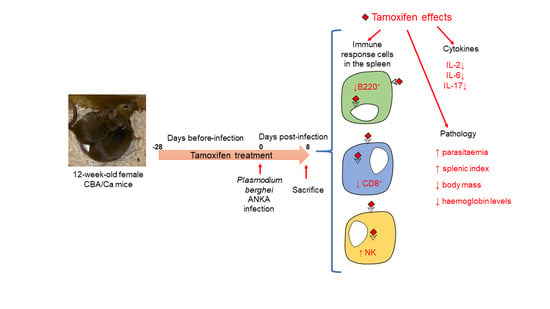
Tamoxifen Suppresses the Immune Response to Plasmodium berghei ANKA and Exacerbates Symptomatology
Abstract
1. Introduction
2. Results
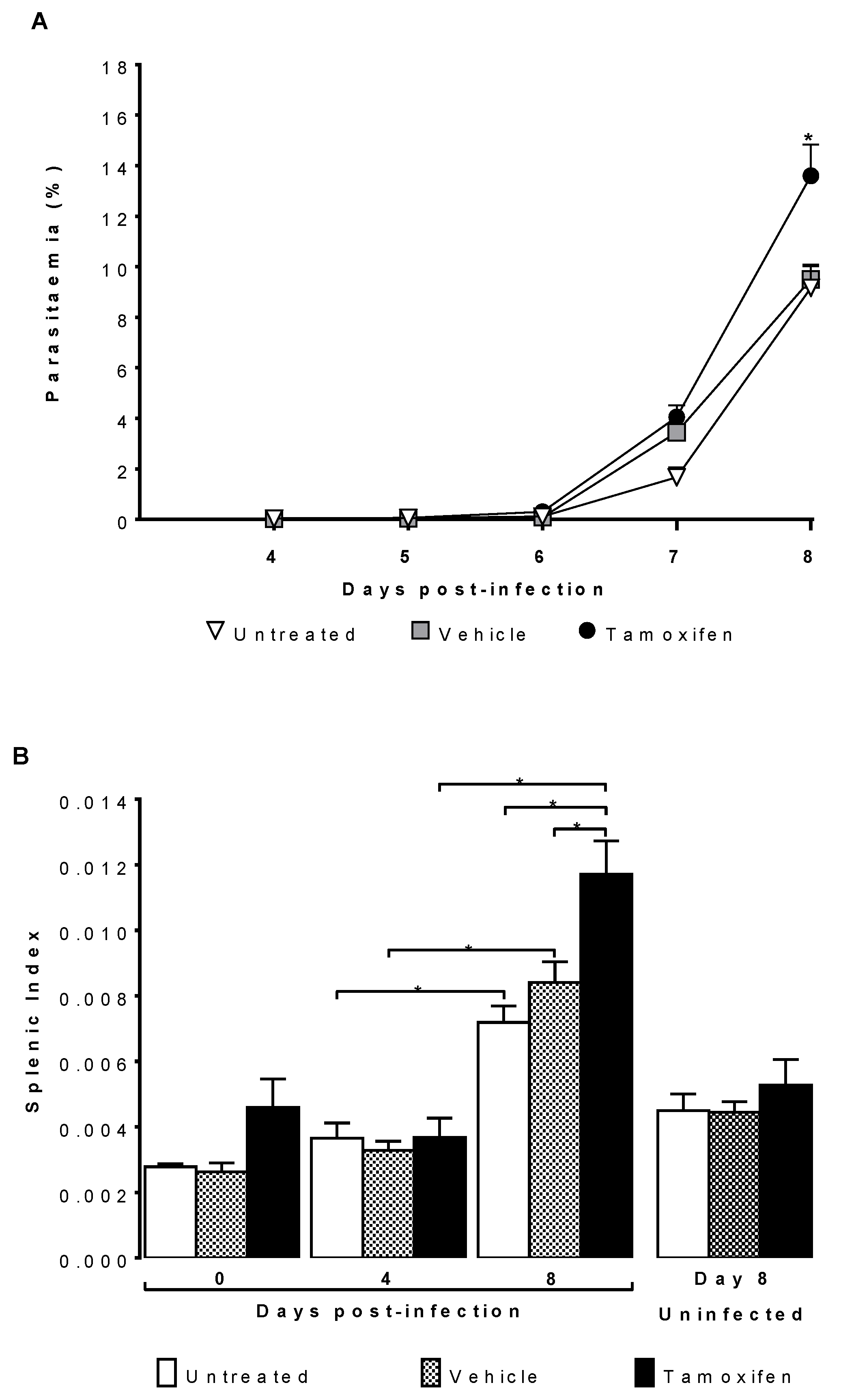
2.1. Tamoxifen Increased Parasitaemia and Splenic Index of CBA/Ca Mice Infected with Plasmodium berghei ANKA
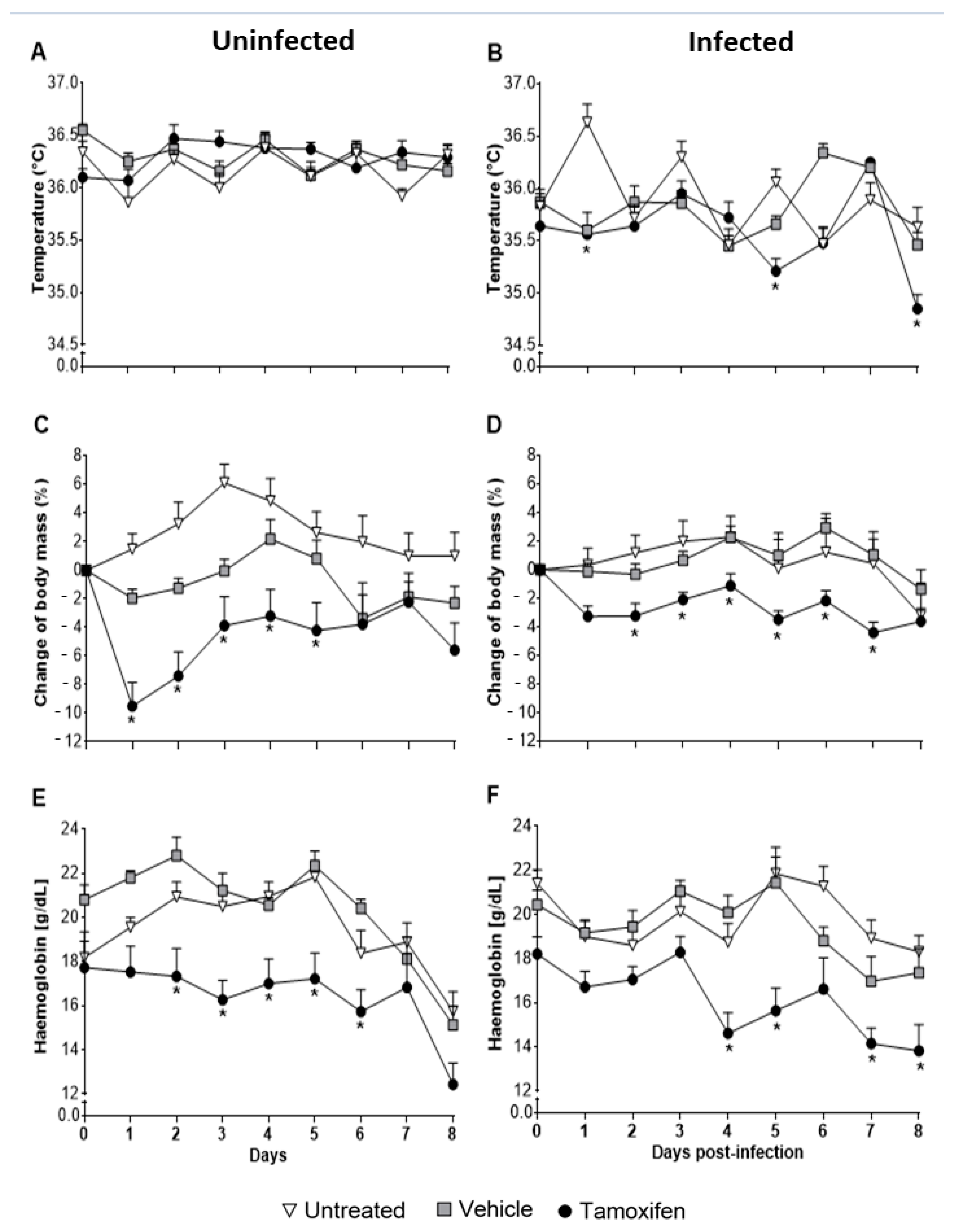
2.2. Tamoxifen Aggravates Pathology by Decreasing Body Temperature, Body Mass, and Haemoglobin Levels in CBA/Ca Mice Infected with Plasmodium berghei ANKA
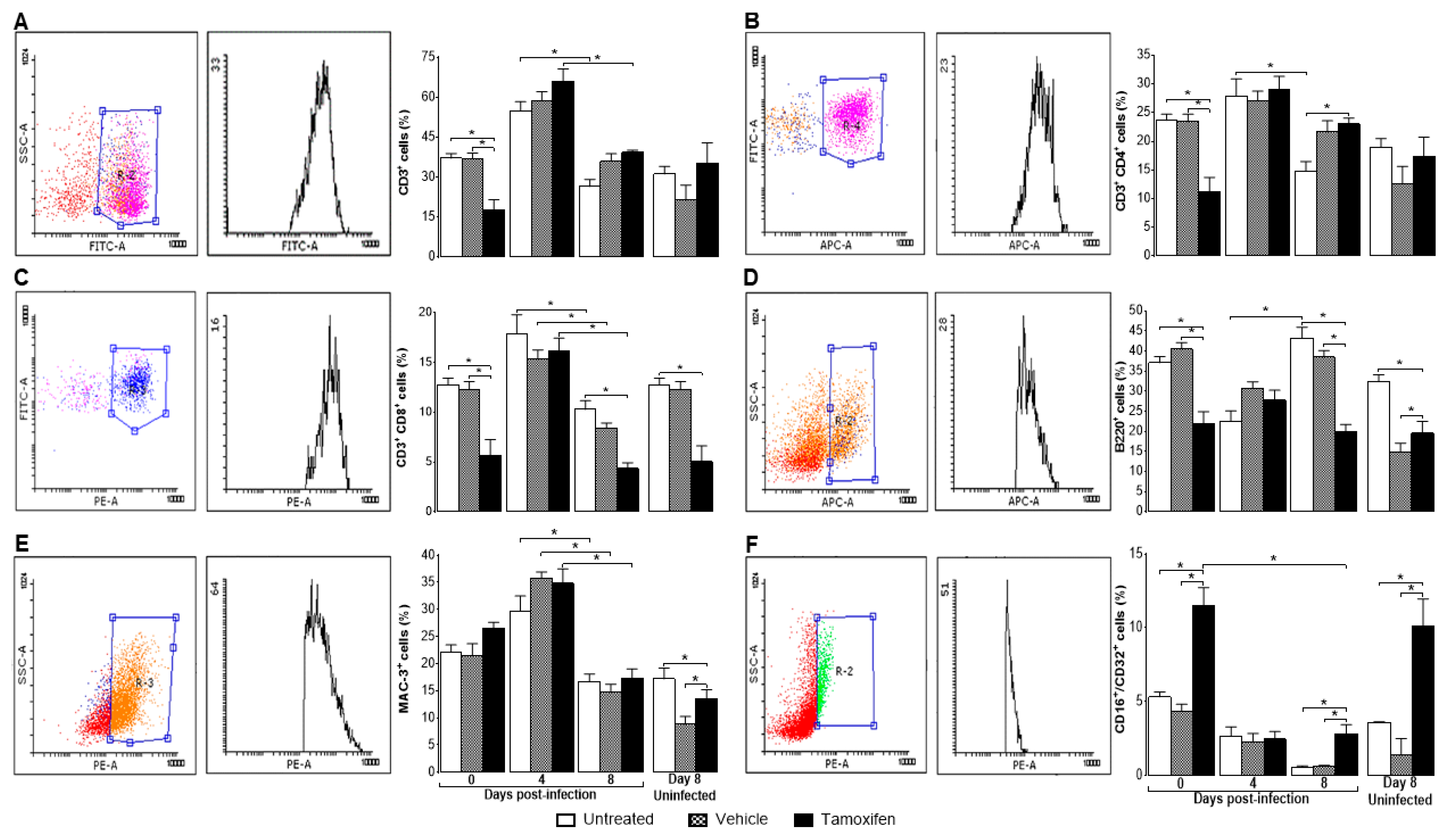
2.3. Tamoxifen Decreased the Percentage of Immune Response Cells in the Spleen of Mice Infected with P. berghei ANKA
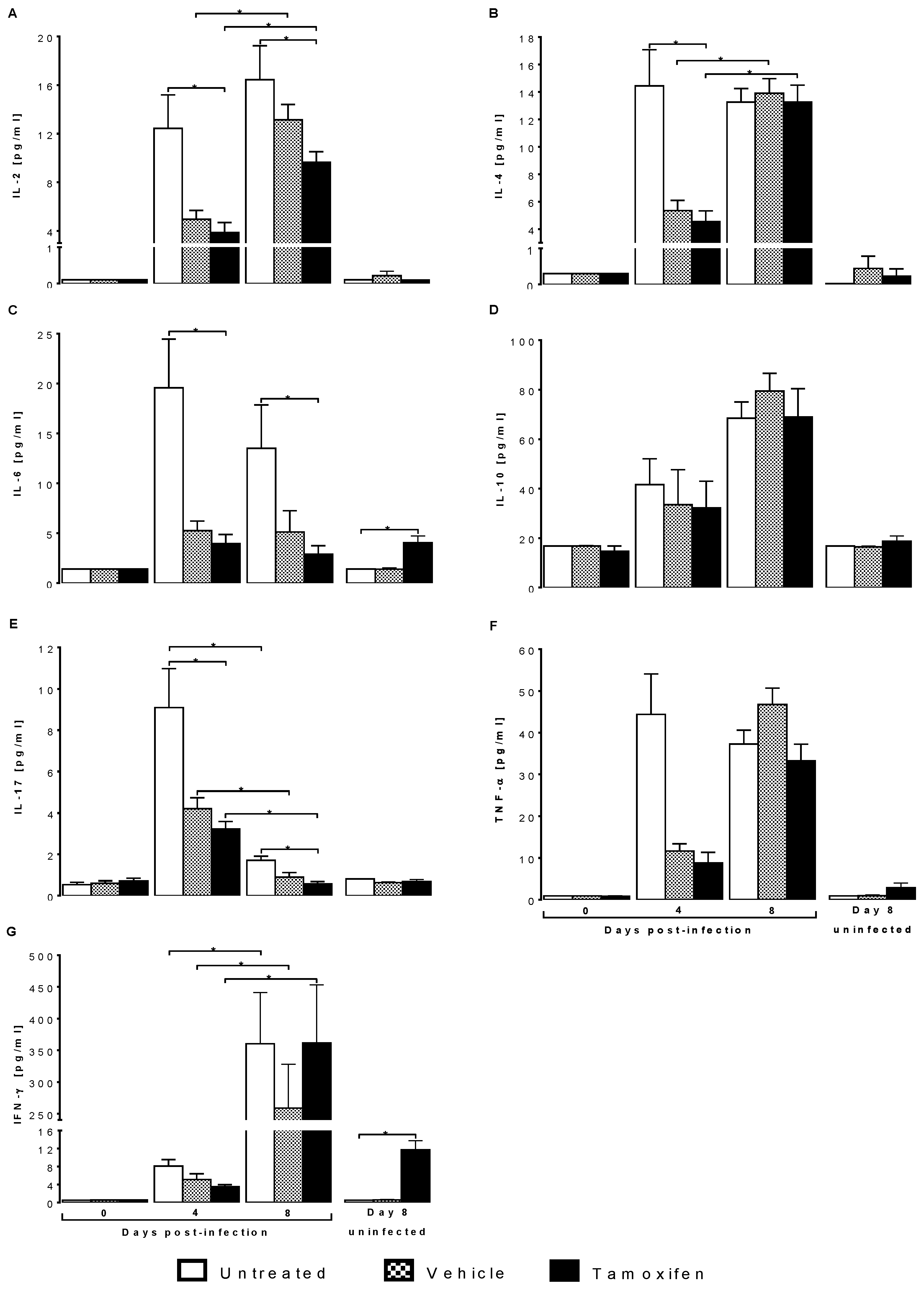
2.4. Tamoxifen Modified Plasma Levels of Th1, Th2 and Th17 Cytokines from CBA/Ca Mice Infected with Plasmodium berghei ANKA
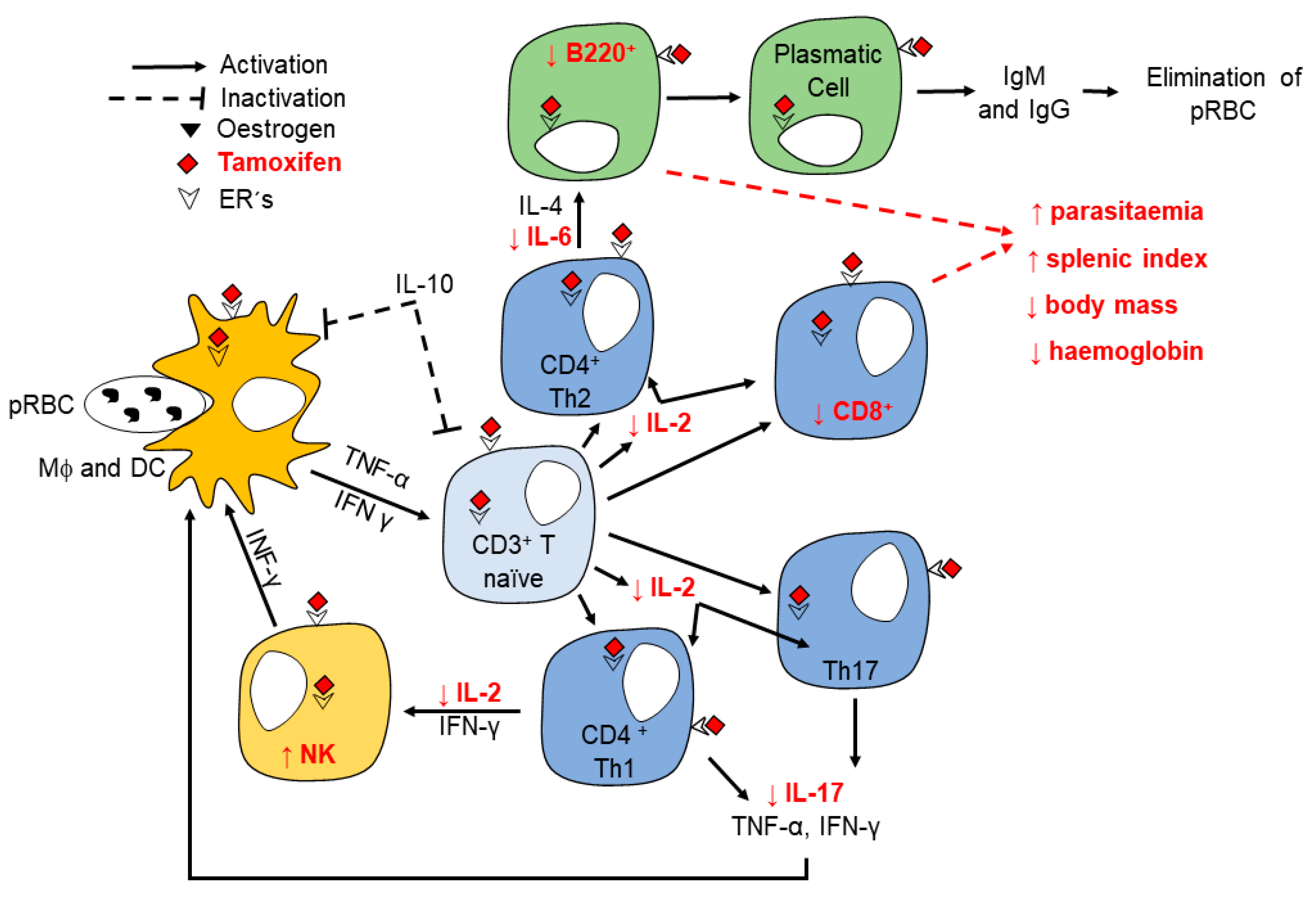
3. Discussion
4. Materials and methods
4.1. Mice and Parasites
4.2. Experimental Design
4.3. Tamoxifen Administration
4.4. Parasitaemia
4.5. Body Temperature
4.6. Body Mass Variation
4.7. Haemoglobin (Hb) Levels
4.8. Splenic Index
4.9. Quantification of Spleen Cell Populations by Flow Cytometry
4.10. Th1/Th2/Th17 Cytokine Quantification
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2020: 20 Years of Global Progress & Challenges; Licence: CCBY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Landgraf, B.; Kollaritsch, H.; Wiedermann, G.; Wernsdorfer, W.H. Parasite density of Plasmodium falciparum malaria in ghanaian schoolchildren: Evidence for influence of sex hormones? Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 73–74. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Saito, S.; Kanagawa, O.; Sendo, F. Possible modulation by male sex hormone of th1/th2 function in protection against Plasmodium chabaudi chabaudi as infection in mice. Exp. Parasitol. 2000, 96, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Cernetich, A.; Garver, L.S.; Jedlicka, A.E.; Klein, P.W.; Kumar, N.; Scott, A.L.; Klein, S.L. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect. Immun. 2006, 74, 3190–3203. [Google Scholar] [CrossRef]
