Bacterial Antibiotic Resistance: The Most Critical Pathogens
Abstract
:1. Introduction
2. What Is It and What Are the Mechanisms by Which Antimicrobial Resistance Is Steadily Increasing?
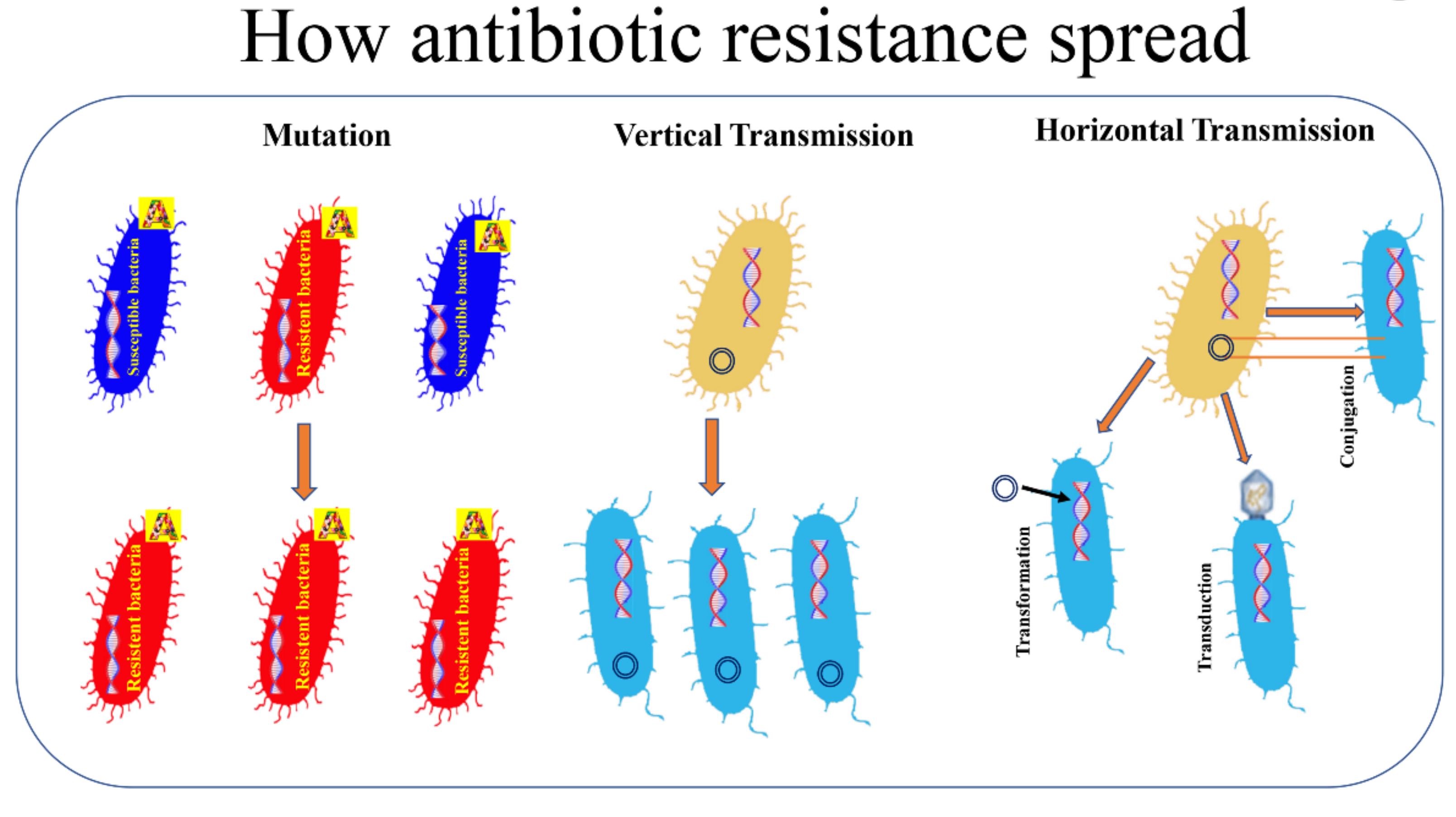
3. How Bacteria Acquire Resistance
4. The Main Difficult-to-Treat Antibiotic-Resistant Pathogens
4.1. Acinetobacter baumannii
- (1)
- the production of enzymes that degrade beta-lactam antibiotics. The production of all four classes of β-lactamases (A, B, C, and D) through the incorporation of exogenous DNA into its genome would underlie the rapid evolution of this strain toward multi-resistance [28,29]. Moreover, in Acinetobacter spp. have been identified both the genes encoding for narrow-spectrum β-lactamases (i.e., TEM-1, SCO-1, and CARB-4) and those encoding for ESBL (GES-11 and CTX-M) [29,30]. As stated above, class B β-lactamases are metallo-β-lactamases (MBLs) that have a broad substrate range, being able to inhibit all β-lactam antibiotics except the monobactams [31]. Class C β-lactamases are a group of broadly disseminated enzymes usually resistant to cephamycins (cefoxitin and cefotetan), penicillins and cephalosporins [32,33]. A. baumannii also possesses Class D or OXAs β-lactamases that can hydrolyze extended-spectrum cephalosporins and carbapenems [33,34]. Moreover, A. baumannii has an intrinsic ampC cephalosporinase [35];
- (2)
- the expression of efflux pumps. In A. baumannii efflux pumps are involved in bacterial resistance to a number of antibiotics belonging to different chemical classes such as aminoglycosides, tetracyclines, erythromycin, chloramphenicol, trimethoprim, fluoroquinolones and different beta-lactams [36,37]. Different studies have shown that at least four classes of efflux pumps are associated with A. baumannii antimicrobial resistance: the major facilitator superfamily (MFS), the resistance nodulation division (RND) superfamily, the multidrug and toxic compound extrusion (MATE) family and the small multidrug resistance (SMR) family transporters [36,38]. More recently, an overexpression of the Ade ABC efflux pump, a member of the RND, was associated with tigecycline resistance in A. baumannii [39];
- (3)
