Natural Transformation as a Mechanism of Horizontal Gene Transfer in Aliarcobacter butzleri
Abstract
1. Introduction
2. Results
2.1. Screening for Aliarcobacter butzleri Isolates with Detectable Levels of Natural Transformation
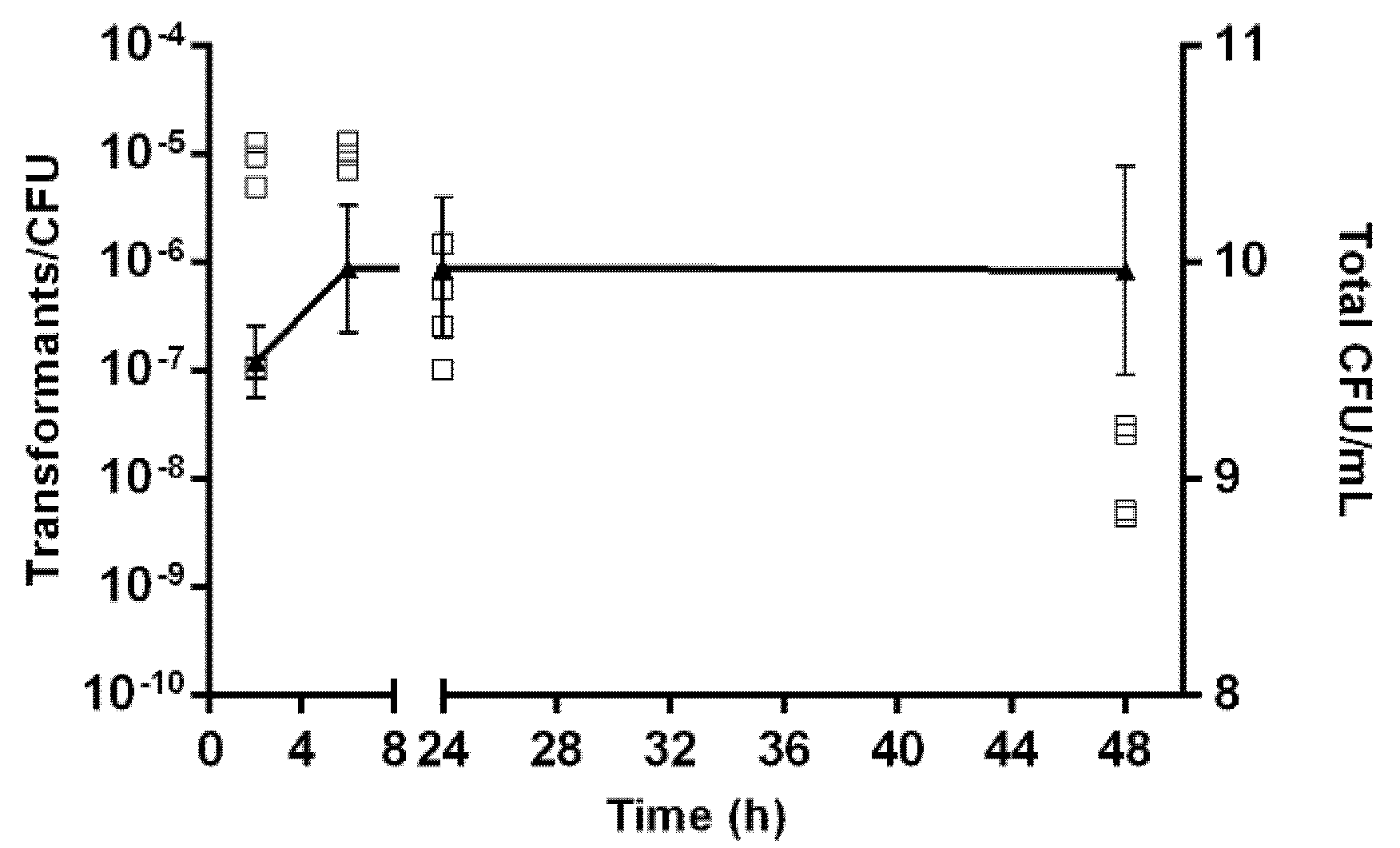
2.2. Effect of Growth Conditions in the Transformation Frequency of Aliarcobacter butzleri
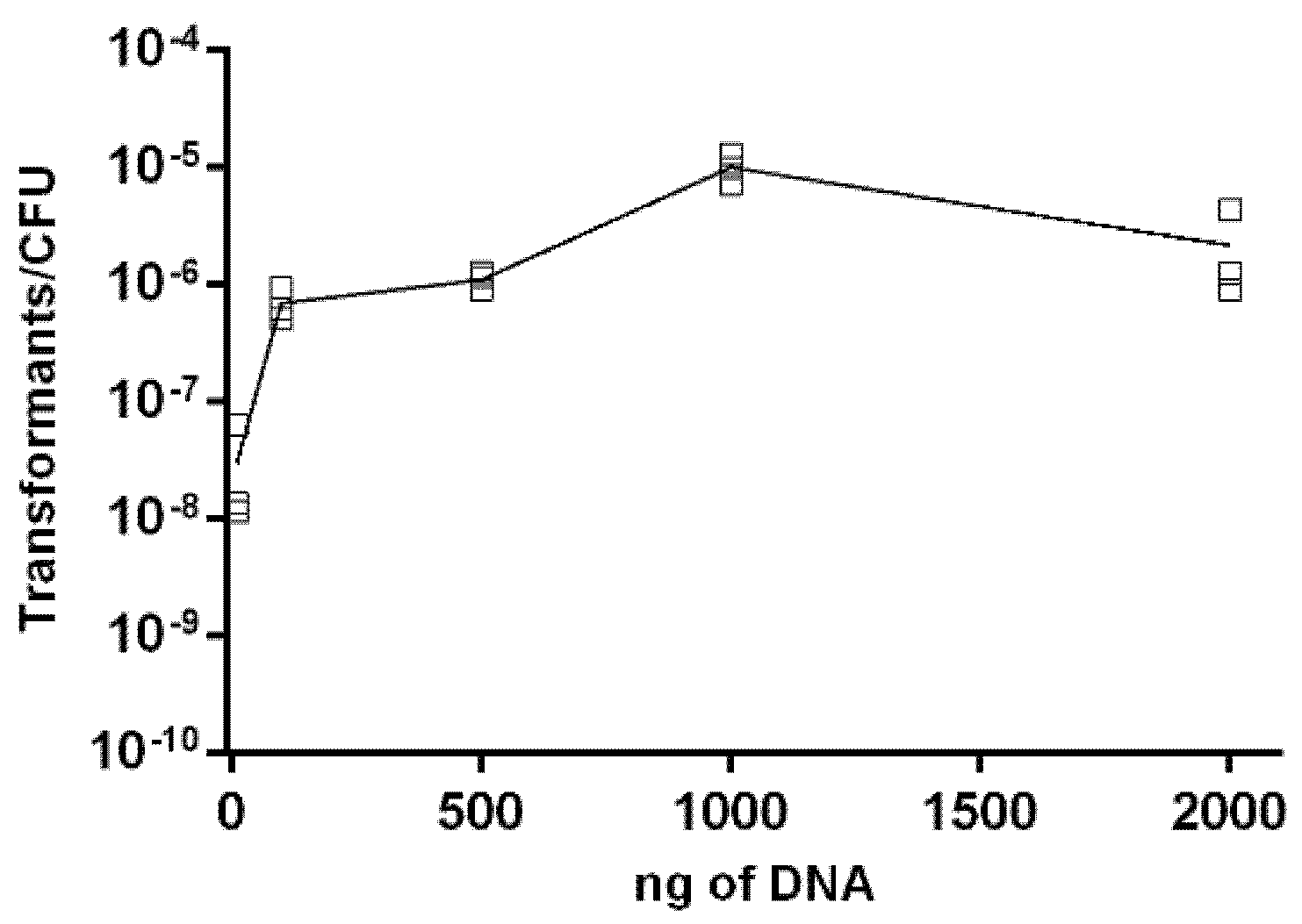
2.3. Effect of DNA Concentration on Transformation Frequency of Aliarcobacter butzleri
2.4. Transformation of Aliarcobacter butzleri with Different Donor DNA
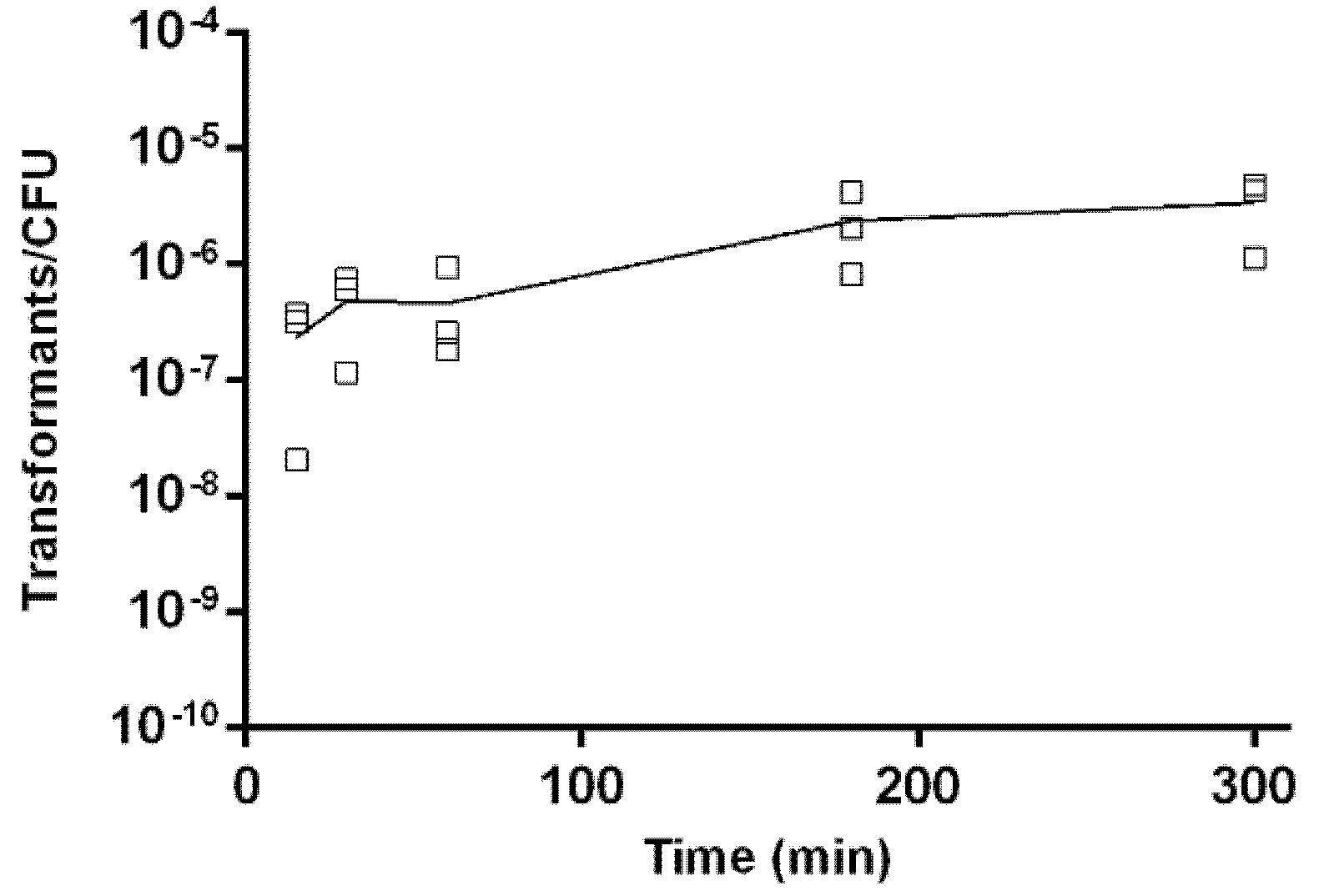
2.5. Kinetics of Natural Transformation
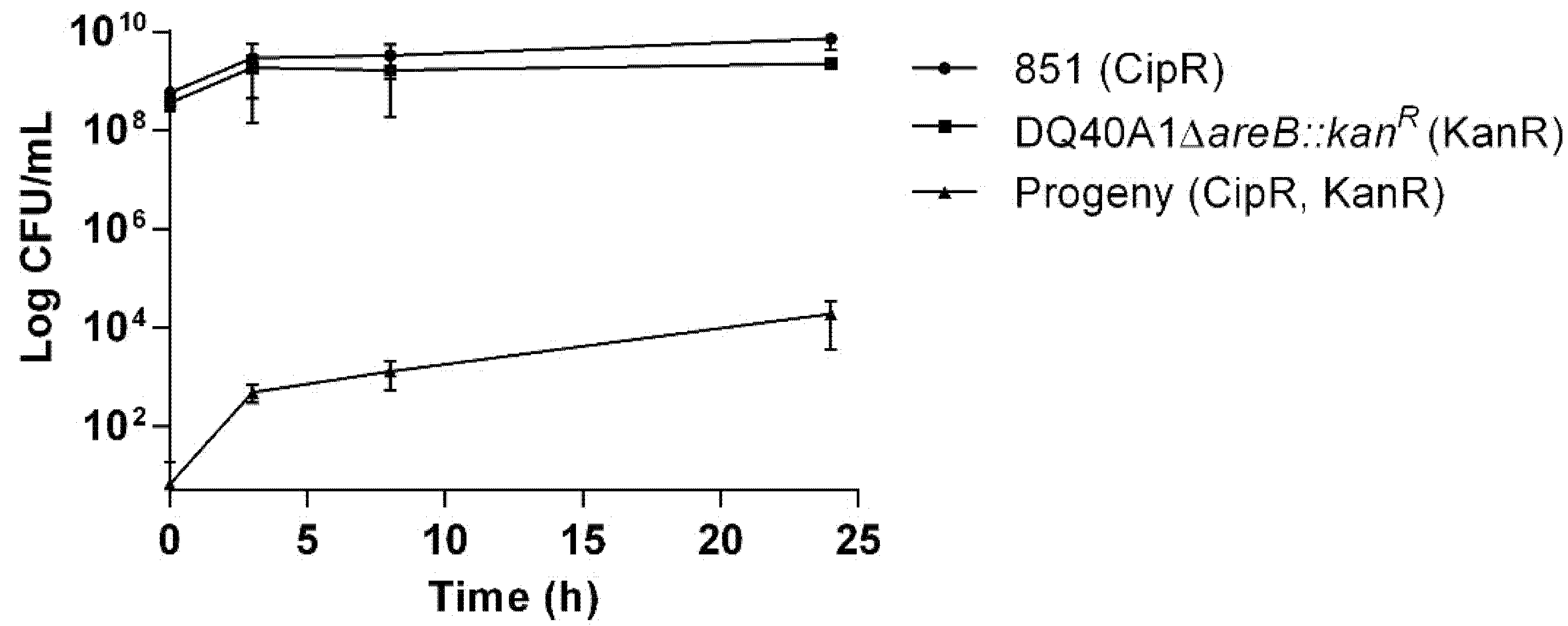
2.6. Transfer of Antibiotic Resistance Determinants in Aliarcobacter butzleri Co-Cultures
2.7. Identification of Putative Competence Genes in Aliarcobacter butzleri DQ40A1
| Campylobacter jejuni | Aliarcobacter butzleri RM4018 a | Aliarcobacter butzleri DQ40A1 (CDS) | ||||
|---|---|---|---|---|---|---|