- Nordqvist, J.; Bernardi, A.; Islander, U.; Carlsten, H. Effects of a tissue-selective estrogen complex on b lymphopoiesis and b cell function. Immunobiology 2017, 222, 918–923. [Google Scholar] [CrossRef]
- Mohammad, I.; Starskaia, I.; Nagy, T.; Guo, J.; Yatkin, E.; Vaananen, K.; Watford, W.T.; Chen, Z. Estrogen receptor alpha contributes to t cell-mediated autoimmune inflammation by promoting t cell activation and proliferation. Sci. Signal. 2018, 11, 9415. [Google Scholar] [CrossRef]
- Ghisletti, S.; Meda, C.; Maggi, A.; Vegeto, E. 17beta-estradiol inhibits inflammatory gene expression by controlling nf-kappab intracellular localization. Mol. Cell Biol. 2005, 25, 2957–2968. [Google Scholar] [CrossRef]
- Acconcia, F.; Totta, P.; Ogawa, S.; Cardillo, I.; Inoue, S.; Leone, S.; Trentalance, A.; Muramatsu, M.; Marino, M. Survival versus apoptotic 17beta-estradiol effect: Role of er alpha and er beta activated non-genomic signaling. J. Cell Physiol. 2005, 203, 193–201. [Google Scholar] [CrossRef]
- Marino, M.; Acconcia, F.; Trentalance, A. Biphasic estradiol-induced akt phosphorylation is modulated by pten via map kinase in hepg2 cells. Mol. Biol. Cell 2003, 14, 2583–2591. [Google Scholar] [CrossRef]
- Robinson, D.P.; Hall, O.J.; Nilles, T.L.; Bream, J.H.; Klein, S.L. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific cd8 t cell responses in the lungs. J. Virol. 2014, 88, 4711–4720. [Google Scholar] [CrossRef]
- Maret, A.; Coudert, J.D.; Garidou, L.; Foucras, G.; Gourdy, P.; Krust, A.; Dupont, S.; Chambon, P.; Druet, P.; Bayard, F.; et al. ; et al. Estradiol enhances primary antigen-specific cd4 t cell responses and th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur. J. Immunol. 2003, 33, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, M.C.; Jonsson, C.A.; Lindberg, M.K.; Ohlsson, C.; Carlsten, H. Raloxifene- and estradiol-mediated effects on uterus, bone and b lymphocytes in mice. J. Endocrinol. 2002, 175, 319–327. [Google Scholar] [CrossRef][Green Version]
