Antimicrob. Agents Chemother. 1996, 40, 1104–1107. [Google Scholar] [CrossRef]
- Sicklinger, M.; Wienecke, R.; Neubert, U. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: A comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. J. Clin. Microbiol. 2003, 41, 1791–1793. [Google Scholar] [CrossRef]
- Dattwyler, R.J.; Luft, B.J.; Kunkel, M.J.; Finkel, M.F.; Wormser, G.P.; Rush, T.J.; Grunwaldt, E.; Agger, W.A.; Franklin, M.; Oswald, D.; et al. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N. Engl. J. Med. 1997, 337, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Ljøstad, U.; Skogvoll, E.; Eikeland, R.; Midgard, R.; Skarpaas, T.; Berg, A.; Mygland, A. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: A multicentre, non-inferiority, double-blind, randomised trial. Lancet Neurol. 2008, 7, 690–695. [Google Scholar] [CrossRef]
- Borg, R.; Dotevall, L.; Hagberg, L.; Maraspin, V.; Lotric-Furlan, S.; Cimperman, J.; Strle, F. Intravenous ceftriaxone compared with oral doxycycline for the treatment of Lyme neuroborreliosis. Scand. J. Infect. Dis. 2005, 37, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kohlhepp, W.; Oschmann, P.; Mertens, H.G. Treatment of Lyme borreliosis. Randomized comparison of doxycycline and penicillin G. J. Neurol. 1989, 236, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Hammers-Berggren, S.; Lindquist, L.; Stiernstedt, G.; Svenungsson, B. Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis. Neurology 1994, 44, 1203. [Google Scholar] [CrossRef]
- Ljøstad, U.; Henriksen, T.-H. Management of neuroborreliosis in European adult patients. Acta Neurol. Scand. Suppl. 2008, 188, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.W.; Preac-Mursic, V.; Wilske, B.; Einhäupl, K.M. Cefotaxime vs penicillin G for acute neurologic manifestations in Lyme borreliosis. A prospective randomized study. Arch. Neurol. 1989, 46, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Nikoskelainen, J.; Hiekkanen, H.; Lauhio, A.; Peltomaa, M.; Pitkäranta, A.; Nyman, D.; Granlund, H.; Carlsson, S.-A.; Seppälä, I.; et al. Duration of antibiotic treatment in disseminated Lyme borreliosis: A double-blind, randomized, placebo-controlled, multicenter clinical study. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 571–581. [Google Scholar] [CrossRef]
- Cadavid, D.; Auwaerter, P.G.; Rumbaugh, J.; Gelderblom, H. Antibiotics for the neurological complications of Lyme disease. Cochrane Database Syst. Rev. 2016, 12, 6978. [Google Scholar] [CrossRef] [PubMed]
- Kortela, E.; Kanerva, M.J.; Puustinen, J.; Hurme, S.; Airas, L.; Lauhio, A.; Hohenthal, U.; Jalava-Karvinen, P.; Nieminen, T.; Finnilä, T.; et al. Oral Doxycycline Compared to Intravenous Ceftriaxone in the Treatment of Lyme Neuroborreliosis: A Multicenter, Equivalence, Randomized, Open-label Trial. Clin. Infect. Dis. 2021, 72, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Dersch, R.; Toews, I.; Sommer, H.; Rauer, S.; Meerpohl, J.J. Methodological quality of guidelines for management of Lyme neuroborreliosis. BMC Neurol. 