Zinc Deprivation as a Promising Approach for Combating Methicillin-Resistant Staphylococcus aureus: A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Assessment of the Effect of Zinc Deprivation on Growth of S. aureus in Chemically Defined Media
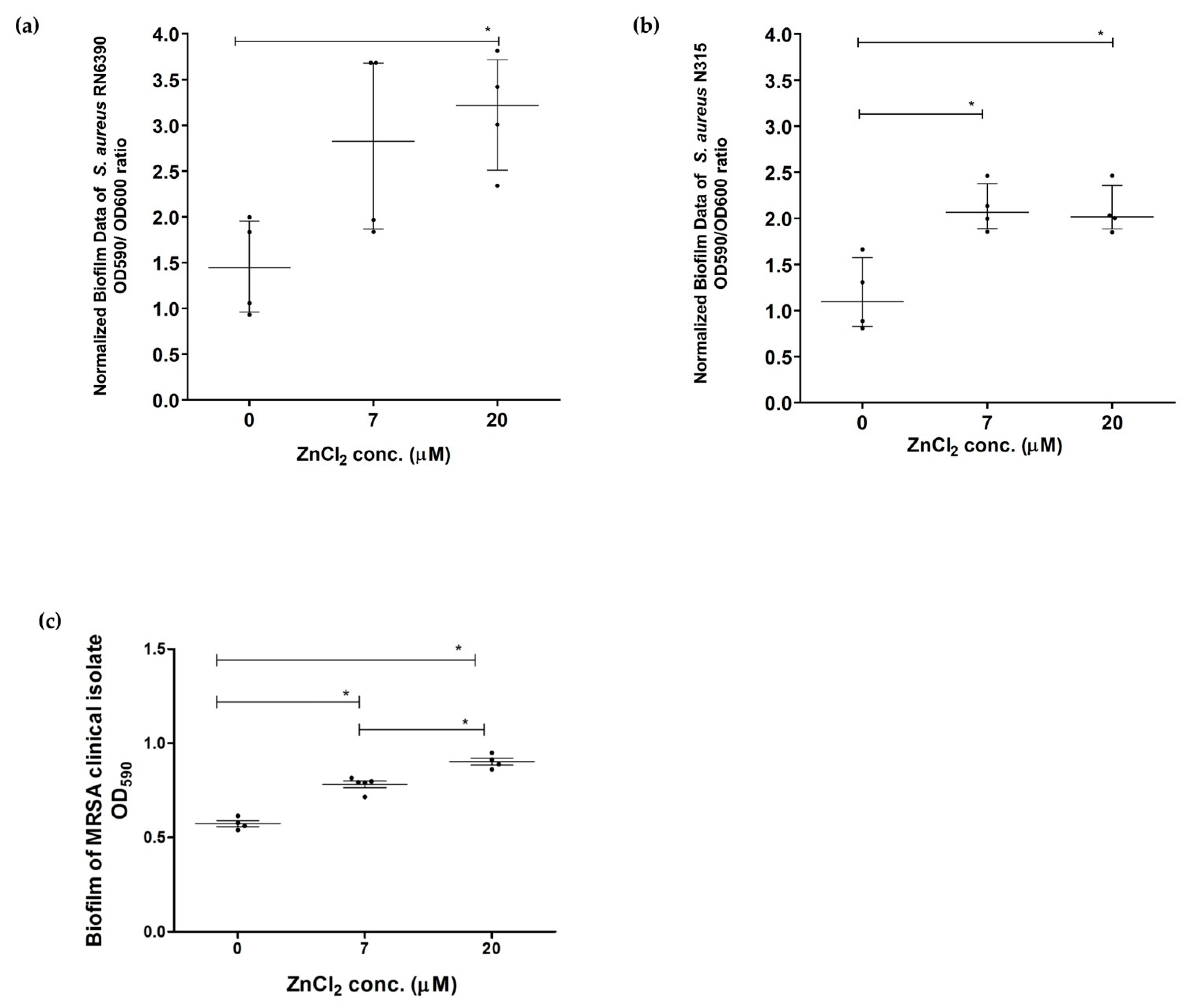
2.2. Assessment of Effect of Zinc Deprivation on Biofilm Formation Ability of S. aureus in Chemically Defined Media Using Crystal Violet Microtiter Plate Method
2.3. Assessment of the Effect of Zinc Deprivation on Oxidative Stress and Metalloproteases Activity of S. aureus Exemplified as Catalase Activity and Protease Activity
2.4. Determination of the Effect of Zinc Deprivation on S. aureus Hemolytic Activity in Chemically Defined Media
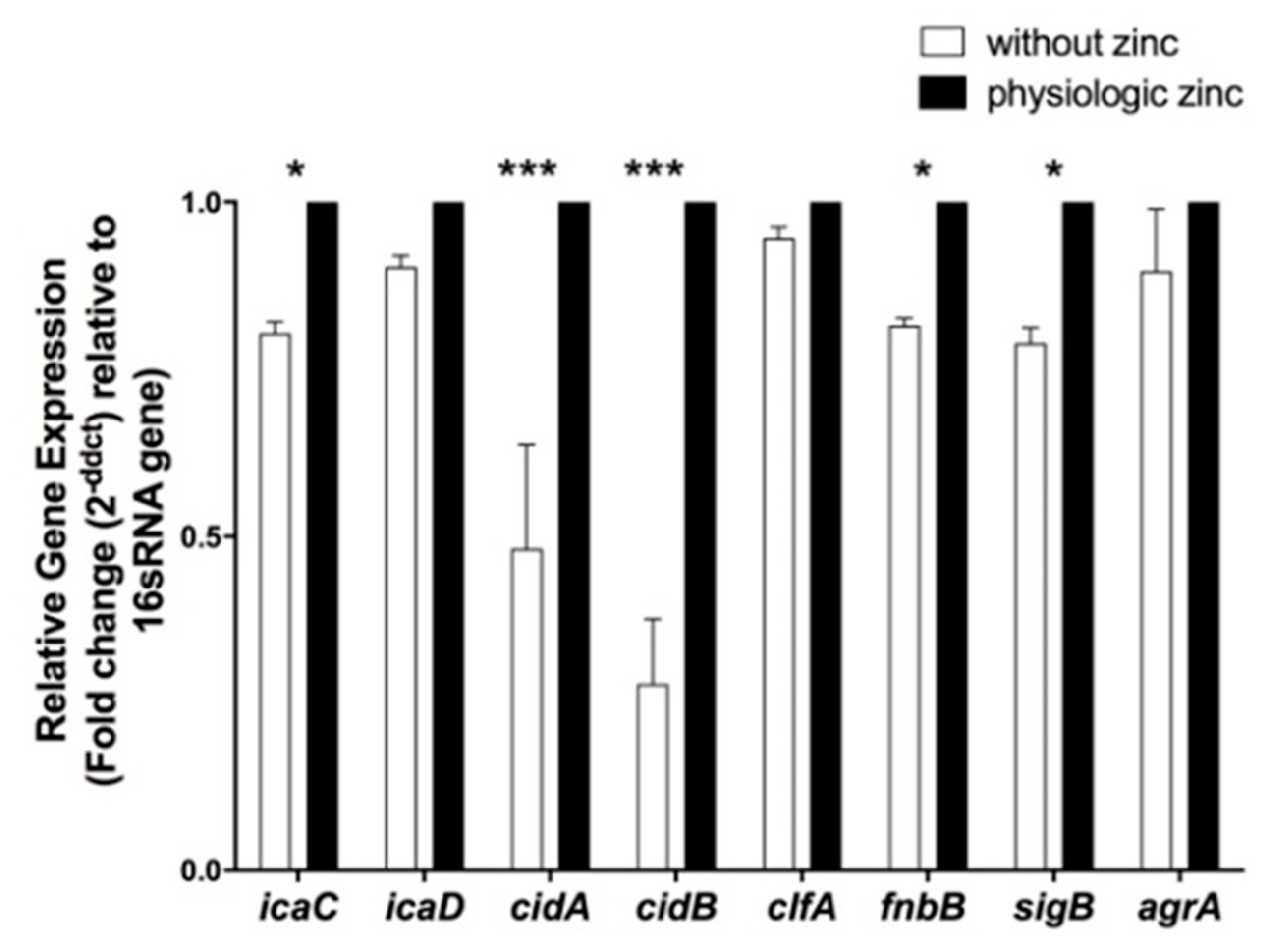
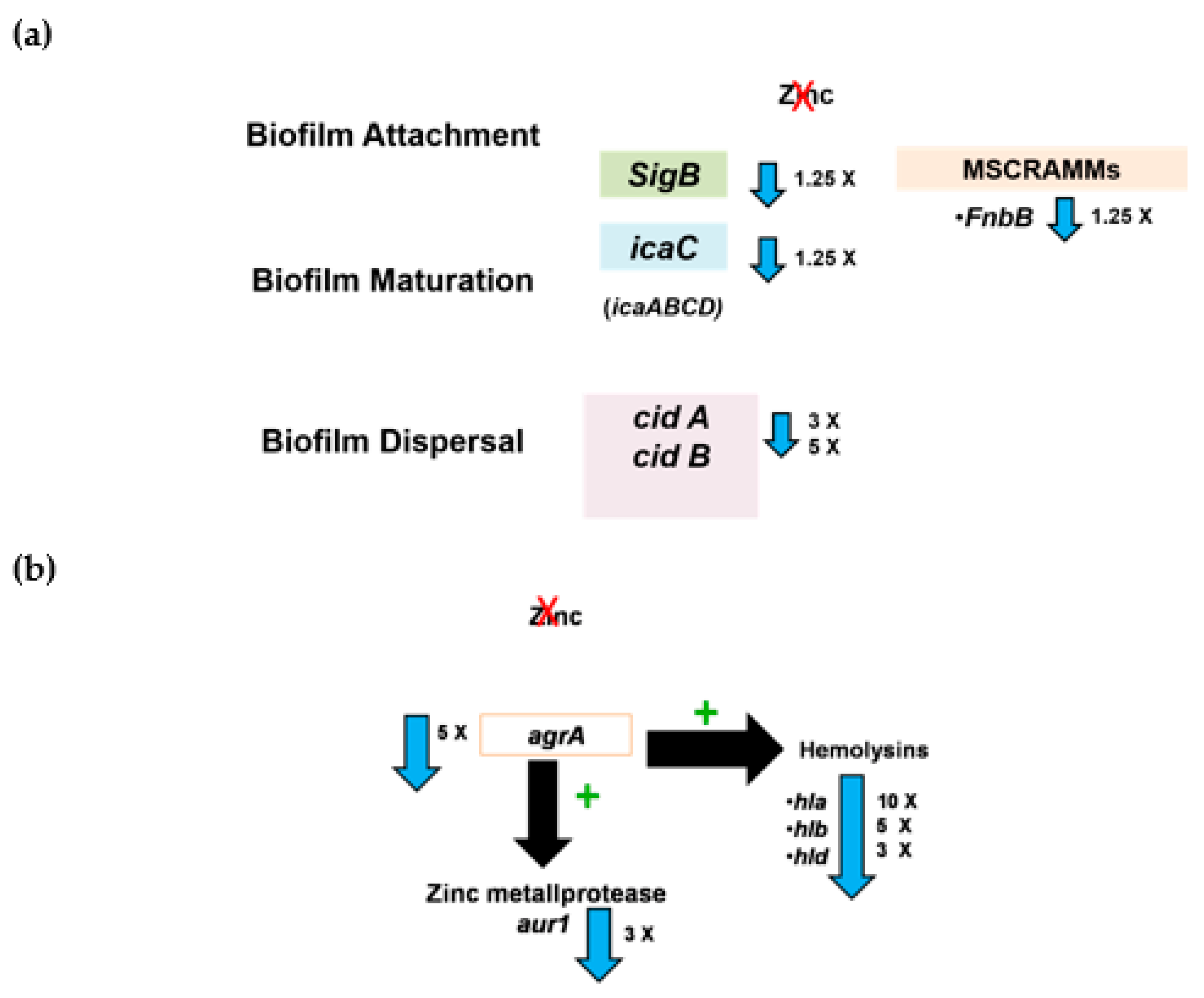
2.5. Determination of the Effect of Zinc Deprivation on Differential Gene Expression of Selected Biofilm Associated Genes in S. aureus N315 under Biofilm Condition
2.6. Determination of the Effect of Zinc Deprivation on Differential Gene Expression of Selected Protease and Hemolysis Associated Genes in S. aureus N315 under Planktonic Condition
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains Maintenance and Culturing Conditions
4.2. Chemically Defined Medium for Assessment of Zinc Deprivation
4.3. Analysis of Zinc Concentrations in the Prepared Chemically Defined Media
4.3.1. Zincon Assay
4.3.2. Inductively Coupled Plasma Spectrometry (ICP)
4.4. Determination of the Effect of Zinc Deprivation on the Growth of Selected S. aureus Strains Using Chemically Defined Media
4.5. Determination of Effect of Zinc Deprivation on the Biofilm Formation of Selected S. aureus Strains Using Crystal Violet Microtiter Plate Assay Method
4.6. Qualitative Assessment of the Effect of Zinc Deprivation on the Oxidative Stress Exemplified as Catalase Activity of Selected S. aureus Strains in Chemically Defined Media
4.7. Qualitative Assessment of the Effect of Zinc Deprivation on the Metalloproteases Exemplified as Protease Activity of Selected S. aureus Strains in Chemically Defined Media
4.8. Quantitative Assessment of the Effect of Zinc Deprivation on the Hemolytic Activity of Selected S. aureus Strains in Chemically Defined Media
4.9. Determination of the Effect of Zinc Deprivation on Differential Gene Expression of Selected Genes in S. aureus N315 under Planktonic and Biofilm Conditions
4.9.1. Total RNA Isolation
4.9.2. Complementary DNA (cDNA) Synthesis
4.9.3. Quantitative Real-Time PCR Analysis of Differentially Expressed Genes
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holden, M.; Lindsay, J.; Bentley, S. The Grapes of Wrath. Nat. Rev. Microbiol. 2006, 4, 806–807. [Google Scholar] [CrossRef]
- Schito, G.C. The Importance of the Development of Antibiotic Resistance in Staphylococcus aureus. Clin. Microbiol. Infect. 2006, 12, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.R.; Batistão, D.W.F.; Ribas, R.M.; Sousa, A.M.; Pereira, O.; Botelho, C.M. Staphylococcus aureus Virulence Factors and Disease. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Formatex Research Center: Badajoz, Spain, 2013; pp. 702–710. [Google Scholar]
