Reporter Virus Neutralization Test Evaluation for Dengue and Zika Virus Diagnosis in Flavivirus Endemic Area
Abstract
1. Introduction
2. Results
2.1. Groups’ Categories
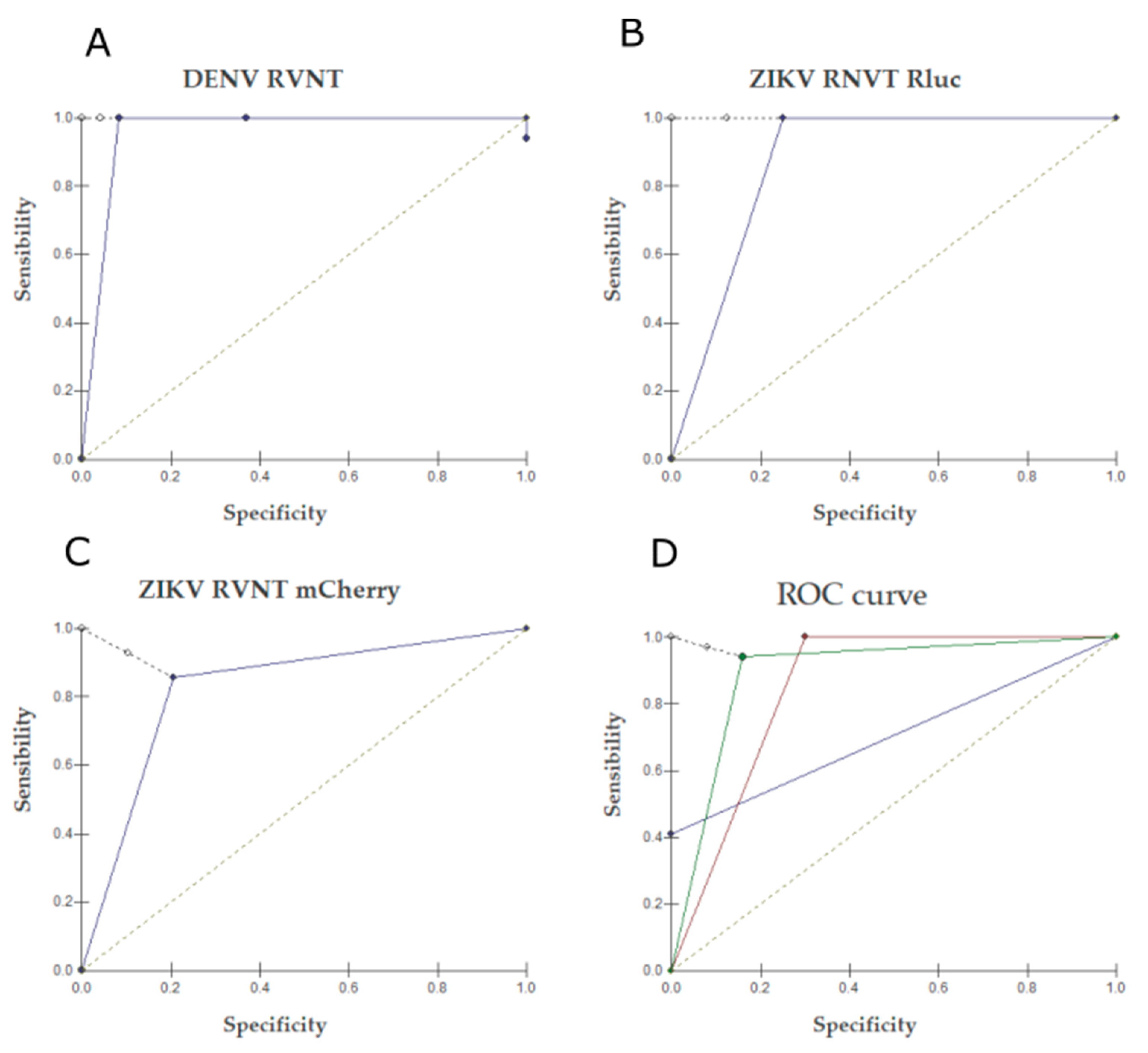
2.2. Neutralization Tests
2.3. RVNT
3. Discussion
4. Materials and Methods
4.1. Specimen Selection
4.2. Cells and Viruses
4.3. Plaque Reduction Neutralization Test
4.4. Reporter Virus-Based Neutralization Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. ZIKA virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of ZIKA virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, C.B.; Ximenes, M.F. ZIKA virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev. Soc. Bras. Med. Trop. 2016, 49, 4–10. [Google Scholar] [CrossRef]
- Basarab, M.; Bowman, C.; Aarons, E.J.; Cropley, I. ZIKA virus. BMJ 2016, 352, i1049. [Google Scholar] [CrossRef]
- Haby, M.M.; Pinart, M.; Elias, V.; Reveiz, L. Prevalence of asymptomatic ZIKA virus infection: A systematic review. Bull. World Health Organ. 2018, 96, 402–413. [Google Scholar] [CrossRef]
- Saiz, J.; Vasquez-Calvo, A.; Blazquez, A.; Merino-Ramos, T.; Escribano-Romero, E.; Martín-Acebes, M.A. ZIKA virus: The latest newcomer. Front. Microbiol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Calvez, E.; O’Connor, O.; Pol, M.; Rousset, D.; Faye, O.; Richard, V.; Tarantola, A.; Dupont-Rouzeyrol, M. Differential transmission of Asian and African ZIKA virus lineages by Aedes aegypti from New Caledonia. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Li, J.; Carr, M.J.; Duchene, S.; Shi, W. The Asian Lineage of Zika Virus: Transmission and Evolution in Asia and the Americas. Virol. Sin. 2019, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Nasar, F.; Guzman, H.; Ponlawat, A.; Jarman, R.G.; Tesh, R.B.; Scott, C.W. Genetic Characterization of Spondweni and ZIKA Viruses and Susceptibility of Geographically Distinct Strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni Virus. PLoS Negl. Trop. Dis. 2016, 10, e0005083. [Google Scholar] [CrossRef]
- Aliota, M.T.; Bassit, L.; Bradrick, S.S.; Cox, B.; Garcia-Blanco, M.A.; Gavegnano, C.; Friedrich, T.C.; Golos, T.G.; Griffin, D.E.; Haddow, A.D.; et al. ZIKA in the Americas, year 2: What have we learned? What gaps remain? A report from the Global Virus Network. Antivir. Res. 2017, 144, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Shi, W.F.; Qin, C.F. The evolution of ZIKA virus from Asia to the Americas. Nat. Rev. Microbiol. 