2015, 15, 242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steere, A.C.; Green, J.; Schoen, R.T.; Taylor, E.; Hutchinson, G.J.; Rahn, D.W.; Malawista, S.E. Successful parenteral penicillin therapy of established Lyme arthritis. N. Engl. J. Med. 1985, 312, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Levin, R.E.; Molloy, P.J.; Kalish, R.A.; Abraham, J.H.; Liu, N.Y.; Schmid, C.H. Treatment of Lyme arthritis. Arthritis Rheum. 1994, 37, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Dattwyler, R.J.; Wormser, G.P.; Rush, T.J.; Finkel, M.F.; Schoen, R.T.; Grunwaldt, E.; Franklin, M.; Hilton, E.; Bryant, G.L.; Agger, W.A.; et al. A comparison of two treatment regimens of ceftriaxone in late Lyme disease. Wien. Klin. Wochenschr. 2005, 117, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Dattwyler, R.J.; Halperin, J.J.; Volkman, D.J.; Luft, B.J. Treatment of late Lyme borreliosis-randomised comparison of ceftriaxone and penicillin. Lancet 1988, 1, 1191–1194. [Google Scholar] [CrossRef]
- Renaud, I.; Cachin, C.; Gerster, J.-C. Good outcomes of Lyme arthritis in 24 patients in an endemic area of Switzerland. Jt. Bone Spine 2004, 71, 39–43. [Google Scholar] [CrossRef]
- Nimmrich, S.; Becker, I.; Horneff, G. Intraarticular corticosteroids in refractory childhood Lyme arthritis. Rheumatol. Int. 2014, 34, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Valesová, H.; Mailer, J.; Havlík, J.; Hulínská, D.; Hercogová, J. Long-term results in patients with Lyme arthritis following treatment with ceftriaxone. Infection 1996, 24, 98–102. [Google Scholar]
- Tory, H.O.; Zurakowski, D.; Sundel, R.P. Outcomes of children treated for Lyme arthritis: Results of a large pediatric cohort. J Rheumatol. 2010, 37, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E.; et al. AGREE II: Advancing guideline development, reporting and evaluation in health care. Can. Med. Assoc. J. 2010, 182, E839–E842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, Y.; Zhong, J.; Wang, K.; Ding, Y.; Li, L.; Pan, X. Quality appraisal of gestational diabetes mellitus guidelines with AGREE II: A systematic review. BMC Pregnancy Childbirth 2019, 19, 478. [Google Scholar] [CrossRef] [PubMed]
| Guidelines | Country | Institution | Year | Type | |
|---|---|---|---|---|---|
| 1 | ILADS guidelines [27] | USA | ILADS | 2014 | Evidence-based and expert consensus |
| 2 | Polish guidelines [20] | Polish | PSEID | 2015 | Evidence-based |
| 3 | Swiss guidelines [17] | Swiss | SSID and SSN | 2016 | Evidence-based |
| 4 | Belgium guidelines [26] | Belgium | BAPCOC | 2016 | Evidence-based |
| 5 | German Dermatology Society guidelines for cutaneous LB [24] | Germany | AWMF | 2017 | Evidence-based |
| 6 | ESGBOR guidelines [18] | Europe | ESGBOR | 2017 | Evidence-based |
| 7 | French High Health Authority guidelines [22] | France | HAS | 2018 | Evidence-based |
| 8 | NICE guidelines [23] | England | NICE | 2018 | Evidence-based |
| 9 | German Neurology Society guidelines for LNB [25] | Germany | AWMF | 2019 | Evidence-based |
| 10 | French scientific societies guidelines [7] | France | French scientific societies | 2019 | Evidence-based |
| 11 | Canadian guidelines, Prince Edward Island [21] | Canada | Department of Health | 2019 | Evidence-based |
| 12 | American guidelines [19] | USA | IDSA | 2020 | Evidence-based |
| Guidelines | Scope and Purpose | Stakeholder Involvement | Rigor of Development | Clarity of Presentation | Applicability | Editorial Independence | Overall Assessment | Recommended |