- Udo, E.E. Community-Acquired Methicillin-Resistant Staphylococcus aureus: The New Face of an Old Foe? Med. Princ. Pract. 2013, 22 (Suppl. S1), 20–29. [Google Scholar] [CrossRef]
- Abouelfetouh, A. The Status of Methicillin Resistance Among Egyptian Staphylococcus aureus Isolates: An Overview. Infect. Disord.-Drug Targets 2017, 17, 67–69. [Google Scholar] [CrossRef]
- Kong, E.F.; Johnson, J.K.; Jabra-Rizk, M.A. Community-Associated Methicillin-Resistant Staphylococcus aureus: An Enemy amidst Us. PLoS Pathog. 2016, 12, e1005837. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Diep, B.A.; Otto, M. Host Defense and Pathogenesis in Staphylococcus aureus Infections. Infect. Dis. Clin. N. Am. 2009, 23, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Fey, P.D.; Olson, M.E. Current Concepts in Biofilm Formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcal Biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [CrossRef]
- Soto, S.M. Importance of Biofilms in Urinary Tract Infections: New Therapeutic Approaches. Adv. Biol. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Trautner, B.; Darouiche, R. Role of Biofilm in Catheter-Associated Urinary Tract Infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef]
- Weinberg, E.D. Iron and Susceptibility to Infectious Disease. Science 1974, 184, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal Chelation and Inhibition of Bacterial Growth in Tissue Abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef]
- Agranoff, D.D.; Krishna, S. Metal Ion Homeostasis and Intracellular Parasitism. Mol. Microbiol. 1998, 28, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Hood, M.I.; Skaar, E.P. Nutritional Immunity: Transition Metals at the Pathogen–Host Interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Mcdevitt, C.A.; Ogunniyi, A.D.; Valkov, E.; Lawrence, M.C.; Kobe, B.; Mcewan, A.G.; Paton, J.C. A Molecular Mechanism for Bacterial Susceptibility to Zinc. PLoS Pathog. 2011, 7, e1002357. [Google Scholar] [CrossRef] [Green Version]
- Hammer, N.D.; Skaar, E.P. The Impact of Metal Sequestration on Staphylococcus aureus Metabolism. Curr. Opin. Microbiol. 2012, 15, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Festa, R.A.; Thiele, D.J. Copper at the Front Line of the Host-Pathogen Battle. PLoS Pathog. 2012, 8, e1002887. [Google Scholar] [CrossRef] [Green Version]
- Cassat, J.E.; Skaar, E.P. Metal Ion Acquisition in Staphylococcus aureus: Overcoming Nutritional Immunity. Semin. Immunopathol. 2012, 34, 215–235. [Google Scholar] [CrossRef] [Green Version]
- Beasley, F.C.; Vinés, E.D.; Grigg, J.C.; Zheng, Q.; Liu, S.; Lajoie, G.A.; Murphy, M.E.P.; Heinrichs, D.E. Characterization of Staphyloferrin A Biosynthetic and Transport Mutants in Staphylococcus aureus. Mol. Microbiol. 2009, 72, 947–963. [Google Scholar] [CrossRef]
- Skaar, E.P. The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts. PLoS Pathog. 2010, 6, e1000949. [Google Scholar] [CrossRef]