- Liao, Z.H.; Huang, T.; Xiao, J.W.; Gu, R.C.; Ouyang, J.; Wu, G.; Liao, H. Estrogen signaling effects on muscle-specific immune responses through controlling the recruitment and function of macrophages and t cells. Skelet. Muscle 2019, 9, 20. [Google Scholar] [CrossRef]
- Tzehoval, E.; De Baetselier, P.; Ron, Y.; Tartakovsky, B.; Feldman, M.; Segal, S. Splenic b cells function as immunogenic antigen-presenting cells for the induction of effector t cells. Eur. J. Immunol. 1983, 13, 89–94. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Li, M.; Fu, Y.; Zhang, T.; Han, Q.; Liu, Q. Role of an estradiol regulatory factor-hydroxysteroid dehydrogenase (hsd) in Toxoplasma gondii infection and pathogenicity. J. Steroid Biochem. Mol. Biol. 2017, 174, 176–182. [Google Scholar] [CrossRef]
- Trinconi, C.T.; Reimao, J.Q.; Coelho, A.C.; Uliana, S.R.B. Efficacy of tamoxifen and miltefosine combined therapy for cutaneous leishmaniasis in the murine model of infection with Leishmania amazonensis. J. Antimicrob. Chemother. 2016, 71, 1314–1322. [Google Scholar] [CrossRef]
- Ambrosio, J.R.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Ruiz-Rosado, A.; Sanchez-Orellana, P.L.; Reynoso-Ducoing, O.; Nava-Castro, K.E.; Martinez-Velazquez, N.; Escobedo, G.; Ibarra-Coronado, E.G.; et al. Oestradiol and progesterone differentially alter cytoskeletal protein expression and flame cell morphology in taenia crassiceps. Int. J. Parasitol. 2014, 44, 687–696. [Google Scholar] [CrossRef]
- Legorreta Herrera, M.; Mosqueda Romo, N.A.; Nava Castro, K.E.; Morales Rodríguez, A.L.; Buendía González, F.O.; Morales Montor, J. Sex hormones modulate the immune response to Plasmodium berghei ANKA in CBA/Ca mice. Parasitol. Res. 2015, 114, 11. [Google Scholar] [CrossRef]