|---|---|---|---|---|---|---|---|---|
| ILADS [27] | 100% | 69% | 58% | 22% | 31% | 13% | 42% | N |
| PSEID [20] | 81% | 31% | 10% | 58% | 44% | 29% | 33% | N |
| SSID and SSN [17] | 100% | 58% | 89% | 67% | 81% | 67% | 83% | Y |
| BAPCOC [26] | 72% | 50% | 40% | 67% | 71% | 58% | 53% | YM |
| AWMF [24] | 100% | 100% | 96% | 86% | 81% | 100% | 100% | Y |
| ESGBOR [18] | 75% | 58% | 66% | 42% | 44% | 79% | 50% | YM |
| HAS [22] | 100% | 100% | 88% | 83% | 79% | 54% | 75% | YM |
| NICE [23] | 100% | 100% | 94% | 89% | 100% | 100% | 100% | Y |
| AWMF [25] | 100% | 100% | 98% | 89% | 94% | 100% | 100% | Y |
| French scientific societies [7] | 100% | 100% | 99% | 87% | 92% | 100% | 100% | Y |
| Canada [21] | 64% | 36% | 17% | 39% | 85% | 42% | 25% | N |
| IDSA [19] | 100% | 100% | 100% | 63% | 94% | 96% | 100% | Y |
| Median ± IQR | 100% ± 22% | 85% ± 46% | 88% ± 48% | 67% ± 35% | 81% ± 36% | 73% ± 52% | 79% ± 54% | |
| Cohen’s kappa | - | - | - | - | - | - | 0.90 (0.84–0.96) |
| Guidelines | Erythema Migrans | Borrelial Lymphocytoma | Acrodermatitis Chronica Atrophicans | |||
|---|---|---|---|---|---|---|
| First Intention | Second Intention | First Intention | Second Intention | First Intention | Second Intention | |
| ILADS [27] | Amoxicillin (1.5–2.0 g/d) or Cefuroxime-axetil (500 mg × 2/d) or Doxycycline (100 mg × 2/d) for 4–6 weeks or Azithromycin (250–500 mg/d) for 21 days minimum level of evidence = very low | NA | NA | NA | NA | |
| PSEID [20] | Doxycycline (100 mg × 2/d), for 14–28 days level of evidence = NA | Amoxicillin (1.5–2 g/d) or Cefuroxime-axetil (500 mg × 2/d),for 14-28 days level of evidence = NA | Doxycycline (100 mg × 2/d) or Amoxicillin (1.5–2 g/d) or Cefuroxime axetil 500 mg × 2/d for 14–28 days level of evidence = NA | Doxycycline (100 mg × 2/d) or Ceftriaxone (2 g/d) or Amoxicillin (1.5–2 g/d) or Cefuroxim-axetil (500 mg × 2/d) for 14-21 days level of evidence = NA | ||
| SSID and SSN [17] | NA | NA | NA | NA | NA | NA |
| BAPCOC [26] | Doxycycline (100 mg × 2/d) for 10 days level of evidence = NA | Amoxicillin (1.5–2 g/d) or Cefuroxime-axetil (500 mg × 2/d), or Clarythromycin (500 mg × 2/d) for 14 days or Azithromycin (1 g on D1, then 500 mg/d) for 4 days or (500 mg/d) for 7 days level of evidence = NA | NA | NA | Doxycycline (100 mg × 2/d) for 21–28 days or Ceftriaxone (2 g/d) for 14 days level of evidence = NA | |
| AWMF [24] | Doxycycline (100 mg × 2/d or 200 mg/d) for 10–14 days or Amoxicillin (0.5–1 g × 3/d) or Cefuroxime axetil (500 mg × 2/d) for 14 days or Azithromycin (250 mg × 2/d) for 5–10 days level of evidence = NA | Doxycycline (100 mg × 2/d or 200 mg/d) or Amoxicillin (0.5–1 g × 3/d) or Cefuroxime-axetil (500 mg × 2/d), for 14–21 days or Azithromycin (250 mg × 2/d) for 5–10 days level of evidence = NA | Doxycycline (100 mg × 2/d or 200 mg/d) or Amoxicillin (0.5–1 g × 3/d) for 30 days If associated with neurological symptoms: Penicillin G (4 × 5MU/d) or Ceftriaxone (2 g/d) or Cefotaxime (2 g × 3/d) for 14–21 days level of evidence = NA | |||