- Ciccaglione, A.F.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Cellini, L.; Marzio, L. Bovine Lactoferrin Enhances the Efficacy of Levofloxacin-Based Triple Therapy as First-Line Treatment of Helicobacter pylori Infection: An in Vitro and in Vivo Study. J. Antimicrob. Chemother. 2019, 74, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Kehl-Fie, T.E.; Chitayat, S.; Hood, M.I.; Damo, S.; Restrepo, N.; Garcia, C.; Munro, K.A.; Chazin, W.J.; Skaar, E.P. Nutrient Metal Sequestration by Calprotectin Inhibits Bacterial Superoxide Defense, Enhancing Neutrophil Killing of Staphylococcus aureus. Cell Host Microbe 2011, 10, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.T.; Karim, M.M. Metallothionein: A Potential Link in the Regulation of Zinc in Nutritional Immunity. Biol. Trace Elem. Res. 2018, 182, 1–13. [Google Scholar] [CrossRef]
- Chowdhury, D.; Alrefai, H.; Landero Figueroa, J.A.; Candor, K.; Porollo, A.; Fecher, R.; Divanovic, S.; Deepe, G.S.; Subramanian Vignesh, K. Metallothionein 3 Controls the Phenotype and Metabolic Programming of Alternatively Activated Macrophages. Cell Rep. 2019, 27, 3873–3886.e7. [Google Scholar] [CrossRef] [Green Version]
- Gaddy, J.A.; Radin, J.N.; Loh, J.T.; Piazuelo, M.B.; Kehl-Fie, T.E.; Delgado, A.G.; Ilca, F.T.; Peek, R.M.; Cover, T.L.; Chazin, W.J.; et al. The Host Protein Calprotectin Modulates the Helicobacter pylori Cag Type IV Secretion System via Zinc Sequestration. PLoS Pathog. 2014, 10, e1004450. [Google Scholar] [CrossRef] [Green Version]
- Velasco, E.; Wang, S.; Sanet, M.; Fernández-Vázquez, J.; Jové, D.; Glaría, E.; Valledor, A.F.; O’Halloran, T.V.; Balsalobre, C. A New Role for Zinc Limitation in Bacterial Pathogenicity: Modulation of α-Hemolysin from Uropathogenic Escherichia coli. Sci. Rep. 2018, 8, 6535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Hastings, J.G.M.; White, P.J. A Chemically Defined Medium for Slime Production by Coagulase-Negative Staphylococci. J. Med. Microbiol. 1991, 34, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, J.A.; Foster, S.J. Zur: A Zn2+-Responsive Regulatory Element of Staphylococcus aureus The GenBank Accession Number for the Sequence Reported in This Paper Is AF101263. Microbiology 2001, 47, 1259–1266. [Google Scholar] [CrossRef] [Green Version]
- Conrady, D.G.; Brescia, C.C.; Horii, K.; Weiss, A.A.; Hassett, D.J.; Herr, A.B. A Zinc-Dependent Adhesion Module Is Responsible for Intercellular Adhesion in Staphylococcal Biofilms. Proc. Natl. Acad. Sci. USA 2008, 2008, 19456–19461. [Google Scholar] [CrossRef] [Green Version]
- Grim, K.P.; San Francisco, B.; Radin, J.N.; Brazel, E.B.; Kelliher, J.L.; Párraga Solórzano, P.K.; Kim, P.C.; McDevitt, C.A.; Kehl-Fie, T.E. The Metallophore Staphylopine Enables Staphylococcus aureus To Compete with the Host for Zinc and Overcome Nutritional Immunity. mBio 2017, 8, e01281-17. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Gifford, A.H.; Hampton, T.H.; O’Toole, G.A. Availability of Zinc Impacts Interactions between Streptococcus Sanguinis and Pseudomonas Aeruginosa in Coculture. J. Bacteriol. 2020, 202, e00618-19. [Google Scholar] [CrossRef]
