Roles of OmpA in Type III Secretion System-Mediated Virulence of Enterohemorrhagic Escherichia coli
Abstract
1. Introduction
2. Results
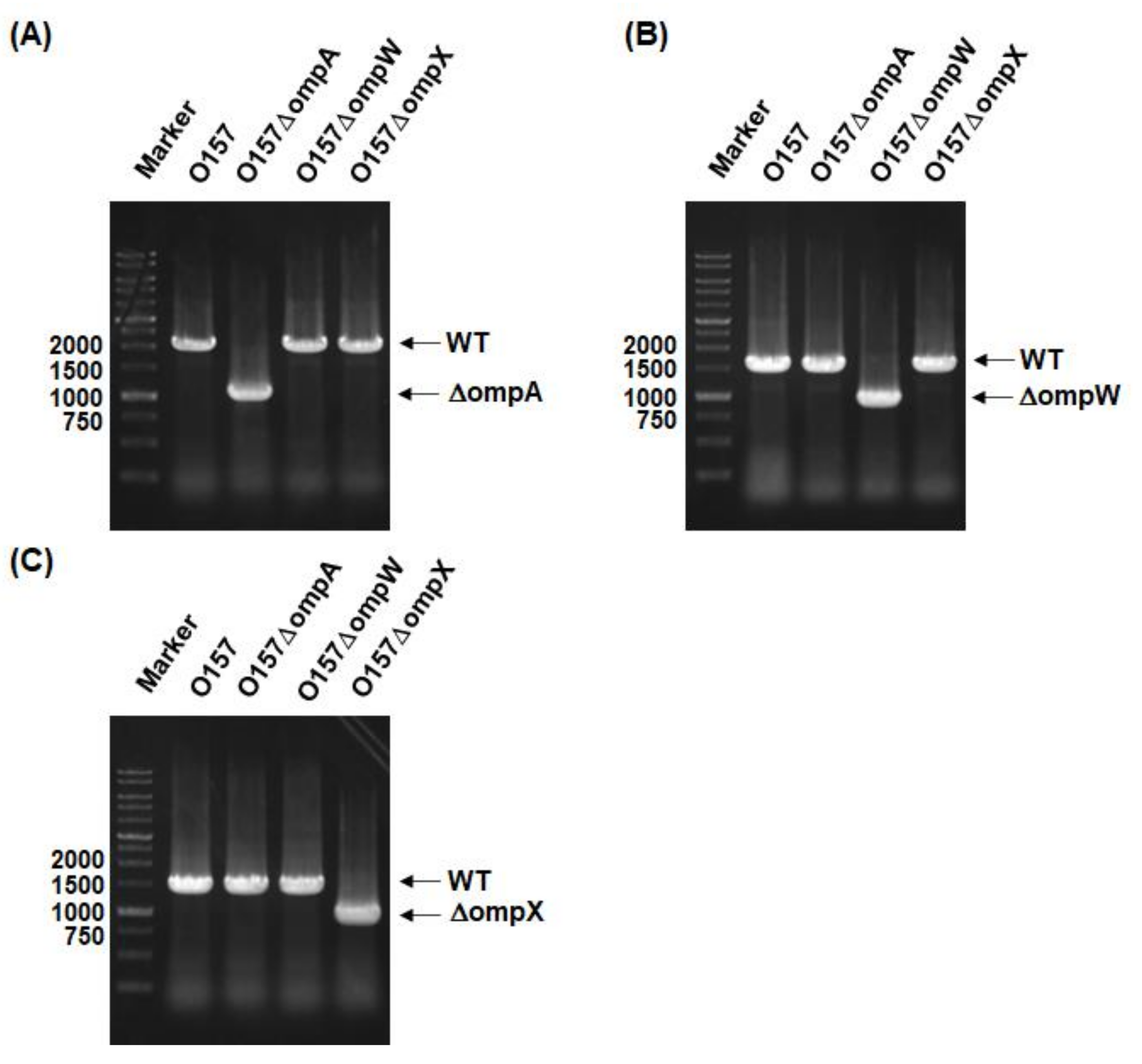
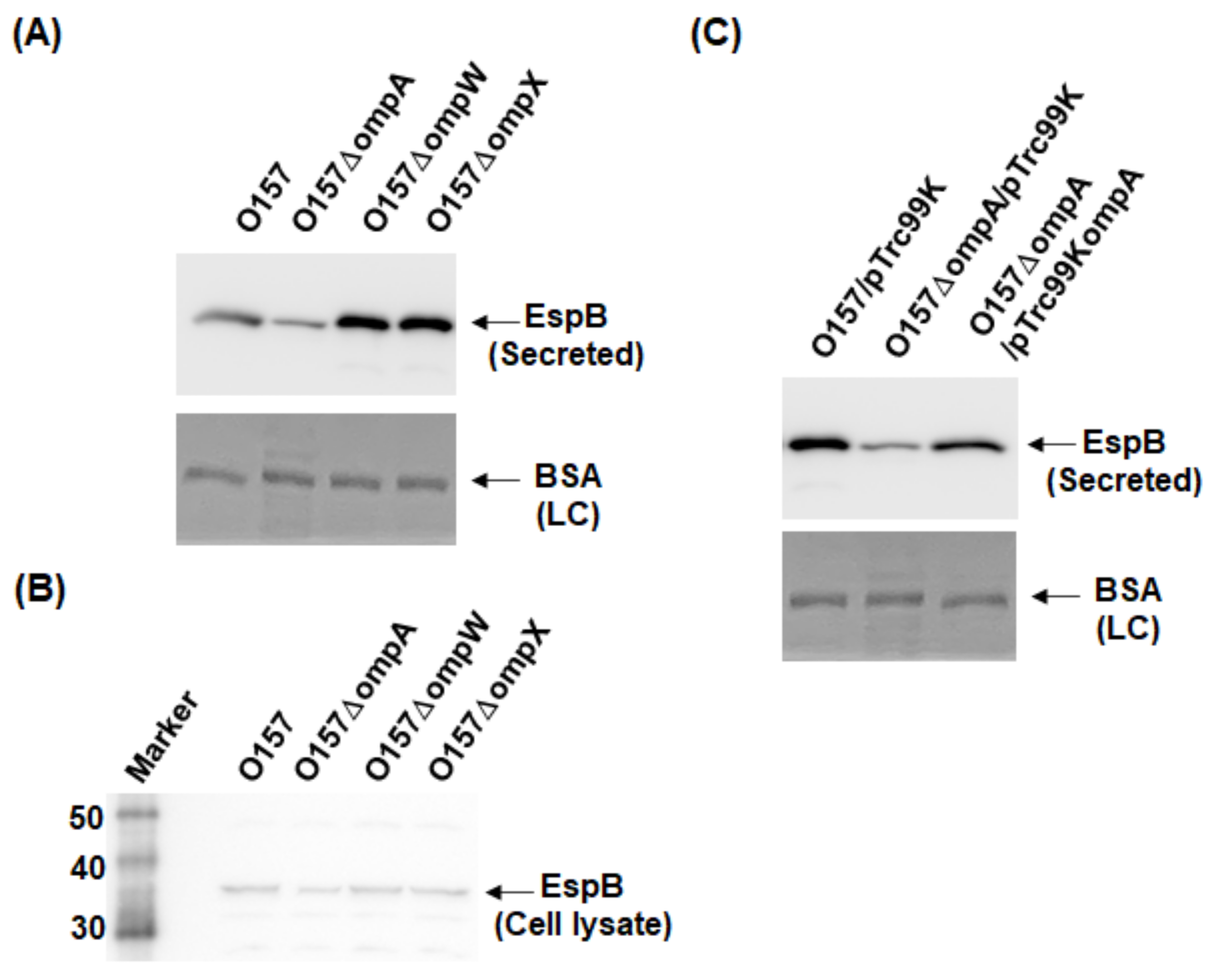
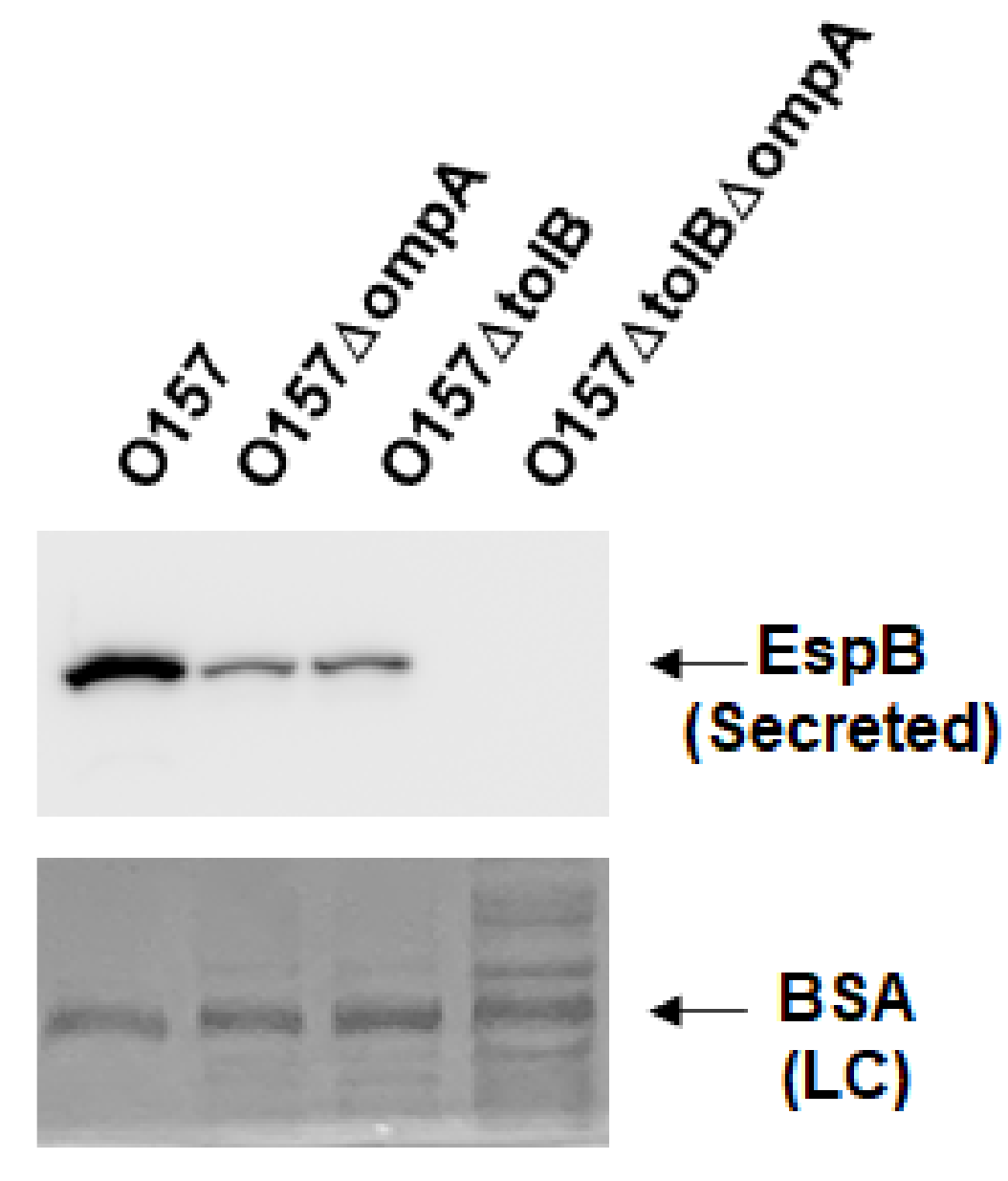
2.1. Deletion of ompA, but Not ompW and ompX, Decreases Extracellular Levels of the Type III Secretory Proteins EspA and EspB
2.2. Deletion of ompA Reduces Abilities of EHEC to Form A/E Lesions and to Lyse Red Blood Cells (RBCs) Associated with the T3SS Activity
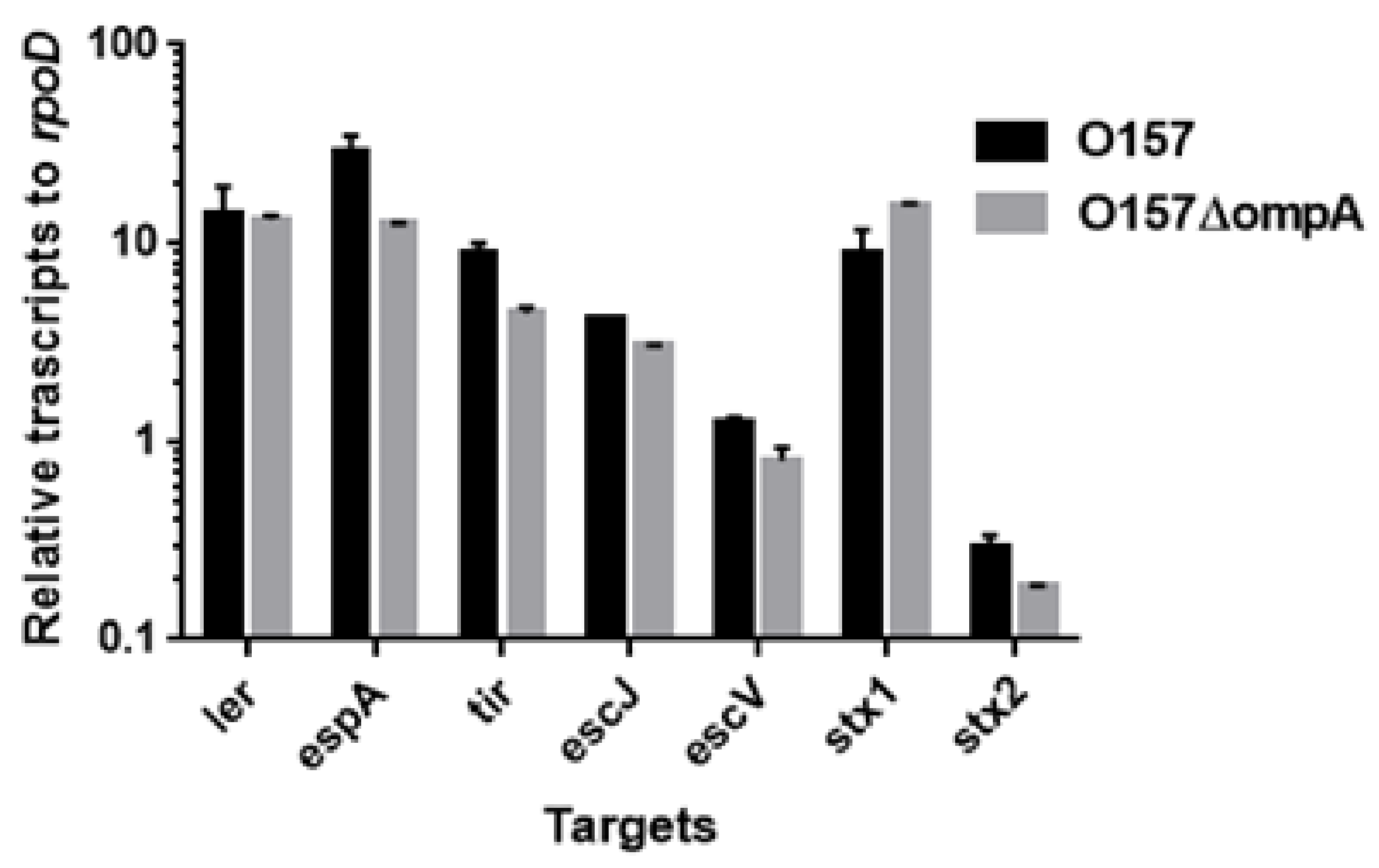
2.3. Neither ompA, Nor ompW, Nor ompX Deletion Decreases the Level of Shiga Toxins
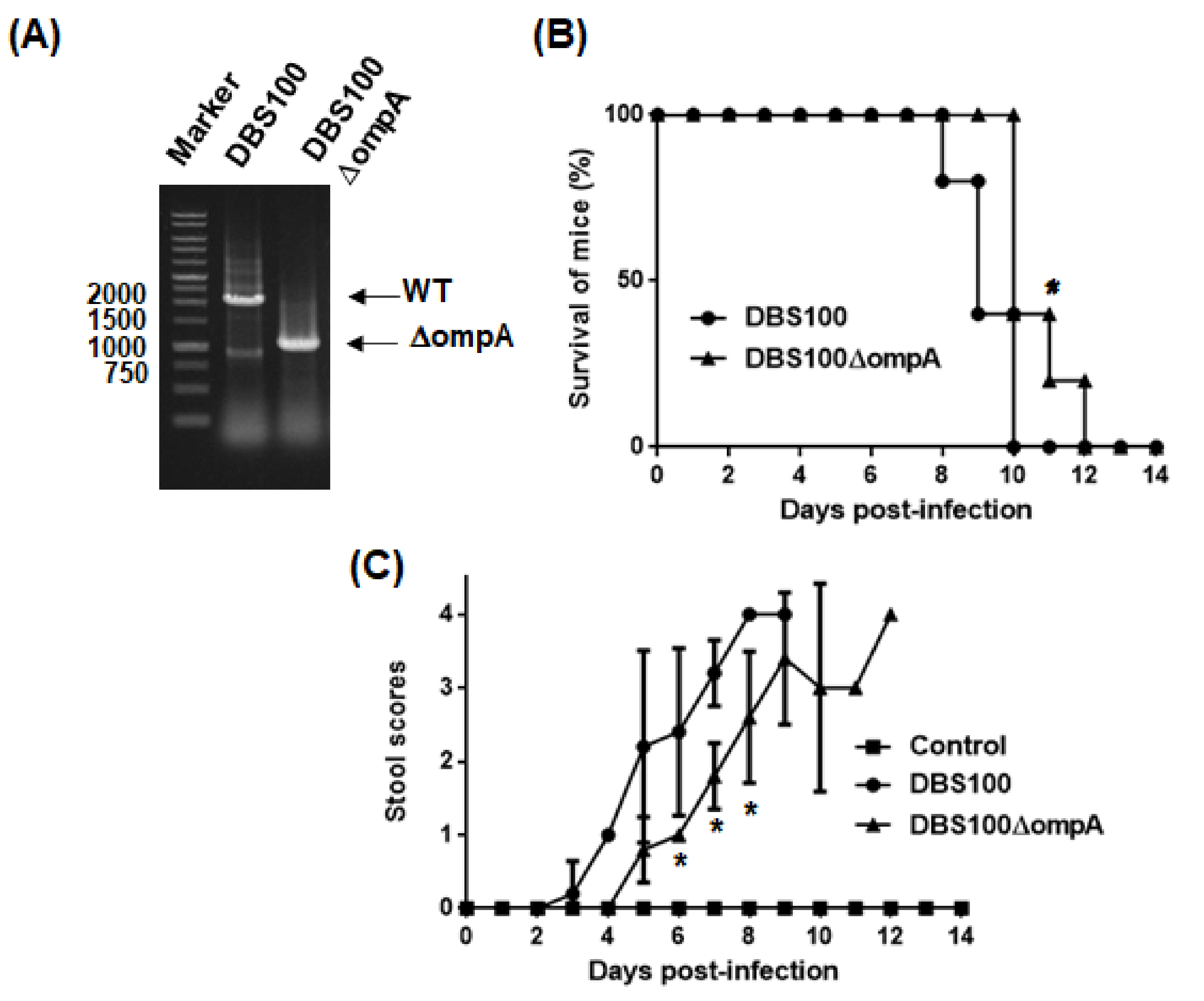
2.4. Virulence of C. rodentium to Mice Is Attenuated by ompA Gene Deletion
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Host Cells, and Culture Conditions
4.2. Cloning and Mutant Construction
4.3. Western Blotting
4.4. Shiga Toxin Assay
4.5. RNA Extraction and Quantitative Real-Time PCR
4.6. A/E Lesion Formation
4.7. Hemolysis Assay
4.8. C. rodentium Infections in Mice
4.9. Bile Salts Susceptibility Assays
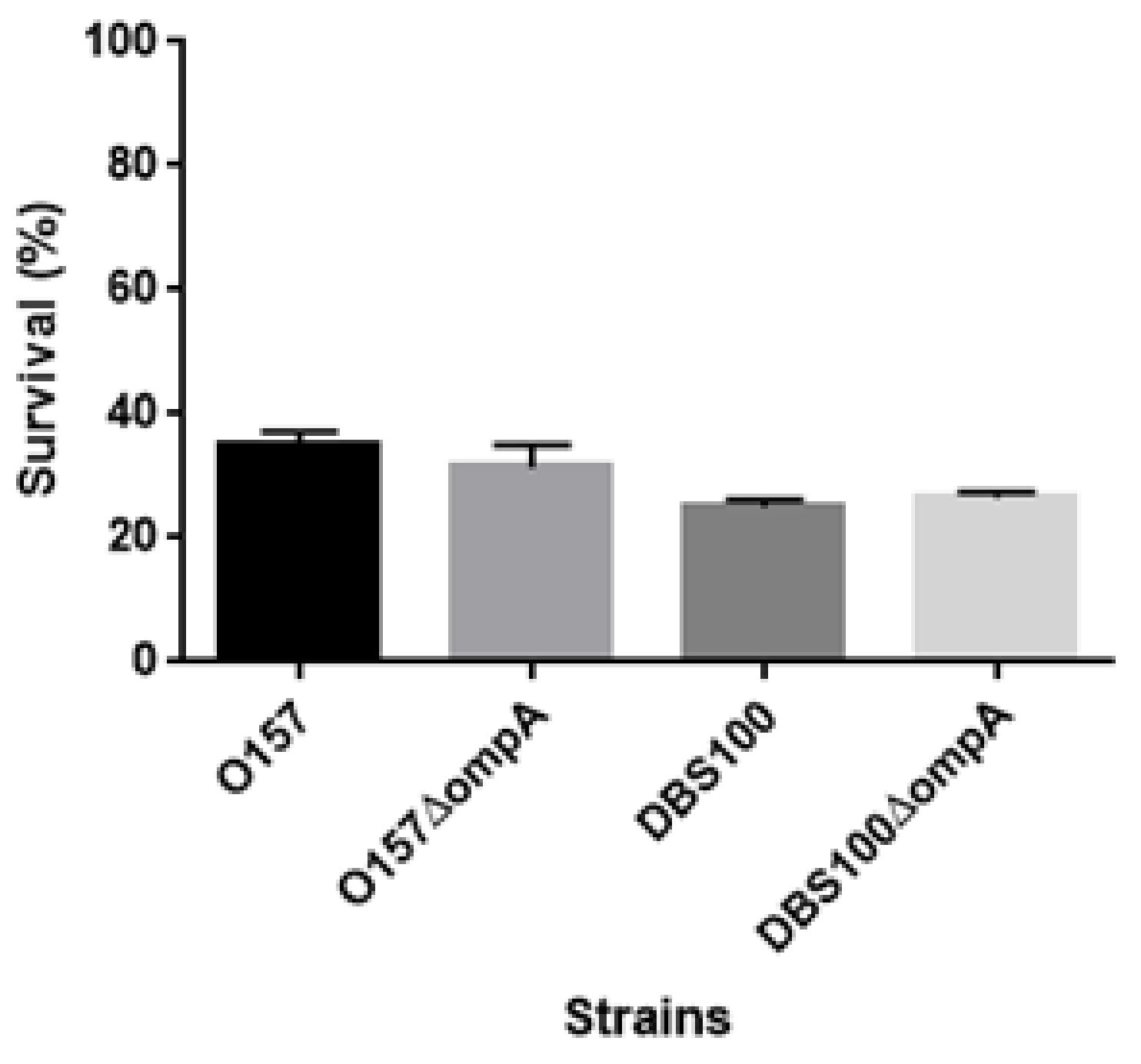
4.10. Acid Survival Assays
4.11. Statistical Analysis
4.12. Ethics Information
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed]
- Hueck, C.J. Type III Protein Secretion Systems in Bacterial Pathogens of Animals and Plants. Microbiol. Mol. Biol. Rev. 1998, 62, 379–433. [Google Scholar] [CrossRef]
- Galán, J.E.; Wolf-Watz, H. Protein delivery into eukaryotic cells by type III secretion machines. Nat. Cell Biol. 2006, 444, 567–573. [Google Scholar] [CrossRef]
- Kenny, B.; DeVinney, R.; Stein, M.; Reinscheid, D.J.; Frey, E.A.; Finlay, B.B. Enteropathogenic E. coli (EPEC) transfers its recep-tor for intimate adherence into mammalian cells. Cell 1997, 91, 511–520. [Google Scholar] [CrossRef]