| ESGBOR [18] | Doxycycline for 10 days or Amoxicillin or Cefuroxime-axetil or Phenoxymethylpenicillin for 14 days level of evidence = NA | Azithromycin for 5–10 days, if CI for β-lactams or tetracyclines level of evidence = NA | Doxycycline or Amoxicillin or Cefuroxime-axetil or Phenoxymethylpenicillin for 14 days level of evidence = NA | Azithromycin for 5–10 days, if CI for β-lactams or tetracyclines level of evidence = NA | Doxycycline or Amoxicillin or Cefuroxime-axetil or Phenoxymethylpenicillin for 21–28 days level of evidence = NA | Azithromycin for 5–10 days, if CI for β-lactams or tetracyclins level of evidence = NA |
| HAS [22] | Doxycycline (100 mg × 2/d or 200 mg/d) or Amoxicillin (1 g × 3/d), for 14 days level of evidence = moderate | Azithromycin (1 g on D1, then 500 mg/d) for 7 days level of evidence = moderate | Doxycycline (100 mg × 2/d or 200 mg/d) or Amoxicillin (1–2 g × 3/d), for 21 days level of evidence = low | Azithromycin (1 g on D1, then 500 mg/d) for 10 days level of evidence = low | Doxycycline (200 mg/d) for 28 days level of evidence = low | Ceftriaxone (2 g/d), for 28 days level of evidence = low |
| NICE [23] | Doxycycline (100 mg × 2/d or 200 mg/d) for 21 days level of evidence = low | Amoxicillin (1 g × 3/d) for 21 days or Azithromycin (500 mg/d) for 17 days level of evidence = low | NA | NA | Doxycycline (100 mg × 2/d or 200 mg/d) for 28 days level of evidence = low | Amoxicillin (1 g × 3/d) or Ceftriaxone (2 g/d), for 28 days level of evidence = low |
| AWMF [25] | NA | NA | NA | NA | NA | NA |
| French scientific societies [7] | Doxycycline (100 mg × 2/d) for 14 days level of evidence = grade B | Amoxicillin (1 g × 3/d) for 14 days level of evidence = grade B | Doxycycline (100 mg × 2/d) for 21 days level of evidence = grade B | Amoxicillin (1 g × 3/d) for 21 days level of evidence = grade B | Doxycycline (200 mg/d) for 28 days level of evidence = grade B | Ceftriaxone (2 g/d) for 28 days level of evidence = grade B |
| Canada [21] | Doxycycline (100 mg × 2/d) for 14 days level of evidence = NA | Amoxicillin (0.5 g × 3/d) or Cefuroxime-axetil (500 mg × 2/d) for penicillin-allergic patient, for 14 days level of evidence = NA | NA | NA | NA | NA |
| IDSA [19] | Doxycycline (100 mg × 2/d or 200 mg/d) for 10 days or Amoxicillin (0.5 g × 3/d) or Cefuroxime-axetil (500 mg × 2/d) for 14 days level of evidence = moderate | Azithromycin (500 mg/d) for 5–10 days level of evidence = moderate | Doxycycline (100 mg × 2/d or 200 mg/d) for 21 days or Amoxicillin (0.5–1 g × 3/d) for 14 days or Cefuroxime-axetil (500 mg × 2/d) for 14–21 days level of evidence = low | Doxycycline (100 mg × 2/d or 200 mg/d) or Amoxicillin (0.5–1 g × 3/d) or Cefuroxime-axetil (500 mg × 2/d) for 21–28 days level of evidence = low | ||
| Guidelines | Early Lyme Neuroborreliosis | Late Lyme Neuroborreliosis | Early (<6 Months) and Late (>6 Months) Lyme Arthritis | |||
|---|---|---|---|---|---|---|
| First Intention | Second Intention | First Intention | Second Intention | First Intention | Second Intention | |
| ILADS [27] | NA | NA | NA | NA | NA | NA |
| PSEID [20] | Cranial nerves deficit: Doxycycline 100 mg × 2/d for 14–28 days Meningitis, radiculitis, vasculitis: Doxycycline (100 mg × 2/d) or Ceftriaxone (2g/d) for 14–28 days level of evidence = NA | Encephalomyelitis, radiculoneuritis, meningitis, occlusive vasculitis stroke: Ceftriaxone (2 g/d) for 21–28 days. level of evidence = NA | Doxycycline (100 mg × 2/d) Or Ceftriaxone (2 g/d) for 28–30 days level of evidence = NA | If failure: continue the antibiotics one more month level of evidence = NA | ||