2019, 17, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. ZIKA Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.S.; Vasconcelos, P.F.C. Microcephaly and ZIKA virus. J. Pediatr. 2016, 92, 103–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Azevedo, R.S.S.; De Sousa, J.R.; Araujo, M.T.F.; Martins Filho, A.J.; De Alcantara, B.N.; Araujo, F.M.C.; Queiroz, M.G.L.; Cruz, A.C.R.; Vasconcelos, B.H.B.; Chiang, J.O.; et al. In situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by ZIKA virus. Sci. Rep. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, B.N.; Imbeloni, A.A.; Durans, D.B.S.; Araújo, M.T.F.; Cruz, E.R.M.; Carvalho, C.A.M.; Mendonça, M.H.R.; Sousa, J.R.; Moraes, A.F.; Martins Filho, A.J.; et al. Histopathological lesions of congenital ZIKA syndrome in newborn squirrel monkeys. Sci Rep. 2021, 11, 6099. [Google Scholar] [CrossRef]
- Kobres, P.Y.; Chretien, J.P.; Johansson, M.A.; Morgan, J.J.; Whung, P.Y.; Mukundan, H.; Dell Valle, S.Y.; Forshey, B.M.; Quandelacy, T.M.; Biggerstaff, M.; et al. A systematic review and evaluation of ZIKA virus forecasting and prediction research during a public health emergency of international concern. PLoS Negl. Trop. Dis. 2019, 13, e0007451. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.M.; Fischer, M.; Muñoz-Jordán, J.L.; Paz-Bailey, G.; Staples, J.E.; Gregory, C.J.; Waterman, S.H. Dengue and ZIKA Virus Diagnostic Testing for Patients with a Clinically Compatible Illness and Risk for Infection with Both Viruses. MMWR Recomm. Rep. 2019, 68, 1–10. [Google Scholar] [CrossRef]
- Panning, M. ZIKA Virus Serology: More Diagnostic Targets, more Reliable Answers? EBioMedicine 2017, 16, 12–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of ZIKA virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Luo, H.; Xie, X.; Medeiros, D.B.A.; Wakamiya, M.; Tesh, R.B.; Barrett, A.D.; Wang, T.; et al. A live-attenuated ZIKA virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med. 2017, 23, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Jagger, B.W.; Richner, J.; Nunes, B.T.D.; Medeiros, D.B.A.; Xie, X.; Nunes, J.G.C.; Morabito, K.M.; Kong, W.P.; et al. A single-dose live-attenuated vaccine prevents ZIKA virus pregnancy transmission and testis damage. Nat. Commun. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Malafa, S.; Medits, I.; Aberle, J.H.; Aberle, S.W.; Haslwanter, D.; Tsouchnikas, G.; Wolfe, S.; Huber, K.L.; Percivalle, E.; Cherpillod, P.; et al. Impact of flavivirus vaccine-induced immunity on primary ZIKA virus antibody response in humans. PLoS Negl. Trop. Dis. 2020, 14, e0008034. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Xie, X.; Ren, P.; Loeffelholz, M.J.; Yang, Y.; Furuya, A.; Dupuis, A.P.; Kramer, L.D.; Wong, S.J.; Shi, P.Y. A Rapid ZIKA Diagnostic Assay to Measure Neutralizing Antibodies in Patients. EBioMedicine 2017, 17, 157–162. [Google Scholar] [CrossRef]
- Ministério da Saúde. Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus ZIKA até a Semana Epidemiológica 10, 2017. Bol. Epidemiológico Ministério Saúde 2017, 48, 1–10. [Google Scholar]
- Salles, T.S.; Sá-Guimarães, T.E.; de Alvarenga, E.S.L.; Guimarães-Ribeiro, V.; de Meneses, M.D.F.; de Castro-Salles, P.F.; Dos Santos, C.R.; Melo, A.C.A.; Soares, M.R.; Ferreira, D.F.; et al. History, epidemiology and diagnostics of dengue in the American and Brazilian contexts: A review. Parasites Vectors 2018, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Brazil. Reemergência da Febre Amarela Silvestre no Brasil, 2014/2015: Situação epidemiológica e a importância da vacinação preventiva e da vigilância intensificada no período sazonal. Bol. Epidemiológico Ministério Saúde 2015, 46, 1–10. [Google Scholar]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; de Albuquerque Motta, M.; Dos Santos, F.B.; Vazeille, M.; da Costa Vasconcelos, P.F.; Lourenço-de-Oliveira, R.; Failloux, A.B. Potencial risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Azevedo, R.D.S.D.S.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. ZIKA virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.R.; Cerutti, C., Jr.; Froschl, G.; Romano, C.M.; Cabidelle, A.S.A.; Herbinger, K.-H. Influence of demographics on clinical outcome of dengue: A cross-sectional study of 6703 confirmed cases in Vitória, Espírito Santo State, Brazil. Epidemiol. Infect. 2016, 145, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.S.S.; Araujo, M.T.; Filho, A.J.M.; Oliveira, C.S.; Nunes, B.T.D.; Cruz, A.C.R.; Nascimento, A.G.P.A.C.; Medeiros, R.C.; Caldas, C.A.M.; Araujo, F.C.; et al. Zika virus epidemic in Brazil. I. Fatal disease in adults: Clinical and laboratorial aspects. J. Clin. Virol. 2016, 85, 56–64. [Google Scholar] [CrossRef]
