Cross-Sectional Study on the Prevalence and Factors Influencing Occurrence of Tick-Borne Encephalitis in Horses in Lithuania
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Sampling Sites
2.2. Data of Human TBEV Cases
2.3. ELISA and Virus Neutralization Test
2.4. TBE Virus Detection and Viral Load Quantification
2.5. Virus Isolation
2.6. Sequencing and Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
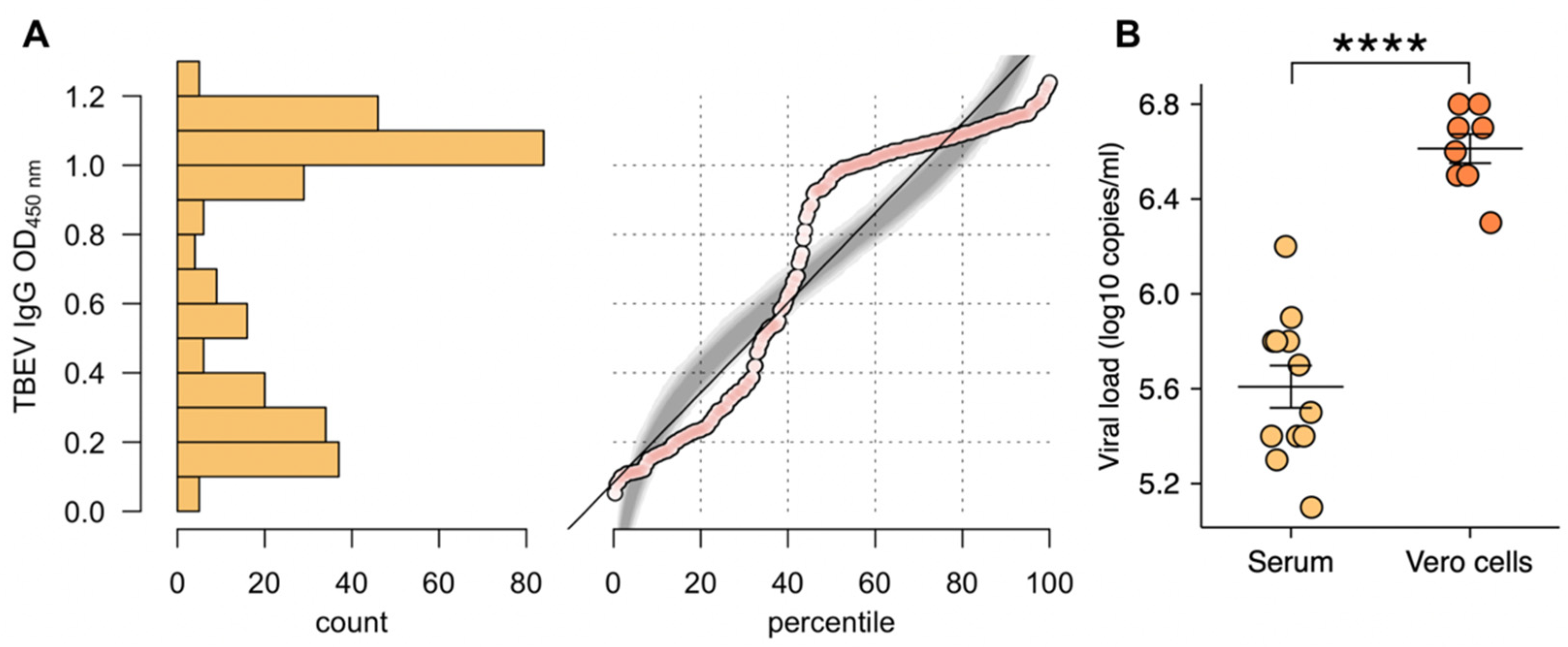
3.1. ELISA and Neutralization Assay
3.2. Virus Detection and Isolation
3.3. Sequencing
3.4. Spatial Distribution and of TBEV Specific Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Süss, J. Tick-Borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Radzišauskienė, D.; Žagminas, K.; Ašoklienė, L.; Jasionis, A.; Mameniškienė, R.; Ambrozaitis, A.; Lancoriene, L.; Jatuzis, D.; Petraityte, I.; Mockiene, E. Epidemiological patterns of tick-borne encephalitis in Lithuania and clinical features in adults in the light of the high incidence in recent years: A retrospective study. Eur. J. Neurol. 2018, 25, 268–274. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Tick-Borne Encephalitis—Annual Epidemiological Report for 2018; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2019.