- the enzymatic modification of aminoglycosides. Enzymatic modification is the most common type of aminoglycoside resistance [40]. Acetyltransferases, adenylyltransferases and phosphotransferases are three classes of enzymes that play a critical role in the resistance of A. baumannii to aminoglycosides [41]. The genes encoding for aminoglycoside modifying enzymes can be transferred through plasmids and transposons [41].
- (4)
- the production of modified porins that decreases the permeability of the outer membrane [42,43]. In A. baumannii the reduced expression of porins, proteins that allow the transport of molecules across the outer membrane, is associated with carbapenem resistance [29,44]. Moreover, A. baumannii may acquire resistance to colistin, a polypeptide antibacterial agent that targets LPS, as a result of mutation of the genes involved in LPS biosynthesis [45,46];
- (5)
4.2. Pseudomonas aeruginosa
4.3. Staphylococcus aureus
4.4. Klebsiella pneumonia
4.5. Enterobacter Spp.
4.6. Enterococci
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Coculescu, B.-I. Antimicrobial resistance induced by genetic changes. J. Med. Life 2009, 2, 114–123. [Google Scholar]
- Collignon, P.; Beggs, J.J. Socioeconomic Enablers for Contagion: Factors Impelling the Antimicrobial Resistance Epidemic. Antibiotics 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, P.D.; Taylor, P.W. Methicillin Resistance in Staphylococcus Aureus: Mechanisms and Modulation. Sci. Prog. 2002, 85, 57–72. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Mulani, M.S.; Kamble, E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, 181. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. W.H.O. 10 Threats to Global Health in 2018. Available online: https://medium.com/@who/10-threats-to-global-health-in-2018-232daf0bbef32018 (accessed on 18 December 2020).
- Abdelaziz, S.; Aboshanab, K.; Yahia, I.; Yassien, M.; Hassouna, N. Correlation between the Antibiotic Resistance Genes and Susceptibility to Antibiotics among the Carbapenem-Resistant Gram-Negative Pathogens. Antibiotics 2021, 10, 255. [Google Scholar] [CrossRef]
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed on 18 December 2020).
- ECDC. Communicable Disease and Threats Report CDTR. 2019. Available online: www.ecdc.europa.e (accessed on 18 December 2020).
- ECDC. Biggest Threats and Data. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf2019 (accessed on 18 December 2020).