- Daniell, S.J.; Kocsis, E.; Morris, E.; Knutton, S.; Booy, F.P.; Frankel, G. 3D structure of EspA filaments from enteropathogenic Escherichia coli. Mol. Microbiol. 2003, 49, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Knutton, S.; Rosenshine, I.; Pallen, M.J.; Nisan, I.; Neves, B.C.; Bain, C.; Wolff, C.; Dougan, G.; Frankel, G. A novel Es-pA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998, 17, 2166–2176. [Google Scholar] [CrossRef]
- Ide, T.; Laarmann, S.; Greune, L.; Schillers, H.; Oberleithner, H.; Schmidt, M.A. Characterization of translocation pores in-serted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell Microbiol. 2001, 3, 669–679. [Google Scholar] [CrossRef]
- Kodama, T.; Akeda, Y.; Kono, G.; Takahashi, A.; Imura, K.; Iida, T.; Honda, T. The EspB protein of enterohaemorrhagic Esche-richia coli interacts directly with alpha-catenin. Cell Microbiol. 2002, 4, 213–222. [Google Scholar] [CrossRef]
- Iizumi, Y.; Sagara, H.; Kabe, Y.; Azuma, M.; Kume, K.; Ogawa, M.; Nagai, T.; Barr-Gillespie, P.; Sasakawa, C.; Handa, H. The Enteropathogenic E. coli Effector EspB Facilitates Microvillus Effacing and Antiphagocytosis by Inhibiting Myosin Function. Cell Host Microbe 2007, 2, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.E.; Brockwell, D.J.; Radford, S.E. Role of the lipid bilayer in outer membrane protein folding in Gram-negative bac-teria. J. Biol. Chem. 2020, 295, 10340–10367. [Google Scholar] [CrossRef]
- Prasadarao, N.V.; Wass, C.A.; Weiser, J.N.; Stins, M.F.; Huang, S.H.; Kim, K.S. Outer membrane protein A of Escherichia coli con-tributes to invasion of brain microvascular endothelial cells. Infect. Immun. 1996, 64, 146–153. [Google Scholar] [CrossRef]