- European Centre for Disease Prevention and Control. Country Profile: Lithuania. Tick-Borne Encephalitis (TBE); European Centre for Disease Prevention and Control: Stockholm, Sweden, 2012.
- Lindquist, L. Tick-Borne encephalitis. In Handbook of Clinical Neurology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; pp. 531–559. [Google Scholar]
- Imhoff, M.; Hagedorn, P.; Schulze, Y.; Hellenbrand, W.; Pfeffer, M.; Niedrig, M. Review: Sentinels of tick-borne encephalitis risk. Ticks Tick-Borne Dis. 2015, 6, 592–600. [Google Scholar] [CrossRef]
- Gerth, H.J.; Grimshandl, D.; Stage, B.; Döller, G.; Kunz, C. Roe deer as sentinels for endemicity of tick-borne encephalitis virus. Epidemiol. Infect. 1995, 115, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Achazi, K.; Růžek, D.; Donoso-Mantke, O.; Schlegel, M.; Ali, H.S.; Wenk, M.; Ruhe, F.; Kallies, R.; Vor, T.; Ulrich, R.G.; et al. Rodents as sentinels for the prevalence of tick-borne encephalitis virus. Vector Borne Zoonotic Dis. 2011, 11, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Knap, N.; Korva, M.; Dolinšek, V.; Sekirnik, M.; Trilar, T.; Avšič-Županc, T. Patterns of Tick-Borne Encephalitis Virus Infection in Rodents in Slovenia. Vector Borne Zoonotic Dis. 2012, 12, 236–242. [Google Scholar] [CrossRef]
- Klaus, C.; Beer, M.; Saier, R.; Schau, U.; Moog, U.; Hoffmann, B.; Diller, R.; Suss, J. Goats and sheep as sentinels for tick-borne encephalitis (TBE) virus—Epidemiological studies in areas endemic and non-endemic for TBE virus in Germany. Ticks Tick Borne Dis. 2012, 3, 27–37. [Google Scholar] [CrossRef]
- Klaus, C.; Hoffmann, B.; Beer, M.; Müller, W.; Stark, B.; Bader, W.; Stiasny, K.; Heinz, F.X.; Suss, J. Seroprevalence of tick-borne encephalitis (TBE) in naturally exposed monkeys (Macaca sylvanus) and sheep and prevalence of TBE virus in ticks in a TBE endemic area in Germany. Ticks Tick Borne Dis. 2010, 1, 141–144. [Google Scholar] [CrossRef]
- Lindhe, K.E.S.; Meldgaard, D.S.; Jensen, P.M.; Houser, G.A.; Berendt, M. Prevalence of tick-borne encephalitis virus antibodies in dogs from Denmark. Acta Vet. Scand. 2009, 51, 56. [Google Scholar] [CrossRef]
- Duscher, G.G.; Wetscher, M.; Baumgartner, R.; Walder, G. Roe deer sera used for TBE surveillance in Austria. Ticks Tick Borne Dis. 2015, 6, 489–493. [Google Scholar] [CrossRef][Green Version]
- Klaus, C.; Hörügel, U.; Hoffmann, B.; Beer, M. Tick-Borne encephalitis virus (TBEV) infection in horses: Clinical and laboratory findings and epidemiological investigations. Vet. Microbiol. 2013, 163, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Rushton, J.O.; Lecollinet, S.; Hubálek, Z.; Svobodová, P.; Lussy, H.; Nowotny, N. Tick-Borne Encephalitis Virus in Horses, Austria, 2011. Emerg. Infect. Dis. 2013, 19, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; König, M.; Thiel, H.J. Tick-Borne encephalitis (TBE) with special emphasis on infection in horses. Dtsch. Tierarztl. Wochenschr. 2006, 113, 147–151. [Google Scholar] [PubMed]
- Setia, M.S. Methodology Series Module 3: Cross-Sectional Studies. Indian J. Dermatol. 2016, 61, 261–264. [Google Scholar] [CrossRef] [PubMed]
- VIII-500 Lietuvos Respublikos Gyvūnų Gerovės ir Apsaugos Įstatymas. (Žin. 2012, Nr. 122-6126). Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.46424/asr (accessed on 12 November 2020).