| Category and Homolog (Locus ID) | Gene Product | Length (AA) | Homolog (Locus ID) | Homolog (Locus ID) | Length (AA) | Sequence ID (%) [DNA (AA)] b |
| DNA Uptake | ||||||
| ctsD (cj1474c) | Component of type II secretion/type IV pilus system (potential outer membrane pore) | 472 | ABU_RS09160 | GDI89_RS08280 | 478 | 46.8 (20.9) |
| ctsP (cj1473c) | Component of type II secretion/type IV pilus system (putative NTPases/NTP binding protein) | 202 | NA | NA | NA | NA |
| ctsX (cj1472c) | Component of type II secretion/type IV pilus system | 195 | NA | NA | NA | NA |
| ctsE (cj1471c) | Component of type II secretion/type IV pilus system (putative NTPases/NTP binding protein) | 519 | ABU_RS08225 | GDI89_RS05100 | 453 | 53.5 (36.1) |
| pilG c | Competence pseudopilus | 410 | ABU_RS08220 | GDI89_RS05105 | 396 | 38.4 (19.9) |
| ctsG (cj1343c) | Putative periplasmic protein | 171 | ABU_RS03485 | GDI89_RS04080 | 132 | 47.5 (28.1) |
| comEC (cj1211) | Membrane transporter protein involved in the transfer of DNA across the membrane | 419 | ABU_RS06285 | GDI89_RS02780 | 409 | 56.6 (36.2) |