- Sukumaran, S.K.; Shimada, H.; Prasadarao, N.V. Entry and Intracellular Replication of Escherichia coli K1 in Macrophages Require Expression of Outer Membrane Protein A. Infect. Immun. 2003, 71, 5951–5961. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Kaper, J.B. Multiple Elements Controlling Adherence of Enterohemorrhagic Escherichia coli O157:H7 to HeLa Cells. Infect. Immun. 2003, 71, 4985–4995. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.F.; Watts, K.M.; Hunstad, D.A. OmpA of Uropathogenic Escherichia coli Promotes Postinvasion Pathogenesis of Cystitis. Infect. Immun. 2009, 77, 5245–5251. [Google Scholar] [CrossRef]
- Meng, X.; Liu, X.; Zhang, L.; Hou, B.; Li, B.; Tan, C.; Li, Z.; Zhou, R.; Li, S. Virulence characteristics of extraintestinal pathogenic Escherichia coli deletion of gene encoding the outer membrane protein X. J. Vet. Med Sci. 2016, 78, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Suzue, K.; Takita, A.; Kamitani, W.; Tomita, H. Roles of OmpX, an Outer Membrane Protein, on Virulence and Flagellar Expression in Uropathogenic Escherichia coli. Infect. Immun. 2021, 89. [Google Scholar] [CrossRef]
- Heffernan, E.J.; Harwood, J.; Fierer, J.; Guiney, D. The Salmonella typhimurium virulence plasmid complement resistance gene rck is homologous to a family of virulence-related outer membrane protein genes, including pagC and ail. J. Bacteriol. 1992, 174, 84–91. [Google Scholar] [CrossRef] [PubMed]
- McPhee, J.B.; Tamber, S.; Bains, M.; Maier, E.; Gellatly, S.; Lo, A.; Benz, R.; Hancock, R.E. The major outer membrane protein OprG of Pseudomonas aeruginosa contributes to cytotoxicity and forms an anaerobically regulated, cation-selective channel. FEMS Microbiol. Lett. 2009, 296, 241–247. [Google Scholar] [CrossRef]
- Nandi, B.; Nandy, R.K.; Sarkar, A.; Ghose, A.C. Structural features, properties and regulation of the outer-membrane pro-tein W (OmpW) of Vibrio cholerae. Microbiology 2005, 151, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Climent, N.; Ferrer, S.; Rubires, X.; Merino, S.; Tomás, J.; Regué, M. Molecular characterization of a 17-kDa outer-membrane protein from Klebsiella pneumoniae. Res. Microbiol. 1997, 148, 133–143. [Google Scholar] [CrossRef]