- Holzmann, H.; Kundi, M.; Stiasny, K.; Clement, J.; McKenna, P.; Kunz, C.; Heinz, F.X. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J. Med. Virol. 1996, 48, 102–107. [Google Scholar] [CrossRef]
- Schwaiger, M.; Cassinotti, P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J. Clin. Virol. 2003, 27, 136–145. [Google Scholar] [CrossRef]
- Puchhammer-Stöckl, E.; Kunz, C.; Mandl, C.W.; Heinz, F.X. Identification of tick-borne encephalitis virus ribonucleic acid in tick suspensions and in clinical specimens by a reverse transcription-nested polymerase chain reaction assay. Clin. Diagn. Virol. 1995, 4, 321–326. [Google Scholar] [CrossRef]
- Šumilo, D.; Bormane, A.; Asokliene, L.; Vasilenko, V.; Golovljova, I.; Avsic-Zupanc, T.; Hubalek, Z.; Randolph, S.E. Socio-Economic factors in the differential upsurge of tick-borne encephalitis in central and Eastern Europe. Rev. Med. Virol. 2008, 18, 81–95. [Google Scholar] [CrossRef]
- Šumilo, D.; Bormane, A.; Asokliene, L.; Lucenko, I.; Vasilenko, V.; Randolph, S. Tick-Borne encephalitis in the Baltic States: Identifying risk factors in space and time. Int. J. Med. Microbiol. 2006, 296, 76–79. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Hjertqvist, M.; Bergström, T.; Lundkvist, A. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasites Vectors 2012, 5, 184. [Google Scholar] [CrossRef]
- Šikutová, S.; Hornok, S.; Hubálek, Z.; Doležálková, I.; Juřicová, Z.; Rudolf, I. Serological survey of domestic animals for tick-borne encephalitis and Bhanja viruses in northeastern Hungary. Vet. Microbiol. 2009, 135, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Stefanoff, P.; Pfeffer, M.; Hellenbrand, W.; Rogalska, J.; Rühe, F.; Makówka, A.; Michalik, J.; Wodecka, B.; Kiewra, D.; Dobler, G.; et al. Virus Detection in Questing Ticks is not a Sensitive Indicator for Risk Assessment of Tick-Borne Encephalitis in Humans. Zoonoses Public Health 2013, 60, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Gäumann, R.; Mühlemann, K.; Strasser, M.; Beuret, C.M. High-Throughput procedure for tick surveys of tick-borne encephalitis virus and its application in a national surveillance study in Switzerland. Appl. Environ. Microbiol. 