- Aguilar-Castro, J.; Cervantes-Candelas, L.A.; Buendia-Gonzalez, F.O.; Nolasco-Perez, T.J.; Lopez-Padilla, M.S.; Fernandez-Rivera, O.; Cervantes-Sandoval, A.; Legorreta-Herrera, M. Dimorphic effect of 17beta-oestradiol on pathology and oxidative stress in experimental malaria. Immunobiology 2020, 225, 151873. [Google Scholar] [CrossRef]
- Cervantes-Candelas, L.A.; Aguilar-Castro, J.; Buendia-Gonzalez, F.O.; Fernandez-Rivera, O.; Nolasco-Perez, T.J.; Lopez-Padilla, M.S.; Chavira-Ramirez, D.R.; Legorreta-Herrera, M. 17beta-estradiol is involved in the sexual dimorphism of the immune response to malaria. Front. Endocrinol. 2021, 12, 643851. [Google Scholar] [CrossRef]
- Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar]
- Behjati, S.; Frank, M.H. The effects of tamoxifen on immunity. Curr. Med. Chem. 2009, 16, 3076–3080. [Google Scholar] [CrossRef]
- Vargas Villavicencio, J.A.; Larralde, C.; Nava, M.A.D.L.; Escobedo, G.; Morales Montor, J. Tamoxifen treatment induces protection in murine cysticercosis. J. Parasitol. 2007, 93, 7. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, G.; Palacios-Arreola, M.I.; Olivos, A.; Lopez-Griego, L.; Morales-Montor, J. Tamoxifen treatment in hamsters induces protection during taeniosis by Taenia solium. Biomed. Res. Int. 2013, 2013, 280496. [Google Scholar] [CrossRef]
- Nicolao, M.C.; Elissondo, M.C.; Denegri, G.M.; Goya, A.B.; Cumino, A.C. In vitro and in vivo effects of tamoxifen against larval stage Echinococcus granulosus. Antimicrob. Agents Chemother. 2014, 58, 5146–5154. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Correa, S.A.P.; Vieira, K.M.; Mendes, T.; Allegretti, S.M.; Miguel, D.C. In vitro schistosomicidal activity of tamoxifen and its effectiveness in a murine model of schistosomiasis at a single dose. Parasitol. Res. 2019, 118, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Landoni, M.; Pinero, T.; Soprano, L.L.; Garcia-Bournissen, F.; Fichera, L.; Esteva, M.I.; Duschak, V.G.; Couto, A.S. Tamoxifen acts on Trypanosoma cruzi sphingolipid pathway triggering an apoptotic death process. Biochem. Biophys. Res. Commun. 