Abstract
The description of Gardnerella vaginalis was recently updated and three new species, including nine genome species within Gardnerella, were defined using whole genome sequences and matrix assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry. A fast and simple method based on readily available techniques would be of immense use to identify Gardnerella species in research and clinical practice. Here we show that 34 previously characterized Gardnerella isolates were assigned to the species using partial chaperonin cpn60 sequences. The MALDI Biotyper from Bruker Daltonik GmbH demonstrated the capability to differentiate the phylogenetically diverse groups composed of G. vaginalis/G. piotii and G. leopoldii/G. swidsinskii. Among the phenotypic properties that characterize Gardnerella species are sialidase and β-galactosidase activities. Our data confirmed that the NanH3 enzyme is responsible for sialidase activity in Gardnerella spp. isolates. Almost all G. piotii isolates displayed a sialidase positive phenotype, whereas the majority of G. vaginalis strains were sialidase negative. G. leopoldii and G. swidskinskii displayed a sialidase negative phenotype. β-galactosidase is produced exclusively in G. vaginalis strains. Earlier determined phenotypic characteristics associated with virulence of Gardnerella isolates now assigned to the defined species may provide insights on how diverse species contribute to shaping the vaginal microbiome.
1. Introduction
Gardnerella vaginalis has been the only identified species in the genus Gardnerella for a long time. While this bacterium is found to be closely associated with bacterial vaginosis (BV), a form of vaginal dysbiosis [1,2], Gardnerella isolates from BV-positive women showed genetic and phenotypic diversity [3,4,5]. Gardnerella has also been detected in vaginal microbial communities of healthy BV-negative women [1,6]. These findings suggest a diverse role of genetic variants of Gardnerella in the vaginal microbiota.
The earlier proposed biotyping [7] and genotyping [8] schemes had limited success revealing Gardnerella diversity. The comparative genomic analysis of the 17 genomes allowed separating Gardnerella isolates into 4 subgroups, which likely are separate species [9,10]. The existence of four subgroups within Gardnerella was confirmed by the sequence analysis of cpn60 gene [11,12]. In 2019, Vaneechoutte and colleagues [13] performed the genome analysis (digital DNA–DNA hybridization (DDH) and average nucleotide identity (ANI)) of 81 whole genomes of Gardnerella isolates and proposed the genus separation into four species: Gardnerella vaginalis, Gardnerella piotii, Gardnerella leopoldii, Gardnerella swidsinskii, and 9 genome species. This work also confirmed earlier findings that Gardnerella spp. cannot be differentiated based on the 16S rRNA gene sequences as they share no less than 98.5% sequence similarity. While all G. vaginalis isolates corresponded to previously described subgroup 1, subgroup 2 included G. piotii and genome species 3, species G. leopoldii and G. swidsinskii corresponded to subgroup 4, and subgroup 3 contained at least three neither named nor formally described species most probably due to the low number of isolates [13]. The colonies of four named Gardnerella species had the same appearance on blood agar plates, but the differences in β-galactosidase and sialidase activities were determined.
Selection of a fast, simple, and not expensive method based on readily available techniques would be of great use to identify Gardnerella species in research and clinical practice. In this study, we aimed to differentiate 34 previously characterized Gardnerella isolates of known subgroups/clades [14,15,16] into newly defined species and genome groups [13] using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry and chaperonin cpn60 universal target (UT) sequences [11]. We also determined how the presence of the genes coding for sialidases NanH1, NanH2, and NanH3 reflects the ability of Gardnerella species to display sialidase activity. Assigning the previously determined phenotypic features [16] of three subgroups/clades to the newly defined Gardnerella species provide an understanding of how these species may impact the development of vaginal dysbiosis.