2010, 76, 4241–4249. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, M.; Radzijevskaja, J.; Mickevičius, S.; Bratčikovienė, N.; Paulauskas, A. Prevalence of tick-borne encephalitis virus in questing Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania. Ticks Tick Borne Dis. 2021, 12, 101594. [Google Scholar] [CrossRef] [PubMed]
- Belova, O.A.; Burenkova, L.A.; Karganova, G.G. Different tick-borne encephalitis virus (TBEV) prevalences in unfed versus partially engorged ixodid ticks--evidence of virus replication and changes in tick behavior. Ticks Tick Borne Dis. 2012, 3, 240–246. [Google Scholar] [CrossRef]
- Balling, A.; Plessow, U.; Beer, M.; Pfeffer, M. Prevalence of antibodies against tick-borne encephalitis virus in wild game from Saxony, Germany. Ticks Tick Borne Dis. 2014, 5, 805–809. [Google Scholar] [CrossRef]
- Klaus, C.; Ziegler, U.; Kalthoff, D.; Hoffmann, B.; Beer, M. Tick-Borne encephalitis virus (TBEV)—Findings on cross reactivity and longevity of TBEV antibodies in animal sera. BMC Vet. Res. 2014, 10, 78. [Google Scholar] [CrossRef]
- Klaus, C.; Beer, M.; Saier, R.; Schubert, H.; Bischoff, S.; Suss, J. Evaluation of serological tests for detecting tick-borne encephalitis virus (TBEV) antibodies in animals. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 443–449. [Google Scholar]
- Pacilly, F.C.A.; Benning, M.E.; Jacobs, F.; Leidekker, J.; Sprong, H.; Van Wieren, S.E.; Takken, W. Blood feeding on large grazers affects the transmission of Borrelia burgdorferi sensu lato by Ixodes ricinus. Ticks Tick Borne Dis. 2014, 5, 810–817. [Google Scholar] [CrossRef]
- Rizzoli, A.; Hauffe, H.C.; Tagliapietra, V.; Neteler, M.; Rosà, R. Forest Structure and Roe Deer Abundance Predict Tick-Borne Encephalitis Risk in Italy. PLoS ONE 2009, 4, e4336. [Google Scholar] [CrossRef]