- Taylor, A. Measurement of Zinc in Clinical Samples. Ann. Clin. Biochem. 1997, 34, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Hussain, W.; Mumtaz, A.; Yasmeen, F.; Khan, S.Q.; Butt, T. Reference Range of Zinc in Adult Population (20–29 years) of Lahore, Pakistan. Pak. J. Med Sci. 2014, 30, 545. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The Metal of Life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current Understanding of ZIP and ZnT Zinc Transporters in Human Health and Diseases. Cell. Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Hajo, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Bose, J.L.; Daly, S.M.; Hall, P.R.; Bayles, K.W. Identification of the Staphylococcus aureus VfrAB Operon, a Novel Virulence Factor Regulatory Locus. Infect. Immun. 2014, 82, 1813–1822. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-C.; Peterson, M.L. New Insights into the Prevention of Staphylococcal Infections and Toxic Shock Syndrome. Expert Rev. Clin. Pharmacol. 2010, 3, 753–767. [Google Scholar] [CrossRef] [Green Version]
- Palmer, L.D.; Skaar, E.P. Transition Metals and Virulence in Bacteria. Annu. Rev. Genet. 2016, 50, 67–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, G.; Carver, P.L. Role of Divalent Metals in Infectious Disease Susceptibility and Outcome. Clin. Microbiol. Infect. 2018, 24, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Platte, J.A.; Marcy, V.M. Photometric Determination of Zinc with Zincon. Application to Water Containing Heavy Metals. Anal. Chem. 1959, 31, 1226–1228. [Google Scholar] [CrossRef]
- Olesik, J.W. ICP-OES Capabilities, Developments, Limitations, and Any Potential Challengers? Spectroscopy 2020, 35, 18–21. [Google Scholar]
- Soltyk, K.; Lozak, A.; Warowna-Grzeskiewicz, M.; Fijalek, Z. The AAS ICP-MS and Electrochemical Determinations of Zinc in Selected Pharmaceutical Preparations. Acta Pol. Pharm. 2000, 57, 261–266. [Google Scholar]
- Zecconi, A.; Scali, F. Staphylococcus aureus Virulence Factors in Evasion from Innate Immune Defenses in Human and Animal Diseases. Immunol. Lett. 2013, 150, 12–22. [Google Scholar] [CrossRef]
- Atshan, S.S.; Shamsudin, M.N.; Karunanidhi, A.; van Belkum, A.; Lung, L.T.T.; Sekawi, Z.; Nathan, J.J.; Ling, K.H.; Seng, J.S.C.; Ali, A.M.; et al. Quantitative PCR Analysis of Genes Expressed during Biofilm Development of Methicillin Resistant Staphylococcus aureus (MRSA). Infect. Genet. Evol. 2013, 18, 106–112. [Google Scholar] [CrossRef]
- Conrady, D.G.; Wilson, J.J.; Herr, A.B. Structural Basis for Zn2+-Dependent Intercellular Adhesion in Staphylococcal Biofilms. Proc. Natl. Acad. Sci. USA 2013, 110, E202–E211. [Google Scholar] [CrossRef] [Green Version]