2. Results and Discussion
2.1. Collection of Gardnerella Isolates
Thirty-three Gardnerella isolates from the characterized vaginal samples were subtyped previously [14] based on the subgroup/clade-specific genes as described earlier [9,10]. The strain GV37 was isolated from blood [15] and its whole genome sequence was deposited in GenBank (acc. no. CP019058.1). Gardnerella isolates were assigned to three subgroups (clade 1, clade 2, and clade 4), whereas isolate 86.1 was negative in all clade-specific PCR assays and defined as an unknown subgroup [14]. The phenotypic characteristics of the isolates and their distribution among subgroups were determined previously: the in vitro ability to produce the toxin vaginolysin, to form a biofilm and express sialidase activity [16]. Vaginolysin was quantified using a monoclonal antibody-based sandwich ELISA. The amount of biofilm produced in brain-heart infusion broth with supplements (BHIs) in 96-well microplate was quantified by safranin staining. The presence of the sialidase A gene was determined by PCR, whereas the sialidase activity in culture supernatants of Gardnerella isolates was quantified using fluorogenic substrate [16].
The resolving power of protein profiling by MALDI-TOF and partial chaperonin cpn60 sequences were used for the separation of 34 Gardnerella isolates into the newly defined species [13].
2.2. Differentiations of Gardnerella Species Based on cpn60 UT Sequences
It was demonstrated that chaperonin cpn60 universal target sequences of 552 bp are a perfect tool for determining Gardnerella subgroups [12] and the newly defined species and genome species [17]. In this study, Gardnerella isolates were differentiated in the phylogenetic tree based on cpn60 UT sequences (Figure 1). The reference sequences from the type strains of four named species and nine genome species [13] were included. Fifteen isolates that correspond to the previously determined subgroup/clade 1 and G. vaginalis type strain (ATCC 14018) share the branch in the tree. Although G. vaginalis and genome species 2 share the same node, they are well separated with high bootstrap support. Ten isolates were clustered with G. piotii type strain. Four strains (63.2, 65.2, 82.2, and 86.3) from this cluster and genome species 3 type strain share the same node. The separation of subgroup/clade 2 isolates into G. piotii and genome species 3 was consistent with the phylogenetic relationship described earlier [17].
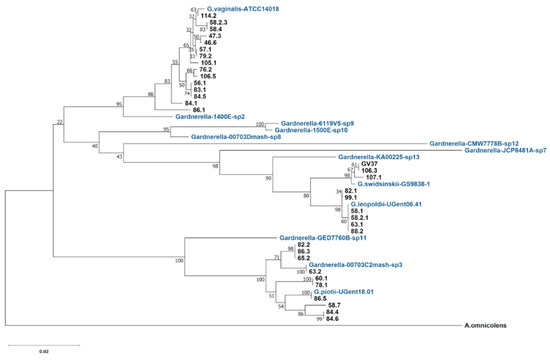
Figure 1.
Phylogenetic relationships of 34 Gardnerella spp. isolates based on cpn60 UT sequences. The type strains of G. vaginalis, G. piotii, G. swidsinskii, G. leopoldii species and nine genome species [13] were included. Evolutionary history was inferred using the neighbor-joining method [18]. The percentage of the replicate trees in which the associated taxa clustered together in the bootstrap test of 500 replicates is indicated. Alloscardovia omnicolens sequence was included as an outgroup [17]. Evolutionary analyses were conducted in MEGA X [19].
The isolates corresponding to the previously determined subgroup/clade 4 were separated into G. swidsinskii and G. leopoldii species by MALDI-TOF and whole genome comparison [13]. Six isolates (58.1, 58.2.1, 63.1, 82.1, 88.2, and 99.1) were grouped with G. leopoldii type strain (UGent 06.41) and three (106.3, 107.1, and GV37) with G. swidsinskii type strain (GS9838-1), although both species displayed close relationship in the phylogeny that is in agreement with the results described in [17]. The GV37 isolate with known whole genome sequence has previously been attributed to G. swidsinskii [13]. Any other genome species except for genome species 3 and four named species were not identified among the isolates.