- Nóbrega, M.E.B.; Araújo, E.L.L.; Wada, M.Y.; Leite, P.L.; Dimech, G.S.; Pércio, J. Outbreak of Guillain-Barré syndrome possibly related to prior Zika virus infection, Metropolitan Region of Recife, Pernambuco, Brazil, 2015. Epidemiol. Serviços Saúde 2017, 27. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Alcina, M.S.; Andrade, M.C.N.; Costa, J.S.M.; Castro, F.L.S.; Oliveira, C.S.B.; Goes, M.M.; Santana, E.B.; Nunes, B.T.D.; Vasconcelos, P.F.C. East/Central/South African Genotype Chikungunya Virus, Brazil, 2014. Emerg. Infect. Dis. 2015, 21, 906. [Google Scholar] [CrossRef] [PubMed]
- Aragão, C.F.; Cruz, A.C.R.; Neto, J.P.N.; Monteiro, H.A.O.; Silva, E.V.P.; Silva, S.P.; Andrade, A.T.S.; Tadei, W.P.; Pinheiro, V.C.S. Circulation of Chikungunya virus in Aedes aegypti in Maranhão, Northeast Brazil. Acta Trop. 2018, 186, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; Melo, V.C.A.; Mosimann, A.L.P.; Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Memórias Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.T. Postnatal Imaging Findings of Congenital Zika Syndrome. Top. Magn. Reson. Imaging 2019, 28, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Roehring, J.T.; Nash, D.; Maldin, B.; Labowitz, A.; Martin, D.A.; Lanciotti, R.S.; Campbell, G.L. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile encephalitis cases. Emerg. Infect. Dis. 2003, 9, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.T.; Brito, C.A.; Pena, L.J.; Pena, L.J.; Castanha, P.M.S.; Gil, L.H.V.G.; Lopes, K.G.S.; Dhalia, R.; Meneses, J.A.; Ishigami, A.C.; et al. Results of a Zika virus (ZIKA) immunoglobulin M–specific diagnostic assay are highly correlated with detection of neutralizing anti-ZIKA antibodies in neonates with congenital disease. J. Infect. Dis. 2016, 214, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Araújo, V.E.M.; Bezerra, J.M.T.; Amâncio, F.F.; Passos, V.M.d.A.; Carneiro, M. Increase in the burden of dengue in Brazil and federated units, 2000 and 2015: Analysis of the Global Burden of Disease Study 2015. Rev. Bras. Epidemiol. 2015, 20, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91, e01469-17. [Google Scholar] [CrossRef] [PubMed]
- Shope, R.E. The use of micro-hemagglutinaation-inhibition test to follow antibody response after arthropod-borne virus infection in a community of forest animais. An. Microbiol. 1963, 11, 167–171. [Google Scholar]
- CDC. Guidance for U.S. Laboratories Testing for Zika Virus Infection. Available online: https://www.cdc.gov/zika/pdfs/laboratory-guidance-zika.pdf (accessed on 16 November 2016).