- Kolodziejek, A.M.; Schnider, D.R.; Rohde, H.N.; Wojtowicz, A.J.; Bohach, G.A.; Minnich, S.A.; Hovde, C.J. Outer membrane pro-tein X (Ail) contributes to Yersinia pestis virulence in pneumonic plague and its activity is dependent on the lipopolysaccha-ride core length. Infect. Immun. 2010, 78, 5233–5243. [Google Scholar] [CrossRef]
- McDaniel, T.K.; Kaper, J.B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 1997, 23, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Germon, P.; Vianney, A.; Portalier, R.; Lazzaroni, J.C. TolB protein of Escherichia coli K-12 interacts with the outer membrane peptidoglycan-associated proteins Pal, Lpp and OmpA. Mol. Microbiol. 1998, 29, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Suzue, K.; Takita, A.; Awazu, C.; Kurushima, J.; Tomita, H. Roles of the Tol-Pal system in the Type III secretion system and flagella-mediated virulence in enterohemorrhagic Escherichia coli. Sci. Rep. 2020, 10, 15173. [Google Scholar] [CrossRef]
- Mundy, R.; MacDonald, T.T.; Dougan, G.; Frankel, G.; Wiles, S. Citrobacter rodentium of mice and man. Cell. Microbiol. 2005, 7, 1697–1706. [Google Scholar] [CrossRef]
- Smajs, D.; Pilsl, H.; Braun, V. Colicin U, a novel colicin produced by Shigella boydii. J. Bacteriol. 1997, 179, 4919–4928. [Google Scholar] [CrossRef][Green Version]
- Sugawara, E.; Nikaido, H. OmpA protein of Escherichia coli outer membrane occurs in open and closed channel forms. J. Biol. Chem. 1994, 269, 17981–17987. [Google Scholar] [CrossRef]
- Sonntag, I.; Schwarz, H.; Hirota, Y.; Henning, U. Cell envelope and shape of Escherichia coli: Multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J. Bacteriol. 1978, 136, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wood, T.K. OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ. Microbiol. 2009, 11, 2735–2746. [Google Scholar] [CrossRef]