Pairwise distances between the cpn60 UT nucleotide sequences were calculated (Table S1). In contrast to the data obtained by Hill et al. [17], we found strains with identical cpn60 UT sequence (Table S1). The sequence of isolate 63.2 fully matched the sequence of genome species 3 type strain 00703C2mash-sp3. Isolates 58.1, 58.2.1, 63.1, 88.2, and G. leopoldii type strain (UGent 06.41) had identical sequence. Isolate 86.5 and G. piotii type strain share the same cpn60 UT. The following pairs and triplets of isolates had identical cpn60 UTs: 58.2.3 and 58.4; 57.1 and 79.2; 56.1, 83.1 and 84.5; GV37 and 106.3; 82.1 and 99.1; 82.2, 86.3 and 65.2; 60.1 and 78.1; 84.4 and 84.6. The isolates sharing the same partial cpn60 sequences represent different strains that were verified by a random amplified polymorphic DNA (RAPD) analysis performed previously [16].
2.3. Resolution of Gardnerella Species Based on MALDI Biotyper Protein Profiling
Vaneechoutte and colleagues [13] demonstrated that Gardnerella species could be distinguished by MALDI-TOF mass spectrometry, an indispensable tool for clinical microbiology laboratories. Four Gardnerella species can be separated mainly into pairs of G. vaginalis/G. piotii and G. leopoldii/G. swidsinskii based on their MALDI spectra. Table 2 in the paper by Vaneechoutte et al. 2019 [13] showed seven peak variations (1 single peak and 3 peak pairs) which differentiate G. vaginalis and G. piotii species: two peak pairs (at mass-to-charge (m/z) 4422/4429 and 8842/8857 representing single and double-charged ions of the same masses) and the presence/absence of the single peak at m/z 5162, and the peak pair at m/z 6855/6885. A unique peak at m/z 2704 was proposed to be characteristic to resolve G. leopoldii and G. swidsinskii species. The presence/absence of a single peak at m/z 5349 and the peak pairs at m/z 4849/4928/(9795/9853) differentiate between G. vaginalis/G. piotii and G. leopoldii/G. swidsinskii [13].
Protein profiling of 34 Gardnerella isolates was performed using the MALDI Biotyper (Bruker Daltonik GmbH) mass spectrometer. The recorded 34 MALDI spectra sets were named based on their cpn60 UT sequences and grouped together. Fifteen G. vaginalis, 10 G. piotii/genome species 3, 6 G. leopoldii and 3 G. swidsinskii strains were subjected for analysis. Figure 2 shows the mass peaks at m/z in the mass spectrum of Gardnerella strains.
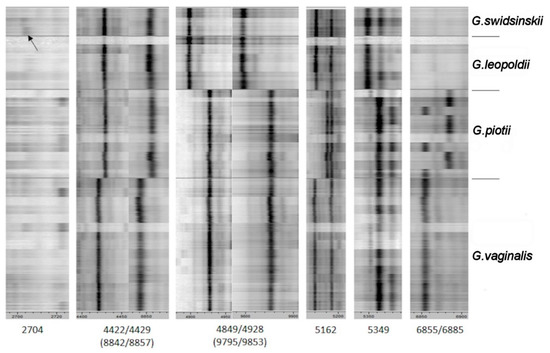
Figure 2.
Mass spectra (n~600) of 34 Gardnerella strains. Peaks at mass-to-charge (m/z) were indicated according to [13]. The arrow indicates the peak at m/z 2704.
All reference spectra (Main Spectrum Profile, MSP) were used for calculation of log(scores) (Table S2) against each other as a taxonomical distance. The log(score) distance was used to demonstrate the taxonomical relationship of Gardnerella strains based on a MALDI Biotyper dendrogram (Figure 3).
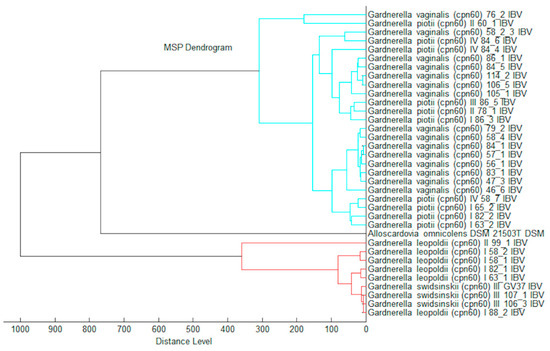
Figure 3.
Matrix assisted laser desorption ionization (MALDI) Biotyper log(score) based dendrogram.