- Formosa-Dague, C.; Speziale, P.; Foster, T.J.; Geoghegan, J.A.; Dufrêne, Y.F. Zinc-Dependent Mechanical Properties of Staphylococcus aureus Biofilm-Forming Surface Protein SasG. Proc. Natl. Acad. Sci. USA 2016, 113, 410–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, S.A.; Huigens, R.W., III; Melander, C. A 2-Aminobenzimidazole That Inhibits and Disperses Gram-Positive Biofilms through a Zinc-Dependent Mechanism. J. Am. Chem. Soc. 2009, 131, 9868–9869. [Google Scholar] [CrossRef]
- Machado, H.; Weng, L.L.; Dillon, N.; Seif, Y.; Holland, M.; Pekar, J.E.; Monk, J.M.; Nizet, V.; Palsson, B.O.; Feist, A.M. Strain-Specific Metabolic Requirements Revealed by a Defined Minimal Medium for Systems Analyses of Staphylococcus aureus. Appl. Environ. Microbiol. 2019, 85, e01773-19. [Google Scholar] [CrossRef] [Green Version]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. In Gram-Positive Pathogens; ASM Press: Washington, DC, USA, 2019; pp. 640–668. [Google Scholar] [CrossRef]
- Mukherji, R.; Patil, A.; Prabhune, A. Role of Extracellular Proteases in Biofilm Disruption of Gram Positive Bacteria with Special Emphasis on Staphylococcus aureus Biofilms. Enzym. Eng. 2015, 4, 126. [Google Scholar] [CrossRef] [Green Version]
- Beaufort, N.; Wojciechowski, P.; Sommerhoff, C.P.; Szmyd, G.; Dubin, G.; Eick, S.; Kellermann, J.; Schmitt, M.; Potempa, J.; Magdolen, V. The Human Fibrinolytic System Is a Target for the Staphylococcal Metalloprotease Aureolysin. Biochem. J. 2008, 410, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laarman, A.J.; Ruyken, M.; Malone, C.L.; van Strijp, J.A.G.; Horswill, A.R.; Rooijakkers, S.H.M. Staphylococcus aureus Metalloprotease Aureolysin Cleaves Complement C3 To Mediate Immune Evasion. J. Immunol. 2011, 186, 6445–6453. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, S.; Saito, M.; Imaizumi, K.; Kaidoh, T.; Higuchi, H.; Inubushi, S. Genetic and Enzymatic Analyses of Metalloprotease (Aureolysin) from Staphylococcus aureus Isolated from Domestic Animals. Vet. Microbiol. 2002, 84, 135–142. [Google Scholar] [CrossRef]
- Pané-Farré, J.; Jonas, B.; Förstner, K.; Engelmann, S.; Hecker, M. The SigB Regulon in Staphylococcus aureus and Its Regulation. Int. J. Med Microbiol. 2006, 296, 237–258. [Google Scholar] [CrossRef]
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus Hemolysins, Bi-Component Leukocidins, and Cytolytic Peptides: A Redundant Arsenal of Membrane-Damaging Virulence Factors? Front. Cell. Infect. Microbiol. 2012, 2, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berube, B.J.; Wardenburg, J.B. Staphylococcus aureus α-Toxin: Nearly a Century of Intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilke, G.A.; Wardenburg, J.B. Role of a Disintegrin and Metalloprotease 10 in Staphylococcus aureus α-Hemolysin-Mediated Cellular Injury. Proc. Natl. Acad. Sci. USA 2010, 107, 13473–13478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menestrina, G.; Bashford, C.L.; Pasternak, C.A. Pore-Forming Toxins: Experiments with S. aureus α-Toxin, C. perfringens θ-Toxin and E. coli Haemolysin in Lipid Bilayers, Liposomes and Intact Cells. Toxicon 1990, 28, 477–491. [Google Scholar] [CrossRef]
- Titball, R.W. Bacterial Phospholipases, C. Microbiol. Rev. 1993, 57, 347–366. [Google Scholar] [CrossRef]