- Pilsl, H.; Smajs, D.; Braun, V. Characterization of Colicin S4 and Its Receptor, OmpW, a Minor Protein of the Escherichia coli Outer Membrane. J. Bacteriol. 1999, 181, 3578–3581. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Schulz, G.E. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mecha-nisms of virulence. Structure 1999, 7, 1301–1309. [Google Scholar] [CrossRef]
- Hirakawa, H.; Suzue, K.; Kurabayashi, K.; Tomita, H. The Tol-Pal System of Uropathogenic Escherichia coli Is Responsible for Optimal Internalization Into and Aggregation Within Bladder Epithelial Cells, Colonization of the Urinary Tract of Mice, and Bacterial Motility. Front. Microbiol. 2019, 10, 1827. [Google Scholar] [CrossRef]
- Wang, Y. The Function of OmpA in Escherichia coli. Biochem. Biophys. Res. Commun. 2002, 292, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Ohno, R.; Sekiya, K.; Kuwae, A.; Matsuzawa, T.; Nonaka, T.; Fukuda, H.; Imajoh-Ohmi, S.; Abe, A. Assembly of the Type III Secretion Apparatus of Enteropathogenic Escherichia coli. J. Bacteriol. 2006, 188, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Tseytin, I.; Dagan, A.; Oren, S.; Sal-Man, N. The role of EscD in supporting EscC polymerization in the type III secretion system of enteropathogenic Escherichia coli. Biochim. et Biophys. Acta (BBA)-Biomembr. 2018, 1860, 384–395. [Google Scholar] [CrossRef]
- Link, A.J.; Phillips, D.; Church, G.M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: Application to open reading frame characterization. J. Bacteriol. 1997, 179, 6228–6237. [Google Scholar] [CrossRef]
- Hirakawa, H.; Nishino, K.; Yamada, J.; Hirata, T.; Yamaguchi, A. Beta-lactam resistance modulated by the overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Antimicrob. Chemother. 2003, 52, 576–582. [Google Scholar] [CrossRef]