- Shan, C.; Xie, X.; Muruato, A.E.; Rossi, S.L.; Roundy, C.M.; Azar, S.R.; Yang, Y.; Tesh, R.B.; Bourne, N.; Barrett, A.D.; et al. An infectious cDNA clone of Zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe 2016, 19, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Xu, H.Y.; Qing, M.; Wang, Q.Y.; Shi, P.Y. Development and characterization of a stable luciferase dengue virus for high-throughput screening. Antivir. Res. 2011, 91, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Castanha, P.M.; Cordeiro, M.T.; Martelli, C.M.; Souza, W.V.; Marques, E.T., Jr.; Braga, C. Force of infection of dengue serotypes in a population-based study in the northeast of Brazil. Epidemiol. Infect. 2013, 141, 1080–1088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabe, I.B.; Staples, J.E.; Villanueva, J.; Hummel, K.B.; Johnson, J.A.; Rose, L.; Hills, S.; Wasley, A.; Fischer, M.; Powers, A.M. Interim Guidance for Interpretation of Zika Virus Antibody Test Results. Morb. Mortal. Wkly. Rep. 2016, 65, 543–546. [Google Scholar] [CrossRef]
- Ayres, M., Jr.; Ayres, D.L.; Santos, A.A.S. BioEstat 5.3: Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas, 5th ed.; Publicações Avulsas do Mamirauá: Belém, Brazil, 2007. [Google Scholar]

| Samples | PRNT90 | RVNT90 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | ID | # | Year | Time of Disease | ZIKV | DENV2 | Rluc DENV2 | Rluc ZIKV | mCherry ZIKV |
| H726608 | 1 | 2007 a | 7 days | Neg | 1:320 | 1:25,600 | Neg | Neg | |
| H769882 | 2 | 2010 a | 4 days | Neg | 1:20 | 1:400 | Neg | Neg | |
| H769929 | 3 | 2010 a | 4 days | Neg | Neg | Neg | Neg | Neg | |
| H770060 | 4 | 2010 a | 2 days | Neg | Neg | 1:200 | Neg | Neg | |
| H770220 | 5 | 2010 a | 3 days | Neg | Neg | Neg | Neg | Neg | |
| H773035 | 6 | 2010 a | 3 days | Neg | 1:80 | 1:1600 | Neg | Neg | |
| H773052 | 7 | 2010 a | 4 days | Neg | 1:20 | 1:12,800 | Neg | Neg | |
| H773054 | 8 | 2010 a | 3 days | Neg | 1:20 | 1:400 | Neg | Neg | |
| H773564 | 9 | 2010 a | 4 days | Neg | Neg | Neg | Neg | Neg | |
| I | H773565 | 10 | 2010 a | 4 days | Neg | 1:320 | 1:12,800 | Neg | Neg |
| H773582 | 11 | 2010 a | 4 days | Neg | Neg | Neg | Neg | Neg | |
| H774749 | 12 | 2010 a | 5 days | Neg | 1:160 | 1:3200 | Neg | Neg | |
| H775262 | 13 | 2010 a | <5 days | Neg | Neg | Neg | Neg | Neg | |
| H775844 | 14 | 2010 a | <5 days | Neg | Neg | Neg | Neg | Neg | |
| H775845 | 15 | 2010 a | <5 days | Neg | Neg | Neg | Neg | Neg | |
| H775848 | 16 | 2010 a | <5 days | Neg | Neg | Neg | Neg | Neg | |
| H775852 | 17 | 2010 a | <5 days | Neg | Neg | Neg | Neg | Neg | |
| H775853 | 18 | 2010 a | <5 days | Neg | 1:80 | 1:400 | Neg | Neg | |
| H775854 | 19 | 2010 a | <5 days | Neg | Neg | Neg | Neg | Neg | |
| H775862 | 20 | 2010 a | <5 days | Neg | Neg | Neg | Neg | Neg | |