In this study, the peak at m/z 2704 proposed as a unique mark to differentiate G. leopoldii and G. swidsinskii species [13] was observed as a very faint peak (indicated by the arrow in Figure 2). Any other peaks suitable for separation of these two species were not determined. Further, the log(score) based routine identification (Table S2) showed the same result.
The single peak at m/z 5162 (Figure 2) is a specific mark for G. vaginalis and G. piotii differentiation. Further, the peak pairs at m/z 4422/4429/(8842/8857) could be used as species-specific signals. The peak pair at m/z 6855/6885 did not increase the discriminatory power as several strains of G. piotii had a peak at 6855 that was characteristic of G. vaginalis in the previous study [13]. In general, very close taxonomic relation of G. vaginalis and G. piotii was demonstrated by the MALDI Biotyper log(score) algorithm for routine species differentiation (Table S2).
Any characteristic peaks suitable to resolve G. piotii and genome species 3 were not observed.
The group G. leopoldii/G. swidsinskii can be distinguished reliably from the group G. vaginalis/G. piotii based on the log(scores) routine identification using the MALDI Biotyper. In the future, G. vaginalis could be separated from G. piotii via the creation of an automated subtyping/differentiation module. Currently, the manual peak picking and peak comparing to the published data could be alternatively used for species discrimination. The next library updates for automated MALDI Biotyper identification will include two species: Gardnerella vaginalis containing the matching hint closely related to Gardnerella piotii and the species Gardnerella leopoldii/Gardnerella swidsinskii.
Thus, the cpn60-based approach showed the capability to separate G. vaginalis, G. piotii/genome species 3, G. swidsinskii, and G. leopoldii species (Figure 1). However, the dendrogram of the MALDI-TOF MS profiles generated using the MALDI Biotyper differentiated the phylogenetically diverse groups composed of species of G. vaginalis/G. piotii and G. leopoldii/G. swidsinskii (Figure 3). The fact that G. vaginalis and G. piotii were not distinguished implies the close relatedness of these species in the genus at the proteome level.
2.4. Phenotypic Characteristics of Gardnerella Species
Sialidase activity is an important phenotypic characteristic of Gardnerella spp. connected with mucus degradation and the development of BV clinical features [20,21,22]. Although the gene nanH1 coding for sialidase NanH1 (former sialidase A [23]) was found in sialidase activity positive strains of Gardnerella subgroups/clades 1, 2, and 3, the gene was also detected in activity-negative strains raising the question about alternative genes responsible for activity or regulation of the nanH1 expression [12,16]. Recently two additional sialidases NanH2 and NanH3 with a broad range of activity were detected in Gardnerella spp. [23]. The genes nanH2, nanH3 or both were found in activity-positive strains, but absent in activity-negative isolates. All this suggests that these enzymes, but not NanH1, are the primary sources of sialidase activity [23]. Sialidase activity in Gardnerella spp. was found to be cell-associated or secreted [20]. Protein organization predicts that NanH2 is a secreted enzyme, NanH3 may be intracellular and/or secreted, whereas NanH1 most probably is intracellular [23].
We found that all G. swidsinskii/G. leopoldii strains did not contain the nanH1-nanH2-nanH3 genes and they were sialidase activity-negative (Figure S1; Table S3) by both quantitative filter spot (this study) and the qualitative fluorometric [16] assays. Even though the nanH1 gene was found in all G. vaginalis strains (n = 15), the sialidase-positive isolates (n = 3) encoded NanH3, except for strain 58.2.3, which was activity negative although possessed nanH3.
None of G. vaginalis isolates contained the nanH2 gene (Figure S1). Nine of ten G. piotii/genome species 3 strains exhibited sialidase activity. The nanH3 gene was found in all sialidase activity-positive isolates except for G. piotii 86.5, which contained nanH1 and nanH2. Isolate 60.1 was activity-negative, although contained the nanH3 gene (Figure S1).