- Hirakawa, H.; Uchida, M.; Kurabayashi, K.; Nishijima, F.; Takita, A.; Tomita, H. In vitro activity of AST-120 that suppresses indole signaling in Escherichia coli, which attenuates drug tolerance and virulence. PLoS ONE 2020, 15, e0232461. [Google Scholar] [CrossRef]
- Kurushima, J.; Kuwae, A.; Abe, A. Btc22 chaperone is required for secretion and stability of the type III secreted protein Bsp22 in Bordetella bronchiseptica. FEMS Microbiol. Lett. 2012, 331, 144–151. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Li, Y.; Guo, X. A mouse model of Citrobacter rodentium oral infection and evaluation of innate and adaptive immune responses. STAR Protoc. 2020, 1, 100218. [Google Scholar] [CrossRef] [PubMed]


| Strains | Shiga Toxin Titers | |
|---|---|---|
| Stx1 | Stx2 | |
| O157 | 128 | 256 |
| O157ΔompA | 128 | 256 |
| O157ΔompW | 128 | 256 |
| O157ΔompX | 128 | 256 |
| Strain | MICs (mg/L) of | |
|---|---|---|
| Sodium Deoxycholate | Sodium Cholate | |
| O157 | >51,200 | >51,200 |
| O157ΔompA | 51,200 | >51,200 |
| DBS100 | 25,600 | 51,200 |
| DBS100ΔompA | 1600 | 6400 |
| Strains or Plasmids | Relevant Genotype/Phenotype | Reference |
|---|---|---|
| Strains | ||
| O157 | EHEC O157:H7 [RIMD0509952] | RIMD 0509952 |
| O157ΔompA | ompA mutant | this work |
| O157ΔompW | ompW mutant | this work |
| O157ΔompX | ompX mutant | this work |
| O157ΔtolB | tolB mutant | [25] |
| O157ΔtolBΔompA | tolB and ompA double mutant | this work |
| DBS100 | Citrobacter rodentium (ATCC 51459) | ATCC51459 |
| DBS100ΔompA | ompA mutant | this work |
| Plasmids | ||
| pKO3 | Temperature-sensitive vector for gene targeting, sacB, CmR | [37] |
| pTrc99K | Vector for IPTG-inducible expression; KmR | [38] |
| pTrc99KompA | ompA expression plasmid; KmR | this work |