- Laffan, A.M.; McKenzie, R.; Forti, J.; Conklin, D.; Marcinko, R.; Shrestha, R.; Bellantoni, M.; Greenough, W.B., III. Lactoferrin for the Prevention of Post-Antibiotic Diarrhoea. J. Health Popul. Nutr. 2011, 29, 547–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilton, C.H.; Crowther, G.S.; Śpiewak, K.; Brindell, M.; Singh, G.; Wilcox, M.H.; Monaghan, T.M. Potential of Lactoferrin to Prevent Antibiotic-Induced Clostridium difficile Infection. J. Antimicrob. Chemother. 2016, 71, 975–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacManus, C.F.; Collins, C.B.; Nguyen, T.T.; Alfano, R.W.; Jedlicka, P.; de Zoeten, E.F. VEN-120, a Recombinant Human Lactoferrin, Promotes a Regulatory T Cell [Treg] Phenotype and Drives Resolution of Inflammation in Distinct Murine Models of Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 1101–1112. [Google Scholar] [CrossRef]
- Pall, E.; Roman, A. Lactoferrin Functionalized Biomaterials: Tools for Prevention of Implant-Associated Infections. Antibiotics 2020, 9, 522. [Google Scholar] [CrossRef]
- Wilson, D.; Varigos, G.; Ackland, M.L. Apoptosis May Underlie the Pathology of Zinc-Deficient Skin. Immunol. Cell Biol. 2006, 84, 28–37. [Google Scholar] [CrossRef]
- Novick, R.P. Genetic Systems in Staphylococci. Methods Enzymol. 1991, 204, 587–636. [Google Scholar] [CrossRef]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole Genome Sequencing of Meticillin-Resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Nour El-Din, H.T.; Yassin, A.S.; Ragab, Y.M.; Hashem, A.M. Phenotype-Genotype Characterization and Antibiotic-Resistance Correlations Among Colonizing and Infectious Methicillin-Resistant Staphylococcus aureus Recovered from Intensive Care Units. Infect. Drug Resist. 2021, 14, 1557–1571. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement, CLSI Document M100-S24; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Vitko, N.P.; Richardson, A.R. Laboratory Maintenance of Methicillin-Resistant Staphylococcus aureus (MRSA). Curr. Protoc. Microbiol. 2013, 28, 9C-2. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.; Holland, K. Amino Acid Requirements for the Growth and Production of Some Exocellular Products of Staphylococcus aureus. J. Appl. Bacteriol. 1989, 66, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Remy, L.; Carrière, M.; Derré-bobillot, A.; Martini, C.; Sanguinetti, M.; Borezée-durant, E.; Yvette, G. The Staphylococcus aureus Opp1 ABC Transporter Imports Nickel and Cobalt in Zinc-Depleted Conditions and Contributes to Virulence. Mol. Microbiol. 2013, 87, 730–743. [Google Scholar] [CrossRef]
- Naves, P.; del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Rodrguez-Cerrato, V.; Ponte, M.C.; Soriano, F. Measurement of Biofilm Formation by Clinical Isolates of Escherichia coli Is Method-Dependent. J. Appl. Microbiol. 2008, 105, 585–590. [Google Scholar] [CrossRef]
- Reiner, K. Catalase-Test-Protocol. Am. Soc. Microbiol. 2010, 1–9. Available online: https://asm.org/Protocols/Catalase-Test-Protocol (accessed on 23 September 2021).