- Bolzoni, L.; Rosà, R.; Cagnacci, F.; Rizzoli, A. Effect of deer density on tick infestation of rodents and the hazard of tick-borne encephalitis. II: Population and infection models. Int. J. Parasitol. 2012, 42, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hofmeester, T.R.; Sprong, H.; Jansen, P.A.; Prins, H.H.T.; van Wieren, S.E. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasites Vectors 2017, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Gassner, F.; Verbaarschot, P.; Smallegange, R.C.; Spitzen, J.; Van Wieren, S.E.; Takken, W. Variations in Ixodes ricinus density and Borrelia infections associated with cattle introduced into a woodland in The Netherlands. Appl. Environ. Microbiol. 2008, 74, 7138–7144. [Google Scholar] [CrossRef] [PubMed]

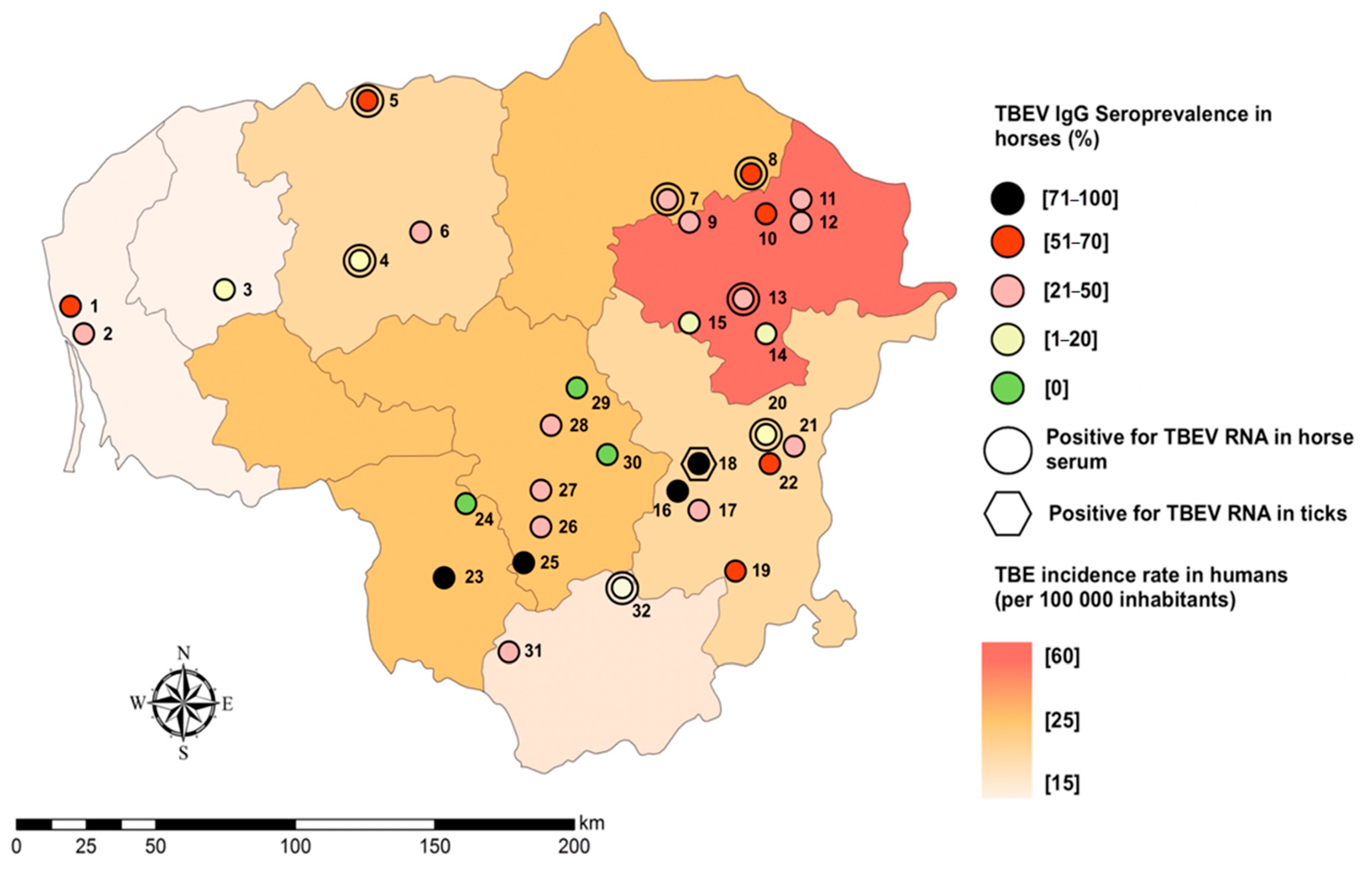
| No. of the Stable | Sample Size of the Stable | County | Seroprevalence in the County | TBEV Seropositive Horses | Seroprevalence in the Stables | CI 95 % | PCR Positive Serum Samples | Tick Pools | PCR Positive Tick Pools |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | Klaipėda | 42.3 | 8 | 66.6 | 34.8–90.0 | 0 | 4 | 0 |
| 2 | 14 | 3 | 21.4 | 0.4–50.8 | 0 | 2 | 0 | ||
| 3 | 12 | Telsiai | 8.3 | 1 | 8.3 | 0.2–38.4 | 0 | 0 | 0 |
| 4 | 7 | Siauliai | 30.6 | 4 | 57.1 | 18.4–90.1 | 1 | 1 | 0 |
| 5 | 10 | 1 | 10.0 | 0.2–44.5 | 3 | 0 | 0 | ||
| 6 | 19 | 6 | 31.5 | 12.5–56.5 | 0 | 0 | 0 | ||
| 7 | 12 | Panevezys | 46.2 | 5 | 41.6 | 15.1–72.3 | 1 | 0 | 0 |
| 8 | 14 | 7 | 51.0 | 23.0–76.9 | 1 | 0 | 0 | ||
| 9 | 13 | Utena | 30.8 | 4 | 30.7 | 0.9–61.4 | 0 | 0 | 0 |
| 10 | 3 | 1 | 33.3 | 0.8–90.5 | 0 | 0 | 0 | ||
| 11 | 4 | 1 | 25.0 | 0.6–80.5 | 0 | 0 | 0 | ||
| 12 | 11 | 4 | 36.3 | 10.9–69.2 | 0 | 3 | 0 | ||
| 13 | 15 | 5 | 33.3 | 11.8–61.6 | 1 | 2 | 0 | ||
| 14 | 6 | 1 | 16.6 | 0.4–64.1 | 0 | 0 | 0 | ||
| 15 | 8 | Vilnius | 50.6 | 1 | 12.5 | 0.3–52.6 | 0 | 1 | 0 |
| 16 | 6 | 6 | 100.0 | 54.0–100 | 0 | 1 | 0 | ||
| 17 | 2 | 1 | 50.0 | 0.1–98.7 | 0 | 0 | 0 | ||
| 18 | 14 | 14 | 100.0 | 0.7–100 | 0 | 22 | 4 | ||
| 19 | 17 | 9 | 52.9 | 27.8–77.0 | 0 | 0 | 0 | ||
| 20 | 17 | 2 | 11.7 | 0.1–36.4 | 2 | 0 | 0 | ||
| 21 | 17 | 7 | 41.1 | 18.4–67.0 | 0 | 0 | 0 | ||
| 22 | 6 | 4 | 66.6 | 22.2–95.6 | 0 | 0 | 0 | ||