Among sialidase-positive strains of Gardnerella species, we did not find strains containing solely nanH1. The nanH2 gene was most often found together with nanH3. Our data confirm the recent findings [23], that the sialidase-positive phenotype correlates with the presence of nanH3. The gene coding for NanH3 prevails in G. piotii and the closely related genome species 3, but it is less common in G. vaginalis. We agree with the assumption that G. vaginalis could gain nanH3 from G. piotii through horizontal gene transfer (HGT) [24]. G. vaginalis participates more frequently in HGT [24] acquiring the genes from other Gardnerella species that co-exist in vaginal microbiota [10,14,17].
The characteristic feature of G. vaginalis is a β-galactosidase activity that is consistent with the data obtained by Vaneechoutte and colleagues [13]. This activity was not found to be present in neither G. piotii/genome species 3 nor G. leopoldii/G. swidsinskii strains.
The phenotypic characteristics previously performed in vitro of three Gardnerella subgroups/clades [16] were assigned to the newly differentiated species (G. vaginalis, G. piotii/genome species 3, and G. swidsinskii/G. leopoldii) (Table S3). G. vaginalis strains except 86.1 contained the vly gene and produced toxin vaginolysin as well as expressed the ability to form a biofilm, but a minority (3/15) of strains was sialidase-positive. A vast majority of G. piotii/genome species 3 strains produced a sialidase and developed a biofilm, whereas the vly gene was absent from nearly half the strains. It was proposed that vaginolysin is not a part of a core genome and may be lost or gained by Gardnerella species [24]. The characteristic feature of G. leopoldii/G. swidsinkii strains is a sialidase activity-negative phenotype. However, the specification of additional isolates is required to differentiate closely related G. leopoldii and G. swidsinkii species.
The Gardnerella isolates classified into species were previously isolated from vaginal samples of BV-positive and BV-negative women [14]. We updated the table provided in [16] placing the species name for each isolate (Table S4). The vast majority of vaginal samples contained multiple Gardnerella clades, however, we isolated the strains of single or several species from these samples. Overall, five Gardnerella species were found in vaginal samples. The strains of three species (G. vaginalis, G. piotii, and G. leopoldii) isolated from the vaginal sample 058S1 (Nugent score = 9) matched the clades identified in that sample by PCR (Table S4). However, some clades (e.g., clade 2) contain several species [13]. A low abundance of particular species in vaginal samples and cultivation issues may result in a loss of isolates. The recent identification of Gardnerella species in noncultured vaginal samples based on the cpn60 UT sequences [17] showed that the most frequently detected species are four named species and genome species 3, an observation that is in agreement with our data. Genome species 2 and 7 to 13 were rarely detected in vaginal samples [17].
3. Materials and Methods
3.1. Bacterial Strains and Cultivation Conditions
Gardnerella spp. isolates were obtained from characterized vaginal samples of women from Lithuania [14]. Bacterial stocks were stored at –80 °C in tryptic soy broth (TSB) (Liofilchem, Roseto degli Abruzzi, Italy) supplemented with 20% (v/v) horse serum (Oxoid, Thermo Fisher Scientific, Waltham, MA, USA) and 15% (v/v) glycerol. The isolates were revived on chocolate agar with Vitox (Oxoid) and incubated at 37 °C in 6% CO2 and 15% O2 atmosphere (CO2 Gen, Oxoid) for 48 h. The isolates 58.2.3, 58.4, 84.4, 84.6, 86.1, and 78.1 were incubated for 48 h in anaerobic conditions generated by AnaeroGen (Oxoid).
3.2. Sequencing of cpn60 Universal Target Regions
The bacterial suspension in water was repeatedly frozen and thawed. After centrifugation, the supernatant was used for PCR. Enzymes and kits were obtained from Thermo Fisher Scientific (Vilnius, Lithuania). The amplification of cpn60 sequence was carried out with primers H729 and H730 [11] using Maxima Hot Start Taq DNA polymerase or Dream Taq Hot Start Taq polymerase in the reaction volume of 15 µL. The reactions included denaturation at 94 °C for 4 min, 40 amplification cycles consisting of denaturation for 30 s at 95 °C, annealing for 30 s at 48 °C, and extension for 30 s at 72 °C. The final extension step was prolonged for 2 min. The PCR products were purified using the GeneJET PCR Purification Kit and sequenced with primer Seq-H729 (5′-CGCCAGGGTTTTCCCAGTCACGAC) to identify the 552-bp universal target (UT) sequence of the cpn60 gene [25]. The cpn60 UT sequences were deposited at GenBank (accession numbers MT501265–MT501298).