| H627940 | 21 | 2000 a | >5 days | Neg | 1:640 | 1:3200 | Neg | Neg | |
| H632195 | 22 | 2000 a | >5 days | Neg | 1:320 | 1:3200 | Neg | Neg | |
| H674584 | 23 | 2004 a | >5 days | Neg | 1:20 | 1:200 | Neg | Neg | |
| H738095 | 24 | 2007 a | >5 days | Neg | 1:80 | 1:400 | Neg | Neg | |
| H739187 | 25 | 2007 a | 6 days | Neg | 1:320 | 1:12,800 | Neg | Neg | |
| H739983 | 26 | 2008 a | >5 days | Neg | 1:640 | 1:6400 | Neg | Neg | |
| H787665 | 27 | 2012 a | 14 days | Neg | 1:10,240 | 1:25,600 | Neg | Neg | |
| H788930 | 28 | 2012 a | >5 days | Neg | 1:640 | 1:6400 | Neg | Neg | |
| H789010 | 29 | 2012 a | >5 days | Neg | 1:5120 | 1:25,600 | Neg | Neg | |
| II | H789197 | 30 | 2012 a | >5 days | Neg | 1:10,240 | >1:51,200 | 1:200 | 1:200 |
| H789912 | 31 | 2012 a | >5 days | Neg | 1:160 | 1:800 | Neg | Neg | |
| H789990 | 32 | 2012 a | >5 days | Neg | 1:640 | 1:12,800 | Neg | Neg | |
| H789997 | 33 | 2012 a | >5 days | Neg | 1:2560 | 1:25,600 | 1:100 | Neg | |
| H790260 | 34 | 2012 a | >5 days | Neg | 1:1280 | 1:6400 | Neg | Neg | |
| H815485 | 35 | 2015 | >5 days | Neg | 1:20 | Neg | Neg | Neg | |
| H817979 | 36 | 2015 | >5 days | Neg | 1:20 | 1:200 | Neg | Neg | |
| H817981 | 37 | 2016 | >5 days | Neg | 1:1280 | 1:12,800 | Neg | Neg | |
| H819129 | 38 | 2015 | >5 days | Neg | 1:160 | 1:1600 | Neg | Neg | |
| H819519 | 39 | 2015 | >5 days | Neg | 1:320 | 1:1600 | Neg | Neg | |
| H817986 | 40 | 2015 | <5 days | Neg | Neg | Neg | Neg | Neg | |
| H819966 | 41 | 2015 | 3 days | Neg | Neg | Neg | Neg | Neg | |
| H820771 | 42 | 2015 | 1 day | Neg | 1:320 | 1:1600 | 1:800 | 1:400 | |
| H821519 | 43 | 2015 | <5 days | Neg | 1:160 | 1:1600 | Neg | Neg | |
| H821557 | 44 | 2015 | 2 days | Neg | 1:80 | 1:800 | 1:200 | 1:100 | |
| H821585 | 45 | 2015 | 3 days | Neg | 1:20 | 1:200 | 1:100 | Neg | |
| H821735 | 46 | 2015 | <5 days | Neg | Neg | 1:400 | Neg | Neg | |
| H821956 | 47 | 2015 | 2 days | Neg | Neg | Neg | Neg | Neg | |
| III | H822137 | 48 | 2015 | 4 days | Neg | Neg | 1:100 | Neg | Neg |
| H822215 | 49 | 2015 | <5 days | Neg | 1:80 | 1:400 | Neg | Neg | |
| H822217 | 50 | 2015 | <5 days | Neg | 1:80 | 1:800 | Neg | Neg | |
| H822226 | 51 | 2015 | 3 days | Neg | Neg | Neg | Neg | Neg | |
| H822604 | 52 | 2015 | 2 days | Neg | Neg | Neg | Neg | Neg | |
| H823608 | 53 | 2015 | 4 days | Neg | Neg | Neg | Neg | Neg | |
| H823390 | 54 | 2015 | 3 days | Neg | 1:80 | 1:800 | 1:200 | Neg | |
| H823594 | 55 | 2015 | 1 day | Neg | Neg | 1:400 | Neg | Neg | |
| H824562 | 56 | 2015 | 3 days | Neg | 1:320 | 1:800 | Neg | Neg | |
| H824564 | 57 | 2015 | 1 day | Neg | Neg | Neg | Neg | Neg | |
| H824708 | 58 | 2015 b | 4 days | Neg | Neg | 1:1600 | 1:6400 | 1:6400 | |
| H816971 | 59 | 2015 | 12 days | 1:20 | 1:1280 | 1:12,800 | 1:1600 | 1:1600 | |
| H819282 | 60 | 2015 | >5 days | 1:40 | 1:5120 | 1:25,600 | 1:6400 | 1:3200 | |