2019, 516, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Miguel, D.C.; Yokoyama-Yasunaka, J.K.; Andreoli, W.K.; Mortara, R.A.; Uliana, S.R. Tamoxifen is effective against Leishmania and induces a rapid alkalinization of parasitophorous vacuoles harbouring Leishmania (leishmania) amazonensis amastigotes. J. Antimicrob. Chemother. 2007, 60, 526–534. [Google Scholar] [CrossRef]
- Trinconi, C.T.; Reimao, J.Q.; Bonano, V.I.; Espada, C.R.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.U.; Uliana, S.R.B. Topical tamoxifen in the therapy of cutaneous leishmaniasis. Parasitology 2018, 145, 490–496. [Google Scholar] [CrossRef]
- Weinstock, A.; Gallego-Delgado, J.; Gomes, C.; Sherman, J.; Nikain, C.; Gonzalez, S.; Fisher, E.; Rodriguez, A. Tamoxifen activity against Plasmodium in vitro and in mice. Malar. J. 2019, 18, 378. [Google Scholar] [CrossRef]
- Singh, N.; Puri, S.K. Interaction between chloroquine and diverse pharmacological agents in chloroquine resistant plasmodium yoelii nigeriensis. Acta Trop. 2000, 77, 185–193. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.M.; Riley, E.M. Innate immunity to malaria. Nat. Rev. Immunol. 2004, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Trinconi, C.T.; Miguel, D.C.; Silber, A.M.; Brown, C.; Mina, J.G.M.; Denny, P.W.; Heise, N.; Uliana, S.R.B. Tamoxifen inhibits the biosynthesis of inositolphosphorylceramide in Leishmania. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 475–487. [Google Scholar] [CrossRef]