| SSID and SSN [17] | NA | NA | NA | NA | NA | NA |
| BAPCOC [26] | Doxycycline (100 mg × 2/d) or Ceftriaxone (2 g/d) for 14 days level of evidence = NA | Ceftriaxone (2 g/d), for 28 days level of evidence = NA | Doxycycline (100 mg × 2/d) or Amoxicillin (500 mg × 3/d), for 28 days level of evidence = NA | If failure: Ceftriaxone (2 g/d) for 14–28 days or another oral line for 28 days level of evidence = NA | ||
| AWMF [24] | NA | NA | NA | NA | NA | NA |
| ESGBOR [18] | For ambulatory patient: Doxycycline (100 mg × 2/d) for 14 days For hospitalized patients: Ceftriaxone (2 g/d) for 14 days level of evidence = NA | Encephalomyelitis: Ceftriaxone (2 g/d) for 14–28 days level of evidence = NA | Doxycycline or Amoxicillin for 28 days level of evidence = NA | If failure: Ceftriaxone for 14–28 days Or Cefuroxime-axetil for 28 days level of evidence = NA | ||
| HAS [22] | Ceftriaxone (2 g/d) or Doxycycline (100 mg × 2/d) for 21 days Children with isolated nerve palsy: Amoxicillin (100 mg/kg/d × 3/d) for 21 days level of evidence = NA | Ceftriaxone (2 g/d) (100 mg/kg/d) for 28 days level of evidence = NA | Doxycycline (100 mg × 2/d or 4 mg/kg/d) or Penicillin G (24 MU/d) for 28 days level of evidence = NA | Doxycycline (200 mg/d) for 28 days level of evidence = low | If failure: Ceftriaxone (2 g/d) for 28 days level of evidence = low | |
| NICE [23] | PNS: Doxycycline (100 mg × 2/d or 200 mg/d) for 21 days CNS: Ceftriaxone (2 g × 2/d or 4 g/d), for 21 days level of evidence = moderate/very low | PNS: Amoxicillin (1 g × 3/d) for 21 days CNS: Doxycycline (200 mg × 2/d or 400 mg/d) for 21 days level of evidence = moderate/ very low | NA | NA | Doxycycline (100 mg × 2/d or 200 mg/d) for 28 days level of evidence = low /very low | 1st Alternative or failure: Amoxicillin (1 g × 3/d) for 28 days 2nd alternative or failure: Ceftriaxone (2 g/d) for 28 days level of evidence = low/very low |
| AWMF [25] | Doxycycline (100 mg × 2/d or 100 mg × 3/d or 200–300 mg/d) or Ceftriaxone (2 g/d) or Penicillin G (5 MU/d) for 14 days level of evidence = grade Ia | Ceftriaxone (2 g/d) or Cefotaxime (2 g × 3/d) or Penicillin G (5 MU/d) or Doxycycline (100 mg × 2/d or 100 mg × 3/d or 200–300mg/d) for 14–21 days level of evidence = grade Ia to grade III | NA | NA | ||
| French scientific societies [7] | Doxycycline (100 mg × 2/d) for 14 days level of evidence = grade EA | Ceftriaxone (2 g/d) for 14 days level of evidence = grade EA | PNS: Doxycycline (100 mg × 2/d) for 21 days CNS: Doxycycline (200 mg × 2/d) for 21 days level of evidence = grade EA | Ceftriaxone (2 g/d) for 21 days level of evidence = grade EA | Doxycycline (200 mg/d) for 28 days level of evidence = grade EA | If failure: Ceftriaxone (2 g/d) for 28 days level of evidence = grade EA |
| Canada [21] | NA | NA | NA | NA | NA | NA |