- Kong, C.; Chee, C.-F.; Richter, K.; Thomas, N.; Rahman, N.A.; Nathan, S. Suppression of Staphylococcus aureus Biofilm Formation and Virulence by a Benzimidazole Derivative, UM-C162. Sci. Rep. 2018, 8, 2758. [Google Scholar] [CrossRef]
- Kateete, D.P.; Kimani, C.N.; Katabazi, F.A.; Okeng, A.; Okee, M.S.; Nanteza, A.; Joloba, M.L.; Najjuka, F.C. Identification of Staphylococcus aureus: DNase and Mannitol Salt Agar Improve the Efficiency of the Tube Coagulase Test. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Bernheimer, A.W. [30] Assay of Hemolytic Toxins. In Microbial Toxins: Tools in Enzymology; Academic Press: Cambridge, MA, USA, 1988; Volume 165, pp. 213–217. [Google Scholar] [CrossRef]
- Costabile, M. Measuring the 50% Haemolytic Complement (CH50) Activity of Serum. J. Vis. Exp. 2010, 37, e1923. [Google Scholar] [CrossRef] [Green Version]
- Attia, A.S.; Benson, M.A.; Stauff, D.L.; Torres, V.J.; Skaar, E.P. Membrane Damage Elicits an Immunomodulatory Program in Staphylococcus aureus. PLoS Pathog. 2010, 6, e1000802. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.; Neoh, H.M.; Nathan, S. Targeting Staphylococcus aureus Toxins: A Potential Form of Anti-Virulence Therapy. Toxins 2016, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.L. Zn Biosorption by Rhizopus arrhizus and Other Fungi. Appl. Microbiol. Biotechnol. 1999, 51, 686–693. [Google Scholar] [CrossRef]



| Dilution | Zinc Depleted Conditions | Physiological Zinc (20 µM zinc) | ||
|---|---|---|---|---|
| Mean Absorbance at 450 nm | Mean Percent Hemolysis (%) * | Mean Absorbance at 450 nm | Mean Percent Hemolysis (%) | |
| 1 ** | 0.969 | 44.09 | 1.389 | 67.492 |
| (1:2) | 0.66 | 26.89 | 0.71 | 29.565 |
| (1:4) | 0.51 | 18.318 | 0.61 | 23.94 |
| (1:8) | 0.38 | 11.19 | 0.39 | 11.8 |
| (1:16) | 0.34 | 9.24 | 0.35 | 9.688 |
| (1:32) | 0.2 | 1.22 | 0.38 | 11.19 |
| Dilution | Zinc Depleted Conditions | Physiological Zinc (20 µM zinc) | ||
|---|---|---|---|---|
| Mean Absorbance at 450 nm | Mean Percent Hemolysis (%) * | Mean Absorbance at 450 nm | Mean Percent Hemolysis (%) | |
| 1 | 1.342 | 77.17 | 1.43 | 80.79 |
| (1:2) | 0.87 | 46.88 | 1.12 | 62.639 |
| (1:4) | 0.8 | 42.817 | 0.95 | 52.817 |
| (1:8) | 0.34 | 12.416 | 0.76 | 37.527 |
| (1:16) ** | 0.24 | 7.01 | 0.668 | 34.3 |
| (1:32) | 0.27 | 6.06 | 0.35 | 13.752 |
| Component | Concentration |
|---|---|
| Salt solution | |
| Ammonium sulfate ≥ 99% | 2 g/L |
| Magnesium sulfate heptahydrate | 0.5 g/L |
| Potassium phosphate monobasic | 4 g/L |
| Sodium phosphate dibasic | 4 g/L |
| Amino acid solution | |
| L (+) Glutamic acid 99% | 10 g/L |
| L (+)-Aspartic acid, 98+% | 9 g/L |
| L-Alanine | 6 g/L |
| L-Arginine | 7 g/L |
| L-Cystine | 2 g/L |
| L-Histidine | 3 g/L |
| L-Isoleucine | 3 g/L |
| L-Leucine | 9 g/L |
| L-Lysine monohydrochloride | 1 g/L |
| L-Methionine | 7 g/L |
| L-Phenylalanine | 5 g/L |
| L-Proline 99% | 1 g/L |
| L-Serine for chemistry | 3 g/L |
| L-Threonine | 3 g/L |
| L-Tryptophan | 1 g/L |
| L-Tyrosine 99+% | 5 g/L |
| L-Valine 99% | 8 g/L |
| Bases | |
| Adenine | 0.5 g/L |
| Cytosine | 0.5 g/L |
| Guanine 99% | 0.5 g/L |
| Thymine | 2g/L |
| Uracil | 0.5 g/L |
| Vitamins solution | |
| Biotin | 5 mg/L |