- Botelho, M.C.; Soares, R.; Vale, N.; Ribeiro, R.; Camilo, V.; Almeida, R.; Medeiros, R.; Gomes, P.; Machado, J.C.; Correia da Costa, J.M. Schistosoma haematobium: Identification of new estrogenic molecules with estradiol antagonistic activity and ability to inactivate estrogen receptor in mammalian cells. Exp. Parasitol. 2010, 126, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Morales-Montor, J.; Larralde, C. The role of sex steroids in the complex physiology of the host-parasite relationship: The case of the larval cestode of Taenia crassiceps. Parasitology 2005, 131, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Nola, M.; Jukic, S.; Ilic-Forko, J.; Babic, D.; Uzarevic, B.; Petrovecki, M.; Suchanek, E.; Skrablin, S.; Dotlic, S.; Marusic, M. Effects of tamoxifen on steroid hormone receptors and hormone concentration and the results of DNA analysis by flow cytometry in endometrial carcinoma. Gynecol. Oncol. 1999, 72, 331–336. [Google Scholar] [CrossRef]
- Thakur, R.S.; Tousif, S.; Awasthi, V.; Sanyal, A.; Atul, P.K.; Punia, P.; Das, J. Mesenchymal stem cells play an important role in host protective immune responses against malaria by modulating regulatory t cells. Eur. J. Immunol. 2013, 43, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Del Portillo, H.A.; Ferrer, M.; Brugat, T.; Martin-Jaular, L.; Langhorne, J.; Lacerda, M.V. The role of the spleen in malaria. Cell Microbiol. 2012, 14, 343–355. [Google Scholar] [CrossRef]
- Zhao, Y.; Hosking, C.; Cunningham, D.; Langhorne, J.; Lin, J.W. Transcriptome analysis of blood and spleen in virulent and avirulent mouse malaria infection. Sci. Data 2020, 7, 253. [Google Scholar] [CrossRef]
- Ageely, H.M.; Dawoud, H.A.; Heiba, A.A. Anemia, interleukin-10, tumor necrosis factor alpha, and erythropoietin levels in children with acute, complicated and uncomplicated malignant malaria in Jazan, Saudi Arabia. J. Egypt. Soc. Parasitol. 2008, 38, 359–370. [Google Scholar]
- Esamai, F.; Mining, S.; Forsberg, P.; Lewis, D.H. A comparison of brain, core and skin temperature in children with complicated and uncomplicated malaria. J. Trop. Pediatr. 2001, 47, 170–175. [Google Scholar] [CrossRef][Green Version]