| comE (cj0011c) | Periplasmic DNA-binding competence protein | 79 | ABU_RS11390 | GDI89_RS07505 | 123 | 43.7 (31.7) |
| priA c | Helicase | 729 | ABU_RS06960 | GDI89_RS02485 | 599 d | 36.6 (26.6) |
| DNA Processing | ||||||
| dprA (cj0634) | DNA processing protein | 257 | ABU_RS01180 | GDI89_RS06255 | 258 | 57.3 (41.3) |
| recA | DNA recombination protein | 343 | ABU_RS11180 | GDI89_RS02220 | 349 | 73.5 (73.4) |
| Others | ||||||
| ctsT (cj1077) | Putative periplasmic protein | 100 | NA | NA | NA | NA |
| ctsW (cj1028c) | Purine/pyrimidine phosphoribosyltransferase | 191 | ABU_RS02380 | GDI89_RS03920 | 190 | 59.2 (44.0) |
| ctsR (cj1475c) | Hypothetical protein | 105 | NA | NA | NA | NA |
| proC (cj1076) | Putative PCA reductase | 243 | ABU_RS02955 | NZ_CABVRU010000127.1 + NZ_CABVRU010000230.1 e | 254 | 53.1 (40.5) |
| ceuB (cj1352) | Enterochelin uptake permease | 322 | ABU_RS05530 | GDI89_RS07350 | 320 | 49.1 (25.4) |
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Genetic Material Preparation