| Primers | DNA Sequence (5′—3′) | Use |
|---|---|---|
| ompA-delta1 | GCGGGATCCTTTGACTGCAGAAGAGCATGC | O157ΔompA construction |
| ompA-delta2 | CCAGACGAGAACTTAAGCCTGCTTTTTCATTTTTTGCGCCTCG | O157ΔompA construction |
| ompA-delta3 | CGAGGCGCAAAAAATGAAAAAGCAGGCTTAAGTTCTCGTCTGG | O157ΔompA construction |
| ompA-delta4 | GCGGTCGACAGCGGTTGGAAATGGAAGTATC | O157ΔompA construction |
| ompW-delta1 | GCGGGATCCACGCACATAGCAACGATACC | O157ΔompW construction |
| ompW-delta2 | TGCAGAGAAAATTAAAAACGATACTTTTTCATATCCGCTCCGTC | O157ΔompW construction |
| ompW-delta3 | CGACGGAGCGGATATGAAAAAGTATCGTTTTTAATTTTCTCTGC | O157ΔompW construction |
| ompW-delta4 | GCGGTCGACGTTATCTTCATGTCGAACAGC | O157ΔompW construction |
| ompX-delta1 | GCGGGATCCGACTTAGCTAACGAGGCTCC | O157ΔompX construction |
| ompX-delta2 | ATATCACCGAAGTGATTAGAAGCGAATTTTTTTCATAACCACCTC | O157ΔompX construction |
| ompX-delta3 | TTTGAGGTGGTTATGAAAAAAATTCGCTTCTAATCACTTCGGTG | O157ΔompX construction |
| ompX-delta4 | GCGGTCGACAAACAGACGATTTACTGCGC | O157ΔompX construction |
| ompA-CR-delta1 | GCGGGATCCGTTAACGGAAGAAGAACACGC | DBS100ΔompA construction |
| ompA-CR-delta2 | GACGGAAACTTAAGCCTGCGGCTTTTTCATTTTTTGCGCCTCG | DBS100ΔompA construction |
| ompA-CR-delta3 | CGAGGCGCAAAAAATGAAAAAGCCGCAGGCTTAAGTTTCCGTC | DBS100ΔompA construction |
| ompA-CR-delta4 | GCGGTCGACAGCGGTTGGAAATGGAAGTATC | DBS100ΔompA construction |
| pTrcompA-F | GCGCCATGGAAAAGACAGCTATCGCG | pTrc99KompA construction |
| pTrcompA-R | GCGGTCGACTTAAGCTTGCGGCTGAGTTAC | pTrc99KompA construction |
| Primer | DNA Sequence (5′—3′) | Use |
|---|---|---|
| rrsA-qPCR-F | CGGTGGAGCATGTGGTTTAA | Quantitative real-time PCR |
| rrsA-qPCR-R | GAAAACTTCCGTGGATGTCAAGA | Quantitative real-time PCR |
| rpoD-qPCR-F | CAAGCCGTGGTCGGAAAA | Quantitative real-time PCR |
| rpoD-qPCR-R | GGGCGCGATGCACTTCT | Quantitative real-time PCR |
| ler-qPCR-F | CGACCAGGTCTGCCCTTCT | Quantitative real-time PCR |