3.3. Phylogenetic Analysis
cpn60 UT sequences from the type strains of four named Gardnerella species (G. vaginalis, G. piotii, G. leopoldii, and G. swidsinskii) and nine genome species [13] were obtained from Chaperonin Database Search (cpnDB) (http://www.cpndb.ca/search.php)(accessed 13 October 2020). A phylogenetic tree based on 552-bp cpn60 UT was built and visualized using MEGA X [19]. Pairwise distances between the sequences were calculated by MEGA X. The type strain Alloscardovia omnicolens (DSM 21503) was included as a root [17].
3.4. MALDI-TOF MS—MALDI Biotyper
For MALDI-TOF MS (Matrix One Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry) analysis, 1 µL inoculation loop of fresh bacterial cells was suspended in 75% ethanol and stored at –20 °C until further processing. The cell suspensions in ethanol were centrifuged at 13.000× g for 2 min, the supernatant was discarded and the residual sample centrifuged again for a short time. The remaining ethanol was discarded and the cell pellet was carefully suspended in 50 µL of 70% formic acid followed by the addition of 50 µL acetonitrile. After mixing, the suspension was centrifuged at 13.000× g for 2 min. One µL of supernatant was transferred to the disposable MALDI target plate (MSP Biotarget 96, Bruker Daltonik GmbH, Bremen, Germany). Eight replicates of each sample were loaded on the plate, dried at room temperature, and overlaid with 1 µL HCCA (a-cyano-4-hydroxycinnamic acid) matrix solution (Bruker Daltonik GmbH). Each assay included the Bruker Bacterial Test Standard (BTS). Spectra for each sample on the target plate were acquired three times, thus resulting in 24 individual MALDI spectra for each strain. All MALDI measurements were performed using the Bruker standard measurement procedures (standard flexControl method, standard AutoX method, standard MBT-Process method) without any alterations. After spectra quality check (QC) and internal recalibration the MALDI Biotyper standard algorithms were used to create the reference spectra (MSPs).
3.5. Detection of the nanH2 and nanH3 Genes and Sialidase Activity by a Filter Spot Test
The nanH2 and nanH3 genes were detected by PCR using primers and cycling conditions described in [23]. A qualitative filter paper spot test using cultures in duplicate was applied as described previously [26].
3.6. β-galactosidase Activity
A colorimetric assay with o-nitrophenol-beta-d-galactosidase (ONPG) tablets (Sigma Aldrich) according to the manufacturer’s instructions was used to detect β-galactosidase activity of Gardnerella spp. strains.
4. Conclusions
The recent amendment of the Gardnerella taxonomic description prompted us to deploy tools for differentiation of characterized 34 Gardnerella isolates of known clade/subgroup into species. Here several techniques were used for species discrimination. Four named Gardnerella species and genome species 3 were resolved in the phylogenetic tree based on cpn60 UT sequences. However, the molecular method utilizing partial cpn60 sequences is a sensitive and specific technique that remains time-consuming. The MALDI Biotyper, based on a sensitive, fast and widely-used MALDI-TOF MS method, demonstrated capability to reliably differentiate the phylogenetically diverse groups composed of species G. leopoldii/G. swidsinskii and G. vaginalis/G. piotii. Our results confirmed recent findings that sialidase NanH3 is responsible for sialidase activity in a collection of 34 Gardnerella isolates. G. leopoldii and G. swidskinskii species do not contain any genes coding for sialidases and display a sialidase activity-negative phenotype. The β-galactosidase activity was detected only in G. vaginalis strains.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/10/3/277/s1, Table S1: Pairwise distances between cpn60 UT nucleotide sequences, Table S2: Comparison of MALDI-TOF spectra of Gardnerella isolates and calculation of log(score), Table S3: Characteristics of Gardnerella isolates, Table S4: Gardnerella species detected in the characterized vaginal samples, Figure S1: PCR detection of the nanH2 and nanH3 genes.