| 23 | 2 | Marijampole | 22.2 | 2 | 100.0 | 15.8–100.0 | 0 | 0 | 0 |
| 24 | 7 | 0 | 0.0 | 0–40.9 | 0 | 0 | 0 | ||
| 25 | 4 | Kaunas | 34.2 | 4 | 100.0 | 39.7–100.0 | 0 | 1 | 0 |
| 26 | 6 | 2 | 33.3 | 0.4–77.7 | 0 | 0 | 0 | ||
| 27 | 8 | 2 | 25.0 | 3.1–65.0 | 0 | 0 | 0 | ||
| 28 | 13 | 5 | 38.4 | 13.8–68.4 | 0 | 0 | 0 | ||
| 29 | 1 | 0 | 0.0 | 0.0–97.5 | 0 | 0 | 0 | ||
| 30 | 6 | 0 | 0.0 | 0.0–45.9 | 0 | 0 | 0 | ||
| 31 | 5 | Alytus | 20.0 | 2 | 40.0 | 0.5–85.3 | 0 | 0 | 0 |
| 32 | 10 | 1 | 10.0 | 0.2–44.5 | 3 | 1 | 0 | ||
| Total | 301 | 113 | 37.5 | 32.3–43.1 | 12 | 38 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pautienius, A.; Armonaite, A.; Simkute, E.; Zagrabskaite, R.; Buitkuviene, J.; Alpizar-Jara, R.; Grigas, J.; Zakiene, I.; Zienius, D.; Salomskas, A.; et al. Cross-Sectional Study on the Prevalence and Factors Influencing Occurrence of Tick-Borne Encephalitis in Horses in Lithuania. Pathogens 2021, 10, 140. https://doi.org/10.3390/pathogens10020140
Pautienius A, Armonaite A, Simkute E, Zagrabskaite R, Buitkuviene J, Alpizar-Jara R, Grigas J, Zakiene I, Zienius D, Salomskas A, et al. Cross-Sectional Study on the Prevalence and Factors Influencing Occurrence of Tick-Borne Encephalitis in Horses in Lithuania. Pathogens. 2021; 10(2):140. https://doi.org/10.3390/pathogens10020140
Chicago/Turabian StylePautienius, Arnoldas, Austeja Armonaite, Evelina Simkute, Ruta Zagrabskaite, Jurate Buitkuviene, Russell Alpizar-Jara, Juozas Grigas, Indre Zakiene, Dainius Zienius, Algirdas Salomskas, and et al. 2021. "Cross-Sectional Study on the Prevalence and Factors Influencing Occurrence of Tick-Borne Encephalitis in Horses in Lithuania" Pathogens 10, no. 2: 140. https://doi.org/10.3390/pathogens10020140
APA StylePautienius, A., Armonaite, A., Simkute, E., Zagrabskaite, R., Buitkuviene, J., Alpizar-Jara, R., Grigas, J., Zakiene, I., Zienius, D., Salomskas, A., & Stankevicius, A. (2021). Cross-Sectional Study on the Prevalence and Factors Influencing Occurrence of Tick-Borne Encephalitis in Horses in Lithuania. Pathogens, 10(2), 140. https://doi.org/10.3390/pathogens10020140