- Cross, C.E.; Langhorne, J. Plasmodium chabaudi chabaudi (AS): Inflammatory cytokines and pathology in an erythrocytic-stage infection in mice. Exp. Parasitol. 1998, 90, 220–229. [Google Scholar] [CrossRef]
- Ferrer-Lorente, R.; Garcia-Pelaez, B.; Fernandez-Lopez, J.A.; Remesar, X.; Alemany, M. Tamoxifen does not prevent the mobilization of body lipids elicited by oleoyl-estrone. Steroids 2004, 69, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Wade, G.N.; Heller, H.W. Tamoxifen mimics the effects of estradiol on food intake, body weight, and body composition in rats. Am. J. Physiol. 1993, 264, R1219–R1223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, B.; Gomez, N.A.; de Avila, J.M.; Zhu, M.J.; Du, M. Even a low dose of tamoxifen profoundly induces adipose tissue browning in female mice. Int. J. Obes. 2020, 44, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Cruz Silva, M.M.; Madeira, V.M.; Almeida, L.M.; Custodio, J.B. Hydroxytamoxifen interaction with human erythrocyte membrane and induction of permeabilization and subsequent hemolysis. Toxicol. In Vitro 2001, 15, 615–622. [Google Scholar] [CrossRef][Green Version]
- Turka, L.A.; Walsh, P.T. Il-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front. Biosci. 2008, 13, 1440–1446. [Google Scholar] [CrossRef]
- Sebina, I.; Fogg, L.G.; James, K.R.; Soon, M.S.F.; Akter, J.; Thomas, B.S.; Hill, G.R.; Engwerda, C.R.; Haque, A. Il-6 promotes cd4(+) t-cell and b-cell activation during Plasmodium infection. Parasite Immunol. 2017, 39, e12455. [Google Scholar] [CrossRef] [PubMed]
- Oyegue-Liabagui, S.L.; Bouopda-Tuedom, A.G.; Kouna, L.C.; Maghendji-Nzondo, S.; Nzoughe, H.; Tchitoula-Makaya, N.; Pegha-Moukandja, I.; Lekana-Douki, J.B. Pro- and anti-inflammatory cytokines in children with malaria in Franceville, Gabon. Am. J. Clin. Exp. Immunol. 2017, 6, 9–20. [Google Scholar]