Author Contributions
Conceptualization, A.B., T.M., and M.P.; methodology, A.B., T.M., and M.P.; software, A.B. and T.M.; formal analysis, A.B., T.M., and M.P.; resources, M.P. and T.M.; writing—original draft preparation, A.B., T.M., and M.P.; writing—M.P.; visualization, A.B. and T.M.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of Lithuania), grant number S-MIP-17-49.
Institutional Review Board Statement
Ethical review and approval were waived for this study as human participants, specimens, or tissue samples were not involved. In this study we analyzed the clinical isolates obtained from the previous study, results of which were published in Janulaitiene et al., BMC Infect Dis, 2017; doi: 10.1186/s12879-017-2501-y. The previous study (Janulaitiene et al., 2017) was approved by the Lithuanian Bioethics Committee (protocol no. 158200-1-3697-223, date of approval 12/11/2013; amendment no. 2, 8/12/2015). Written informed consent was obtained from all study participants prior to enrollment.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary material.
Acknowledgments
We thank Carlos Cordeiro for his advice.
Conflicts of Interest
Thomas Maier is the head of Microbiological Laboratory, R&D Bioanalytics, MALDI Biotyper Business Area Microbiology & Diagnostics, Bruker Daltonik GmbH. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial Communities in Women with Bacterial Vaginosis: High Resolution Phylogenetic Analyses Reveal Relationships of Microbiota to Clinical Criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef]
- Santiago, G.L.D.S.; Deschaght, P.; El Aila, N.; Kiama, T.N.; Verstraelen, H.; Jefferson, K.K.; Temmerman, M.; Vaneechoutte, M. Gardnerella vaginalis comprises three distinct genotypes of which only two produce sialidase. Am. J. Obstet. Gynecol. 2011, 204, 450.e1–450.e7. [Google Scholar] [CrossRef]
- Pleckaityte, M.; Janulaitiene, M.; Lasickiene, R.; Zvirbliene, A. Genetic and biochemical diversity of Gardnerella vaginalis strains isolated from women with bacterial vaginosis. FEMS Immunol. Med. Microbiol. 2012, 65, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Jefferson, K.K.; Cerca, N. Genetic Heterogeneity and Taxonomic Diversity among Gardnerella Species. Trends Microbiol. 2020, 28, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Zozaya-Hinchliffe, M.; Lillis, R.; Martin, D.H.; Ferris, M.J. Quantitative PCR Assessments of Bacterial Species in Women with and without Bacterial Vaginosis. J. Clin. Microbiol. 2010, 48, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Piot, P.; Van Dyck, E.; Peeters, M.; Hale, J.; A Totten, P.; Holmes, K.K. Biotypes of Gardnerella vaginalis. J. Clin. Microbiol. 1984, 20, 677–679. [Google Scholar] [CrossRef]
- Ingianni, A.; Petruzzelli, S.; Morandotti, G.; Pompei, R. Genotypic differentiation of Gardnerella vaginalis by amplified ribosomal DNA restriction analysis (ARDRA). FEMS Immunol. Med. Microbiol. 1997, 18, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Earl, J.; Retchless, A.; Hillier, S.L.; Rabe, L.K.; Cherpes, T.L.; Powell, E.; Janto, B.; Eutsey, R.; Hiller, N.L.; et al. Comparative Genomic Analyses of 17 Clinical Isolates of Gardnerella vaginalis Provide Evidence of Multiple Genetically Isolated Clades Consistent with Subspeciation into Genovars. J. Bacteriol. 2012, 194, 3922–3937. [Google Scholar] [CrossRef] [PubMed]