| H819284 | 61 | 2015 b | >5 days | 1:80 | 1:1280 | 1:6400 | >1:51,200 | 1:3200 | |
| H819360 | 62 | 2015 | 8 days | Neg | 1:640 | 1:12,800 | 1:1600 | 1:400 | |
| H820967 | 63 | 2015 | 7 days | Neg | 1:1280 | 1:12,800 | 1:1600 | 1:800 | |
| H821489 | 64 | 2016 | >5 days | 1:320 | 1:2560 | 1:12,800 | 1:12,800 | 1:1600 | |
| H821491 | 65 | 2015 b | >5 days | Neg | 1:160 | 1:800 | 1:1600 | 1:3200 | |
| H822200 | 66 | 2015 | >5 days | Neg | 1:160 | 1:1600 | 1:1600 | 1:1600 | |
| H822413 | 67 | 2015 | >5 days | Neg | 1:1280 | 1:6400 | 1:1600 | 1:6400 | |
| VI | H822540 | 68 | 2015 | >5 days | 1:20 | 1:640 | 1:6400 | 1:1600 | Neg |
| H823176 | 69 | 2015 b | 50 days | 1:160 | 1:640 | 1:6400 | >1:51,200 | 1:12,800 | |
| H824696 | 70 | 2015 | 7 days | Neg | 1:320 | 1:1600 | 1:200 | 1:400 | |
| H825051 | 71 | 2015 | 11 days | Neg | 1:640 | 1:12,800 | 1:1600 | 1:3200 | |
| H826145 | 72 | 2016 b | >5 days | Neg | 1:20 | 1:400 | 1:1600 | 1:1600 | |
| H828107 | 73 | 2016 | >5 days | Neg | 1:1280 | 1:25,600 | 1:1600 | 1:3200 | |
| H828108 | 74 | 2016 | >5 days | Neg | 1:1280 | 1:6400 | 1:6400 | 1:6400 | |
| H829179 | 75 | 2016 | 19 days | 1:20 | 1:1280 | 1:6400 | 1:1600 | 1:3200 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, J.G.C.; Nunes, B.T.D.; Shan, C.; Moraes, A.F.; Silva, T.R.; de Mendonça, M.H.R.; das Chagas, L.L.; Silva, F.A.e.; Azevedo, R.S.S.; da Silva, E.V.P.; et al. Reporter Virus Neutralization Test Evaluation for Dengue and Zika Virus Diagnosis in Flavivirus Endemic Area. Pathogens 2021, 10, 840. https://doi.org/10.3390/pathogens10070840
Nunes JGC, Nunes BTD, Shan C, Moraes AF, Silva TR, de Mendonça MHR, das Chagas LL, Silva FAe, Azevedo RSS, da Silva EVP, et al. Reporter Virus Neutralization Test Evaluation for Dengue and Zika Virus Diagnosis in Flavivirus Endemic Area. Pathogens. 2021; 10(7):840. https://doi.org/10.3390/pathogens10070840
Chicago/Turabian StyleNunes, Jannyce G. C., Bruno T. D. Nunes, Chao Shan, Adriana F. Moraes, Tais R. Silva, Maria H. R. de Mendonça, Liliane L. das Chagas, Franco A. e Silva, Raimunda S. S. Azevedo, Eliana V. P. da Silva, and et al. 2021. "Reporter Virus Neutralization Test Evaluation for Dengue and Zika Virus Diagnosis in Flavivirus Endemic Area" Pathogens 10, no. 7: 840. https://doi.org/10.3390/pathogens10070840
APA StyleNunes, J. G. C., Nunes, B. T. D., Shan, C., Moraes, A. F., Silva, T. R., de Mendonça, M. H. R., das Chagas, L. L., Silva, F. A. e., Azevedo, R. S. S., da Silva, E. V. P., Martins, L. C., Chiang, J. O., Casseb, L. M. N., Henriques, D. F., Vasconcelos, P. F. C., Burbano, R. M. R., Shi, P.-Y., & Medeiros, D. B. A. (2021). Reporter Virus Neutralization Test Evaluation for Dengue and Zika Virus Diagnosis in Flavivirus Endemic Area. Pathogens, 10(7), 840. https://doi.org/10.3390/pathogens10070840