- Cepas, V.; Soto, S.M. Relationship between Virulence and Resistance among Gram-Negative Bacteria. Antibiotics 2020, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Mir Saleem, B.D.; de la Bastide, A.; Korzen, M. Antibiotics Overuse and Bacterial Resistance. Ann. Microbiol. Res. 2019, 3, 93–99. [Google Scholar]
- Iramiot, J.S.; Kajumbula, H.; Bazira, J.; Kansiime, C.; Asiimwe, B.B. Antimicrobial resistance at the human–animal interface in the Pastoralist Communities of Kasese District, South Western Uganda. Sci. Rep. 2020, 10, 14737. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.; Bhattacharyya, S. Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 2019, 9, 9788. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Sandner-Miranda, L.; Vinuesa, P.; Cravioto, A.; Morales-Espinosa, R. The Genomic Basis of Intrinsic and Acquired Antibiotic Resistance in the Genus Serratia. Front. Microbiol. 2018, 9, 828. [Google Scholar] [CrossRef] [Green Version]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Friedrich, A.W. Control of hospital acquired infections and antimicrobial resistance in Europe: The way to go. Wien. Med. Wochenschr. 2019, 169, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Editorial: Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef] [Green Version]
- Benkő, R.; Gajdács, M.; Matuz, M.; Bodó, G.; Lázár, A.; Hajdú, E.; Papfalvi, E.; Hannauer, P.; Erdélyi, P.; Pető, Z. Prevalence and Antibiotic Resistance of ESKAPE Pathogens Isolated in the Emergency Department of a Tertiary Care Teaching Hospital in Hungary: A 5-Year Retrospective Survey. Antibiotics 2020, 9, 624. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The Complex Relationship between Virulence and Antibiotic Resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.; Mishra, S.K.; Shrestha, A. Characterisation of ESKAPE Pathogens with Special Reference to Multidrug Resistance and Biofilm Production in a Nepalese Hospital. Infect. Drug Resist. 2021, 14, 2201–2212. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Genet. 2018, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.F.; Bonomo, R.A.; Fast, W. B1-Metallo-β-Lactamases: Where Do We Stand? Curr. Drug Targets 2016, 17, 1029–1050. [Google Scholar] [CrossRef]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.D.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [Green Version]
- Halat, D.H.; Moubareck, C.A. The Current Burden of Carbapenemases: Review of Significant Properties and Dissemination among Gram-Negative Bacteria. Antibiotics 2020, 9, 186. [Google Scholar] [CrossRef]
- Beceiro, A.; Dominguez, L.; Ribera, A.; Vila, J.; Molina, F.; Villanueva, R.; Eiros, J.M.; Bou, G. Molecular Characterization of the Gene Encoding a New AmpC β-Lactamase in a Clinical Strain of Acinetobacter Genomic Species 3. Antimicrob. Agents Chemother. 2004, 48, 1374–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdi, S.N.; Ghotaslou, R.; Ganbarov, K.; Mobed, A.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Acinetobacter baumannii Efflux Pumps and Antibiotic Resistance. Infect. Drug Resist. 2020, 13, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Basatian-Tashkan, B.; Niakan, M.; Khaledi, M.; Afkhami, H.; Sameni, F.; Bakhti, S.; Mirnejad, R. Antibiotic resistance assessment of Acinetobacter baumannii isolates from Tehran hospitals due to the presence of efflux pumps encoding genes (adeA and adeS genes) by molecular method. BMC Res. Notes 2020, 13, 543. [Google Scholar] [CrossRef]