| IDSA [19] | Meningitidis or radiculitis: Doxycycline (100 mg × 2/d or 200mg/d) or Ceftriaxone (2 g/d) for 14–21 days Cranial nerve palsy: Doxycycline (100 mg × 2/d or 200mg/d) for 14–21 days Parenchymal involvement of the brain or spinal cord: Ceftriaxone (2 g/d) or Cefotaxime (2 g × 3/d) or Penicillin G (18–24 MU) for 14–21 days level of evidence = strong recommendation moderate-quality evidence | Meningitidis or radiculitis: Cefotaxime (2 g × 3/d) or Penicillin G (18–24 MU) for 14-21 days level of evidence = strong recommendation moderate-quality evidence | NA | NA | Doxycycline (200 mg/d or 100mg × 2/d) or Amoxicillin (500 mg × 3/d) or Cefuroxim axetil (500 mg × 2/d) for 28 days level of evidence = strong recommendation moderate-quality evidence | If partial response (mild residual joint swelling): no antibiotic, search for differential diagnosis, and then eventually discuss a 2nd line of oral antibiotics level of evidence = strong recommendation moderate-quality evidence |
| Guidelines | Lyme Carditis | Ophtalmological Lyme Borreliosis | Post-Treatment Lyme Disease Syndrome | |||
|---|---|---|---|---|---|---|
| First Intention | Second Intention | First Intention | Second Intention | First Intention | Second Intention | |
| ILADS [27] | NA | NA | NA | If persistent symptoms, treatment options are extensive and choices must be individualized: oral antibiotics alone or in combination or injectable penicillin G benzathine or ceftriaxone alone or in combination with other antibiotics for 4–6 weeks level of evidence = low | ||
| PSEID [20] | Doxycycline (100 mg × 2/d) or Amoxicillin (1.5–2g/d) or Ceftriaxone (2 g/d) for 28–30 days level of evidence = NA | NA | NA | NA | NA | |
| SSID and SSN [17] | NA | NA | NA | NA | Antibiotic retreatment is not recommended after appropriate initial antibiotics for LB. No evidence for specific treatment. level of evidence = strong | |
| BAPCOC [26] | Doxycycline (100 mg × 2/d) for 21 days Or Ceftriaxone (2 g/d) for 14 days (preferred in more severe cases) level of evidence = NA | NA | NA | Antibiotic therapy is not recommended level of evidence = NA | ||
| AWMF [24] | NA | NA | NA | NA | Benefit of repeated and long-term antibiotics not verified. Level of evidence = NA | |
| ESGBOR [18] | Outpatients: Doxycycline or Amoxicillin or Cefuroxime-axetil Hospitalized patients: Ceftriaxone for 14–21 days A switch to oral antibiotic can be made if improvement level of evidence = NA | NA | NA | Antibiotic therapy is not recommended level of evidence = NA | ||
| HAS [22] | Outpatients: Doxycycline (200 mg/d) or Amoxicillin (3 g/d) Hospitalized patients: Ceftriaxone (2 g/d) with a switch as soon as possible to oral antibiotics for 21 days level of evidence = NA | Ceftriaxone (2 g/d or 100 mg/kg/d) for 28 days level of evidence = NA | After having eliminated differential diagnosis to LB, consider: Doxycycline (200 mg/d) for 28 days level of evidence = NA | If allergy to doxycycline: Azithromycin (1 g on D1, then then 500 mg/d) for 15 days. level of evidence = NA | ||
| NICE [23] | Stable patients: Doxycycline (100 mg 2 × /d or 200 mg/d) Hemodynamically unstable patients: Ceftriaxone (2 g/d) for 21 days level of evidence = grade EA | Stable patients: Ceftriaxone (2 g/d) for 21 days level of evidence = grade EA | NA | NA | Consider a second course of antibiotics for people with ongoing symptoms if treatment may have failed. Use an alternative antibiotic to the initial course. If a person has ongoing symptoms following 2 completed courses of antibiotics for LB: do not routinely offer further antibiotics and consider discussion with a national reference laboratory or discussion or referral to a specialist level of evidence = EA | |