| D-Calcium pantothenate 98% | 0.25 g/L |
| Nicotinic acid 99.5% | 1.2 g/L |
| Thiamine hydrochloride | 1 g/L |
| Trace elements | |
| Calcium chloride dihydrate | 0.5 g/L |
| Cobalt chloride hexahydrate | 0.4 g/L |
| Copper (II) sulfate pentahydrate | 0.05 g/L |
| Iron (III) chloride hexahydrate | 8 g/L |
| Manganese (II) sulfate monohydrate | 0.56 g/L |
| Nickel (II) chloride hexahydrate | 0.023 g/L |
| Zinc chloride ≥ 98% | 0.0695 g/L |
| Carbon source | |
| Glucose anhydrous | 4 g/L |
| Ultrapure double-distilled deionized water to | 1L |
| Gene Name | Gene Abbreviation | Primer Sequence 5′–3′ Forward and Reverse | Annealing Temperature °C | Expected Amplicon Size |
|---|---|---|---|---|
| Accessory gene regulator | agrA | CAAAGTTGCAGCGATGGATTT | 62 | 92 |
| AGCGTGTATGTGCAGTTTCT | ||||
| Alpha-hemolysin | hla | GGCTCTATGAAAGCAGCAGAT | 61 | 88 |
| CTGTAGCGAAGTCTGGTGAAA | ||||
| Beta-hemolysin | hlb | ATCCTTACCAAACACCTGTACTC | 60 | 269 |
| AGCACCACAACGTGAATCT | ||||
| Delta-hemolysin | hld | GGAGTGATTTCAATGGCACAAG | 60 | 83 |
| GTGAATTTGTTCACTGTGTCGATAA | ||||
| Clumping factor A | clfA | GAATCAGCTCCACAGAGTACAG | 62 | 106 |
| CAGCTACTGCCGCTAAACTAA | ||||
| Fibronectin binding protein B | fnbpB | AGCTCAACCAAGTAACGTCTC | 62 | 114 |
| ACATCTGTACCTGTCGCTTTAG | ||||
| Gyrase enzyme | gyr A | TCCCAACTGCTGGACTTATTT | 62 | 111 |
| CGCCTCCACGTTCTTCAATA | ||||
| Intercellular Adhesion molecule C | icaC | GCGTTAGCAAATGGAGACTATTG | 63 | 79 |
| GCGTGCAAATACCCAAGATAAC | ||||
| Intercellular Adhesion molecule D | icaD | AAGCCCAGACAGAGGGAATA | 62 | 85 |
| AGACACAAGATATAGCTAAGTGC | ||||
| Murein hydrolase activator A | cidA | GTACCGCTAACTTGGGTAGAAG | 62 | 109 |
| GCGTAATTTCGGAAGCAACAT | ||||
| Murein hydrolase activator B | cidB | ACGCAACGGTCGTATGTTTAG | 63 | 105 |
| TCAGCATGACGCCAGTTAATAC | ||||
| Sigma factor B | sigB | AAGGACAATCACATCACGAAGA | 62 | 101 |
| GGCTTCAAACTTCCGTTCAAA | ||||
| Zinc metalloprotease Aureolysin | aur1 | GGTCGCACATTCACAAGTTTATC | 62 | 84 |
| CGCCTGACTGGTCCTTATATTC | ||||
| 16s Ribosomal RNA | 16s | TGAGATGTTGGTTAAGTCCCGCA | 60 | 188 |
| CGGTTTCGCTGCCCTTTGTATTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhakim, Y.A.; Ali, A.E.; Hosny, A.E.-D.M.S.; Abdeltawab, N.F. Zinc Deprivation as a Promising Approach for Combating Methicillin-Resistant Staphylococcus aureus: A Pilot Study. Pathogens 2021, 10, 1228. https://doi.org/10.3390/pathogens10101228
Elhakim YA, Ali AE, Hosny AE-DMS, Abdeltawab NF. Zinc Deprivation as a Promising Approach for Combating Methicillin-Resistant Staphylococcus aureus: A Pilot Study. Pathogens. 2021; 10(10):1228. https://doi.org/10.3390/pathogens10101228
Chicago/Turabian StyleElhakim, Yomna A., Amal E. Ali, Alaa El-Dien M. S. Hosny, and Nourtan F. Abdeltawab. 2021. "Zinc Deprivation as a Promising Approach for Combating Methicillin-Resistant Staphylococcus aureus: A Pilot Study" Pathogens 10, no. 10: 1228. https://doi.org/10.3390/pathogens10101228
APA StyleElhakim, Y. A., Ali, A. E., Hosny, A. E.-D. M. S., & Abdeltawab, N. F. (2021). Zinc Deprivation as a Promising Approach for Combating Methicillin-Resistant Staphylococcus aureus: A Pilot Study. Pathogens, 10(10), 1228. https://doi.org/10.3390/pathogens10101228