- Pérez-Varela, M.; Corral, J.; Aranda, J.; Barbé, J. Roles of Efflux Pumps from Different Superfamilies in the Surface-Associated Motility and Virulence of Acinetobacter baumannii ATCC 17978. Antimicrob. Agents Chemother. 2019, 63, e02190-18. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Bilya, S.; Xu, W. adeABC efflux gene in Acinetobacter baumannii. New Microbes New Infect. 2019, 30, 100549. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Nikolaidis, N.; Tolmasky, M.E. Rise and dissemination of aminoglycoside resistance: The aac(6′)-Ib paradigm. Front. Microbiol. 2013, 4, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Tan, P.; Zeng, J.; Yu, X.; Cai, Y.; Liao, K.; Guo, P.; Chen, Y.; Wu, Z.; Qu, P.; et al. Impact of an Intervention to Control Imipenem-Resistant Acinetobacter baumannii and Its Resistance Mechanisms: An 8-Year Survey. Front. Microbiol. 2021, 11, 610109. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- Almasaudi, S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, E.; Nikaido, H. OmpA Is the Principal Nonspecific Slow Porin of Acinetobacter baumannii. J. Bacteriol. 2012, 194, 4089–4096. [Google Scholar] [CrossRef] [Green Version]
- Kamoshida, G.; Akaji, T.; Takemoto, N.; Suzuki, Y.; Sato, Y.; Kai, D.; Hibino, T.; Yamaguchi, D.; Kikuchi-Ueda, T.; Nishida, S.; et al. Lipopolysaccharide-Deficient Acinetobacter baumannii Due to Colistin Resistance Is Killed by Neutrophil-Produced Lysozyme. Front. Microbiol. 2020, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Lertsrisatit, Y.; Santimaleeworagun, W.; Thunyaharn, S.; Traipattanakul, J. In vitro activity of colistin mono- and combination therapy against colistin-resistant Acinetobacter baumannii, mechanism of resistance, and clinical outcomes of patients infected with colistin-resistant A. baumannii at a Thai university hospital. Infect. Drug Resist. 2017, 10, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Vignon-Whaley, J.J.J.; Vaamonde, J.A.A.; Alonzo, L.A.P.; Reséndiz, A.R.; Álvarez, M.M.; López, E.N.V.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef]
- Shankar, C.; Nabarro, L.E.B.; Anandan, S.; Veeraraghavan, B. Minocycline and Tigecycline: What Is Their Role in the Treatment of Carbapenem-Resistant Gram–Negative Organisms? Microb. Drug Resist. 2017, 23, 437–446. [Google Scholar] [CrossRef]
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The “Old” and the “New” Antibiotics for MDR Gram-Negative Pathogens: For Whom, When, and How. Front. Public Health 2019, 7, 151. [Google Scholar] [CrossRef] [Green Version]
- Bagińska, N.; Cieślik, M.; Górski, A.; Jończyk-Matysiak, E. The Role of Antibiotic Resistant A. baumannii in the Pathogenesis of Urinary Tract Infection and the Potential of Its Treatment with the Use of Bacteriophage Therapy. Antibiotics 2021, 10, 281. [Google Scholar] [CrossRef]
- Konca, C.; Tekin, M.; Geyik, M. Susceptibility Patterns of Multidrug-Resistant Acinetobacter baumannii. Indian J. Pediatr. 2021, 88, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nepka, M.; Perivolioti, E.; Kraniotaki, E.; Politi, L.; Tsakris, A.; Pournaras, S. In Vitro Bactericidal Activity of Trimethoprim-Sulfamethoxazole Alone and in Combination with Colistin against Carbapenem-Resistant Acinetobacter baumannii Clinical Isolates. Antimicrob. Agents Chemother. 2016, 60, 6903–6906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recio, R.; Mancheño, M.; Viedma, E.; Villa, J.; Orellana, M.Á.; Lora-Tamayo, J.; Chaves, F. Predictors of Mortality in Bloodstream Infections Caused by Pseudomonas aeruginosa and Impact of Antimicrobial Resistance and Bacterial Virulence. Antimicrob. Agents Chemother. 2020, 64, e01759-19. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.; Yoon, S.S. Virulence Characteristics and an Action Mode of Antibiotic Resistance in Multidrug-Resistant Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Henrichfreise, B.; Wiegand, I.; Pfister, W.; Wiedemann, B. Resistance Mechanisms of Multiresistant Pseudomonas aeruginosa Strains from Germany and Correlation with Hypermutation. Antimicrob. Agents Chemother. 2007, 51, 4062–4070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langendonk, R.F.; Neill, D.R.; Fothergill, J.L. The Building Blocks of Antimicrobial Resistance in Pseudomonas aeruginosa: Implications for Current Resistance-Breaking Therapies. Front. Cell. Infect. Microbiol. 2021, 11, 665759. [Google Scholar] [CrossRef]