| AWMF [25] | NA | NA | NA | NA | Patients should not be treated with antibiotics. level of evidence = NA | |
| French scientific societies [7] | Patient with syncope, type 2 or 3 AVB, or type 1 AVB > 30 ms: Ceftriaxone (2 g/d) with a switch to oral antibiotics as soon as continuous cardiac monitoring is no longer required Patients with other manifestations: Doxycycline (100 mg × 2/d) or Amoxicillin (1 g × 3/d) for a total of 21 days level of evidence = grade C to EA | Lesions on the surface of the eyes: Doxycycline (200 mg/d) or Ceftriaxone (2 g/d) for 14 days Keratitis, intraocular, orbital, neuro-ophthalmological lesions: Ceftriaxone (2 g/d) for 21 days level of evidence = NA | Doxycycline (100 mg × 2/d) for 21 days level of evidence = NA | Patients should not receive repeated or prolonged courses of antibiotics. level of evidence = grade A | ||
| Canada [21] | NA | NA | NA | NA | NA | NA |
| IDSA [19] | Outpatients: Doxycycline (100 mg × 2/d or 200 mg/d) or Amoxicillin (500 mg × 3/d) or Cefuroxime axetil (500 mg × 2/d) Hospitalized patients: initially Ceftriaxone (2 g/d) then switching to oral antibiotics For a total of 14–21 days Level of evidence = weak recommendation, very low-quality evidence | NA | NA | Additional antibiotic therapy is not recommended. Level of evidence = strong recommendation, moderate-quality evidence. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguala, S.; Baux, E.; Patrat-Delon, S.; Saunier, F.; Schemoul, J.; Tattevin, P.; Cazorla, C.; Eldin, C.; Bouiller, K.; Raffetin, A. Methodological Quality Assessment with the AGREE II Scale and a Comparison of European and American Guidelines for the Treatment of Lyme Borreliosis: A Systematic Review. Pathogens 2021, 10, 972. https://doi.org/10.3390/pathogens10080972
Nguala S, Baux E, Patrat-Delon S, Saunier F, Schemoul J, Tattevin P, Cazorla C, Eldin C, Bouiller K, Raffetin A. Methodological Quality Assessment with the AGREE II Scale and a Comparison of European and American Guidelines for the Treatment of Lyme Borreliosis: A Systematic Review. Pathogens. 2021; 10(8):972. https://doi.org/10.3390/pathogens10080972
Chicago/Turabian StyleNguala, Steve, Elisabeth Baux, Solène Patrat-Delon, Florian Saunier, Julien Schemoul, Pierre Tattevin, Céline Cazorla, Carole Eldin, Kevin Bouiller, and Alice Raffetin. 2021. "Methodological Quality Assessment with the AGREE II Scale and a Comparison of European and American Guidelines for the Treatment of Lyme Borreliosis: A Systematic Review" Pathogens 10, no. 8: 972. https://doi.org/10.3390/pathogens10080972
APA StyleNguala, S., Baux, E., Patrat-Delon, S., Saunier, F., Schemoul, J., Tattevin, P., Cazorla, C., Eldin, C., Bouiller, K., & Raffetin, A. (2021). Methodological Quality Assessment with the AGREE II Scale and a Comparison of European and American Guidelines for the Treatment of Lyme Borreliosis: A Systematic Review. Pathogens, 10(8), 972. https://doi.org/10.3390/pathogens10080972