- Raballah, E.; Kempaiah, P.; Karim, Z.; Orinda, G.O.; Otieno, M.F.; Perkins, D.J.; Ong’echa, J.M. CD4 T-cell expression of ifn-gamma and il-17 in pediatric malarial anemia. PLoS ONE 2017, 12, e0175864. [Google Scholar] [CrossRef]
- Herbert, F.; Tchitchek, N.; Bansal, D.; Jacques, J.; Pathak, S.; Becavin, C.; Fesel, C.; Dalko, E.; Cazenave, P.A.; Preda, C.; et al. Evidence of il-17, ip-10, and il-10 involvement in multiple-organ dysfunction and il-17 pathway in acute renal failure associated to Plasmodium falciparum malaria. J. Transl. Med. 2015, 13, 369. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes-Candelas, L.A.; Aguilar-Castro, J.; Buendía-González, F.O.; Fernández-Rivera, O.; Cervantes-Sandoval, A.; Morales-Montor, J.; Legorreta-Herrera, M. Tamoxifen Suppresses the Immune Response to Plasmodium berghei ANKA and Exacerbates Symptomatology. Pathogens 2021, 10, 743. https://doi.org/10.3390/pathogens10060743
Cervantes-Candelas LA, Aguilar-Castro J, Buendía-González FO, Fernández-Rivera O, Cervantes-Sandoval A, Morales-Montor J, Legorreta-Herrera M. Tamoxifen Suppresses the Immune Response to Plasmodium berghei ANKA and Exacerbates Symptomatology. Pathogens. 2021; 10(6):743. https://doi.org/10.3390/pathogens10060743
Chicago/Turabian StyleCervantes-Candelas, Luis Antonio, Jesús Aguilar-Castro, Fidel Orlando Buendía-González, Omar Fernández-Rivera, Armando Cervantes-Sandoval, Jorge Morales-Montor, and Martha Legorreta-Herrera. 2021. "Tamoxifen Suppresses the Immune Response to Plasmodium berghei ANKA and Exacerbates Symptomatology" Pathogens 10, no. 6: 743. https://doi.org/10.3390/pathogens10060743
APA StyleCervantes-Candelas, L. A., Aguilar-Castro, J., Buendía-González, F. O., Fernández-Rivera, O., Cervantes-Sandoval, A., Morales-Montor, J., & Legorreta-Herrera, M. (2021). Tamoxifen Suppresses the Immune Response to Plasmodium berghei ANKA and Exacerbates Symptomatology. Pathogens, 10(6), 743. https://doi.org/10.3390/pathogens10060743