- Sader, H.S.; Huband, M.D.; Castanheira, M.; Flamm, R.K. Pseudomonas aeruginosa Antimicrobial Susceptibility Results from Four Years (2012 to 2015) of the International Network for Optimal Resistance Monitoring Program in the United States. Antimicrob. Agents Chemother. 2017, 61, e02252-16. [Google Scholar] [CrossRef] [Green Version]
- Dehbashi, S.; Tahmasebi, H.; Alikhani, M.Y.; Keramat, F.; Arabestani, M.R. Distribution of Class B and Class A β-Lactamases in Clinical Strains of Pseudomonas aeruginosa: Comparison of Phenotypic Methods and High-Resolution Melting Analysis (HRMA) Assay. Infect. Drug Resist. 2020, 13, 2037–2052. [Google Scholar] [CrossRef]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef]
- Berrazeg, M.; Jeannot, K.; Enguéné, V.Y.N.; Broutin, I.; Loeffert, S.; Fournier, D.; Plésiat, P. Mutations in β-Lactamase AmpC Increase Resistance of Pseudomonas aeruginosa Isolates to Antipseudomonal Cephalosporins. Antimicrob. Agents Chemother. 2015, 59, 6248–6255. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Sony, S.A.; Chowdhury, B.; Ullah, M.; Paul, S.; Hossain, T. Retention of antibiotic activity against resistant bacteria harbouring aminoglycoside-N-acetyltransferase enzyme by adjuvants: A combination of in-silico and in-vitro study. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Ontong, J.C.; Ozioma, N.F.; Voravuthikunchai, S.P.; Chusri, S. Synergistic antibacterial effects of colistin in combination with aminoglycoside, carbapenems, cephalosporins, fluoroquinolones, tetracyclines, fosfomycin, and piperacillin on multidrug resistant Klebsiella pneumoniae isolates. PLoS ONE 2021, 16, e0244673. [Google Scholar] [CrossRef] [PubMed]
- Pungcharoenkijkul, S.; Traipattanakul, J.; Thunyaharn, S.; Santimaleeworagun, W. Antimicrobials as Single and Combination Therapy for Colistin-Resistant Pseudomonas aeruginosa at a University Hospital in Thailand. Antibiotics 2020, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Tigabu, A.; Getaneh, A. Staphylococcus aureus, ESKAPE Bacteria Challenging Current Health Care and Community Settings: A Literature Review. Clin. Lab. 2021, 67, 930. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimza, B.D.; Cassat, J.E. Mechanisms of Antibiotic Failure During Staphylococcus aureus Osteomyelitis. Front. Immunol. 2021, 12, 638085. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 57. [Google Scholar] [CrossRef]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 18033. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.; Eichenberger, E.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Genet. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Mühlberg, E.; Umstätter, F.; Kleist, C.; Domhan, C.; Mier, W.; Uhl, P. Renaissance of vancomycin: Approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can. J. Microbiol. 2020, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Peérichon, B.; Courvalin, P. VanA-Type Vancomycin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 4580–4587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kest, H.; Kaushik, A. Vancomycin-Resistant Staphylococcus aureus: Formidable Threat or Silence before the Storm? Infect. Dis. Epidemiol. 2019, 5, 93. [Google Scholar] [CrossRef]
- Gardete, S.; Tomasz, A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Investig. 2014, 124, 2836–2840. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Dadashi, M.; Moghadam, M.T.; Van Belkum, A.; Yaslianifard, S.; Darban-Sarokhalil, D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yaw, L.K.; Robinson, J.O.; Ho, K.M. A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: An observational cohort study. Lancet Infect. Dis. 2014, 14, 967–975. [Google Scholar] [CrossRef]
- Nicolas, I.; Bordeau, V.; Bondon, A.; Baudy-Floc’H, M.; Felden, B. Novel antibiotics effective against gram-positive and -negative multi-resistant bacteria with limited resistance. PLoS Biol. 2019, 17, e3000337. [Google Scholar] [CrossRef]