- Balashov, S.V.; Mordechai, E.; Adelson, M.E.; Gygax, S.E. Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. J. Med. Microbiol. 2014, 63, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, T.P.; Schellenberg, J.J.; Hill, J.E. Resolution and Characterization of Distinct cpn60-Based Subgroups of Gardnerella vaginalis in the Vaginal Microbiota. PLoS ONE 2012, 7, e43009. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.J.; Jayaprakash, T.P.; Gamage, N.W.; Patterson, M.H.; Vaneechoutte, M.; Hill, J.E. Gardnerella vaginalis Subgroups Defined by cpn60 Sequencing and Sialidase Activity in Isolates from Canada, Belgium and Kenya. PLoS ONE 2016, 11, e0146510. [Google Scholar] [CrossRef]
- Vaneechoutte, M.; Guschin, A.; Van Simaey, L.; Gansemans, Y.; Van Nieuwerburgh, F.; Cools, P. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int. J. Syst. Evol. Microbiol. 2019, 69, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Janulaitiene, M.; Paliulyte, V.; Grinceviciene, S.; Zakareviciene, J.; Vladisauskiene, A.; Marcinkute, A.; Pleckaityte, M. Prevalence and distribution of Gardnerella vaginalis subgroups in women with and without bacterial vaginosis. BMC Infect. Dis. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tankovic, J.; Timinskas, A.; Janulaitiene, M.; Zilnyte, M.; Baudel, J.-L.; Maury, E.; Zvirbliene, A.; Pleckaityte, M. Gardnerella vaginalis bacteremia associated with severe acute encephalopathy in a young female patient. Anaerobe 2017, 47, 132–134. [Google Scholar] [CrossRef]
- Janulaitiene, M.; Gegzna, V.; Baranauskiene, L.; Bulavaitė, A.; Simanavicius, M.; Pleckaityte, M. Phenotypic characterization of Gardnerella vaginalis subgroups suggests differences in their virulence potential. PLoS ONE 2018, 13, e0200625. [Google Scholar] [CrossRef]
- Hill, J.E.; Albert, A.Y.K.; VOGUE Research Group. Resolution and Cooccurrence Patterns of Gardnerella leopoldii, G. swidsinskii, G. piotii, and G. vaginalis within the Vaginal Microbiome. Infect. Immun. 2019, 87, e00532-19. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Lewis, W.G.; Robinson, L.S.; Gilbert, N.M.; Perry, J.C.; Lewis, A.L. Degradation, Foraging, and Depletion of Mucus Sialoglycans by the Vagina-adapted Actinobacterium Gardnerella vaginalis. J. Biol. Chem. 2013, 288, 12067–12079. [Google Scholar] [CrossRef]
- Gilbert, N.M.; Lewis, W.G.; Lewis, A.L. Clinical Features of Bacterial Vaginosis in a Murine Model of Vaginal Infection with Gardnerella vaginalis. PLoS ONE 2013, 8, e59539. [Google Scholar] [CrossRef]
- Lewis, W.G.; Robinson, L.S.; Perry, J.; Bick, J.L.; Peipert, J.F.; Allsworth, J.E.; Lewis, A.L. Hydrolysis of Secreted Sialoglycoprotein Immunoglobulin A (IgA) in ex Vivo and Biochemical Models of Bacterial Vaginosis. J. Biol. Chem. 2012, 287, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.S.; Schwebke, J.; Lewis, W.G.; Lewis, A.L. Identification and characterization of NanH2 and NanH3, enzymes responsible for sialidase activity in the vaginal bacterium Gardnerella vaginalis. J. Biol. Chem. 2019, 294, 5230–5245. [Google Scholar] [CrossRef] [PubMed]
- Bohr, L.L.; Mortimer, T.D.; Pepperell, C.S. Lateral Gene Transfer Shapes Diversity of Gardnerella spp. Front. Cell. Infect. Microbiol. 2020, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.H.; Potter, S.; O Wood, J.; Hemmingsen, S.M.; Reynolds, R.P.; Chow, A.W. HSP60 gene sequences as universal targets for microbial species identification: Studies with coagulase-negative staphylococci. J. Clin. Microbiol. 1996, 34, 818–823. [Google Scholar] [CrossRef]
- Moncla, B.J.; Braham, P. Detection of sialidase (neuraminidase) activity in Actinomyces species by using 2′-(4-methylumbelliferyl)alpha-D-N-acetylneuraminic acid in a filter paper spot test. J. Clin. Microbiol. 1989, 27, 182–184. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).