| ler-qPCR-R | TCGCTCGCCGGAACTC | Quantitative real-time PCR |
| espA-qPCR-F | CCGTTGTCAGGTTATTCGCTTT | Quantitative real-time PCR |
| espA-qPCR-R | TGATTTAAGCGCTGGTGATCTG | Quantitative real-time PCR |
| tir-qPCR-F | TTTTTGCGCCTGAGCATTATT | Quantitative real-time PCR |
| tir-qPCR-R | GCTAAAGCAGCAGGCGAAGA | Quantitative real-time PCR |
| escJ-qPCR-F | AAAGAAGCTAATCAGATGCAAGCA | Quantitative real-time PCR |
| escJ-qPCR-R | TCCACTTTTGTCCATTTCTTTGG | Quantitative real-time PCR |
| escV-qPCR-F | CGCCTGCGGTGACAGAA | Quantitative real-time PCR |
| escV-qPCR-R | TTGTCTGTCGGTGATGCTTTG | Quantitative real-time PCR |
| stx1-qPCR-F | TCGCGAGTTGCCAGAATG | Quantitative real-time PCR |
| stx1-qPCR-R | TTCCATCTGCCGGACACAT | Quantitative real-time PCR |
| stx2-qPCR-F | TTCGCGCCGTGAATGAA | Quantitative real-time PCR |
| stx2-qPCR-R | CAGGCCTGTCGCCAGTTATC | Quantitative real-time PCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirakawa, H.; Suzue, K.; Takita, A.; Tomita, H. Roles of OmpA in Type III Secretion System-Mediated Virulence of Enterohemorrhagic Escherichia coli. Pathogens 2021, 10, 1496. https://doi.org/10.3390/pathogens10111496
Hirakawa H, Suzue K, Takita A, Tomita H. Roles of OmpA in Type III Secretion System-Mediated Virulence of Enterohemorrhagic Escherichia coli. Pathogens. 2021; 10(11):1496. https://doi.org/10.3390/pathogens10111496
Chicago/Turabian StyleHirakawa, Hidetada, Kazutomo Suzue, Ayako Takita, and Haruyoshi Tomita. 2021. "Roles of OmpA in Type III Secretion System-Mediated Virulence of Enterohemorrhagic Escherichia coli" Pathogens 10, no. 11: 1496. https://doi.org/10.3390/pathogens10111496
APA StyleHirakawa, H., Suzue, K., Takita, A., & Tomita, H. (2021). Roles of OmpA in Type III Secretion System-Mediated Virulence of Enterohemorrhagic Escherichia coli. Pathogens, 10(11), 1496. https://doi.org/10.3390/pathogens10111496