- Kim, B.-N.; Kim, E.S.; Oh, M.-D. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J. Antimicrob. Chemother. 2014, 69, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Khodabandeh, M.; Mohammadi, M.; Abdolsalehi, M.R.; Alvandimanesh, A.; Gholami, M.; Bibalan, M.H.; Pournajaf, A.; Kafshgari, R.; Rajabnia, R. Analysis of Resistance to Macrolide–Lincosamide–Streptogramin B Among mecA-Positive Staphylococcus Aureus Isolates. Osong Public Health Res. Perspect. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Timsina, R.; Shrestha, U.; Singh, A.; Timalsina, B. Inducible clindamycin resistance and erm genes in Staphylococcus aureus in school children in Kathmandu, Nepal. Futur. Sci. OA 2021, 7, FSO361. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Jorgensen, J.H. Inducible Clindamycin Resistance in Staphylococci: Should Clinicians and Microbiologists be Concerned? Clin. Infect. Dis. 2005, 40, 280–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, J.H.; Crawford, S.A.; McElmeel, M.L.; Fiebelkorn, K.R. Detection of Inducible Clindamycin Resistance of Staphylococci in Conjunction with Performance of Automated Broth Susceptibility Testing. J. Clin. Microbiol. 2004, 42, 1800–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and Hospital-Acquired Klebsiella pneumoniae Urinary Tract Infections in Portugal: Virulence and Antibiotic Resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Eghbalpoor, F.; Habibi, M.; Azizi, O.; Karam, M.R.A.; Bouzari, S. Antibiotic resistance, virulence and genetic diversity of Klebsiella pneumoniae in community- and hospital-acquired urinary tract infections in Iran. Acta Microbiol. Immunol. Hung. 2019, 66, 349–366. [Google Scholar] [CrossRef] [Green Version]
- Young, T.M.; Bray, A.S.; Nagpal, R.K.; Caudell, D.L.; Yadav, H.; Zafar, M.A. Animal Model to Study Klebsiella pneumoniae Gastrointestinal Colonization and Host-to-Host Transmission. Infect. Immun. 2020, 88, e00071-20. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Lasko, M.J.; Nicolau, D.P. Carbapenem-Resistant Enterobacterales: Considerations for Treatment in the Era of New Antimicrobials and Evolving Enzymology. Curr. Infect. Dis. Rep. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Gualtero, S.; Valderrama, S.; Valencia, M.; Rueda, D.; Muñoz-Velandia, O.; Ariza, B.; Cortes, G.; Salgado, D.; Porras, Y.; Niño, A. Factors associated with mortality in Infections caused by Carbapenem-resistant Enterobacteriaceae. J. Infect. Dev. Ctries. 2020, 14, 654–659. [Google Scholar] [CrossRef]
- Sheu, C.-C.; Chang, Y.-T.; Lin, S.-Y.; Chen, Y.-H.; Hsueh, P.-R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Davin-Regli, A. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Marín, R.; Lepe, J.A.; Gasch-Blasi, O.; Rodríguez-Martínez, J.M.; Calvo-Montes, J.; Lara-Contreras, R.; Martín-Gandul, C.; Tubau-Quintano, F.; Cano-García, M.E.; Rodríguez-López, F.; et al. Clinical characteristics and outcome of bacteraemia caused by Enterobacter cloacae and Klebsiella aerogenes: More similarities than differences. J. Glob. Antimicrob. Resist. 2021, 25, 351–358. [Google Scholar] [CrossRef]
- Reza, A.; Sutton, J.M.; Rahman, K.M. Effectiveness of Efflux Pump Inhibitors as Biofilm Disruptors and Resistance Breakers in Gram-Negative (ESKAPEE) Bacteria. Antibiotics 2019, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Uzunović, S.; Ibrahimagić, A.; Bedenić, B. Antibiotic resistance in Enterobacter cloacae strains with derepressed/partly derepressed/inducible AmpC and extendedspectrum beta-lactamases in Zenica-Doboj Canton, Bosnia and Herzegovina. Medicinski Glasnik 2018, 15, 37–45. [Google Scholar]
- Di Franco, S.; Alfieri, A.; Pace, M.; Sansone, P.; Pota, V.; Fittipaldi, C.; Fiore, M.; Passavanti, M. Blood Stream Infections from MDR Bacteria. Life 2021, 11, 575. [Google Scholar] [CrossRef]
- Band, V.I.; Crispell, E.K.; Napier, B.A.; Herrera, C.M.; Tharp, G.K.; Vavikolanu, K.; Pohl, J.; Read, T.D.; Bosinger, S.E.; Trent, M.S.; et al. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat. Microbiol. 2016, 1, 16053. [Google Scholar] [CrossRef] [PubMed]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [Green Version]
- Shiadeh, S.M.J.; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef] [Green Version]
- Pöntinen, A.K.; Top, J.; Arredondo-Alonso, S.; Tonkin-Hill, G.; Freitas, A.R.; Novais, C.; Gladstone, R.A.; Pesonen, M.; Meneses, R.; Pesonen, H.; et al. Apparent nosocomial adaptation of Enterococcus faecalis predates the modern hospital era. Nat. Commun. 2021, 12, 1523. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Silva, V.; Dapkevicius, M.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020, 10, 3937. [Google Scholar] [CrossRef] [PubMed]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Stolarzewicz, I.; Cannaerts, D.; Schuermans, J.; Lavigne, R.; Looz, Y.; Landuyt, B.; Schoofs, L.; Schols, D.; Paeshuyse, J.; et al. Iterative Chemical Engineering of Vancomycin Leads to Novel Vancomycin Analogs with a High in Vitro Therapeutic Index. Front. Microbiol. 2018, 9, 1175. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-Resistant Enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Moon, D.; Kim, S.-J.; Mechesso, A.; Song, H.-J.; Kang, H.; Choi, J.-H.; Yoon, S.-S.; Lim, S.-K. Nationwide Surveillance on Antimicrobial Resistance Profiles of Enterococcus faecium and Enterococcus faecalis Isolated from Healthy Food Animals in South Korea, 2010 to 2019. Microorganisms 2021, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Nicoloff, H.; Hjort, K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat. Rev. Genet. 2019, 17, 479–496. [Google Scholar] [CrossRef]
- Metzler, K.; Hansen, G.; Hedlin, P.; Harding, E.; Drlica, K.; Blondeau, J. Comparison of minimal inhibitory and mutant prevention drug concentrations of 4 fluoroquinolones against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2004, 24, 161–167. [Google Scholar] [CrossRef]
- Gianvecchio, C.; Lozano, N.A.; Henderson, C.; Kalhori, P.; Bullivant, A.; Valencia, A.; Su, L.; Bello, G.; Wong, M.; Cook, E.; et al. Variation in Mutant Prevention Concentrations. Front. Microbiol. 2019, 10, 42. [Google Scholar] [CrossRef] [Green Version]

| Antimicrobial Groups | Mechanism of Action | Resistance Mechanism |
|---|---|---|
| β-Lactams Penicillins | Inhibits cell wall production | Beta-lactamase production Penicillinase |
| Cephalosporins Carbapenems | Cephalosporinase Carbapenemase | |
| β-Lactamase inhibitors | Block the activity of beta-lactamase enzymes | Extended-spectrum beta-lactamase (ESBL) |
| Aminoglycosides, Chloramphenicol Macrolides, Tetracyclines | Inhibit ribosome assembly by binding to the bacterial 30S or 50S (inhibit protein synthesis) | Multifactorial (enzymatic modification, target site modification and efflux pumps) |
| Fluoroquinolone | Inhibit DNA replication | Multifactorial (target-site gene mutations, efflux pumps and modifying enzyme) |
| Sulfonamides and trimethoprim | Inhibit folic acid metabolism | Horizontal spread of resistance genes, mediated by transposons and plasmids, expressing drug-insensitive variants of the target enzymes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. https://doi.org/10.3390/pathogens10101310
Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens. 2021; 10(10):1310. https://doi.org/10.3390/pathogens10101310
Chicago/Turabian StyleMancuso, Giuseppe, Angelina Midiri, Elisabetta Gerace, and Carmelo Biondo. 2021. "Bacterial Antibiotic Resistance: The Most Critical Pathogens" Pathogens 10, no. 10: 1310. https://doi.org/10.3390/pathogens10101310
APA StyleMancuso, G., Midiri, A., Gerace, E., & Biondo, C. (2021). Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens, 10(10), 1310. https://doi.org/10.3390/pathogens10101310