4.3. Natural Transformation Using the Agar Transformation Method
4.4. Natural Transformation Using a Biphasic System
4.5. Effect of Growth Conditions
4.6. Kinetics of Natural Transformation
4.7. Influence of Genetic Material on Natural Transformation
4.8. Exchange of Genetic Material Between Bacterial Co-Cultures
4.9. Bioinformatics Analyses—Identification of Homologs Genes Presence/Absence
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandamme, P.; Falsen, E.; Rossau, R.; Hoste, B.; Segers, P.; Tytgat, R.; De Ley, J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: Emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 1991, 41, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cataluña, A.; Salas-Massó, N.; Diéguez, A.L.; Balboa, S.; Lema, A.; Romalde, J.L.; Figueras, M.J. Revisiting the taxonomy of the genus Arcobacter: Getting order from the chaos. Front. Microbiol. 2018, 9, 2077. [Google Scholar] [CrossRef]
- Waite, D.W.; Vanwonterghem, I.; Rinke, C.; Parks, D.H.; Zhang, Y.; Takai, K.; Sievert, S.M.; Simon, J.; Campbell, B.J.; Hanson, T.E.; et al. Comparative genomic analysis of the class Epsilonproteobacteria and proposed reclassification to Epsilonbacteraeota (phyl. nov.). Front. Microbiol. 2017, 8, 682. [Google Scholar] [CrossRef]
- Collado, L.; Figueras, M.J. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin. Microbiol. Rev. 2011, 24, 174–192. [Google Scholar] [CrossRef]
- Ferreira, S.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Insights in the pathogenesis and resistance of Arcobacter: A review. Crit. Rev. Microbiol. 2016, 42, 364–383. [Google Scholar]
- Van den Abeele, A.M.; Vogelaers, D.; Van Hende, J.; Houf, K. Prevalence of Arcobacter species among humans, Belgium, 2008–2013. Emerg. Infect. Dis. 2014, 20, 1731–1734. [Google Scholar] [CrossRef]
- Collado, L.; Gutiérrez, M.; González, M.; Fernández, H. Assessment of the prevalence and diversity of emergent campylobacteria in human stool samples using a combination of traditional and molecular methods. Diagn. Microbiol. Infect. Dis. 2013, 75, 434–436. [Google Scholar] [CrossRef]
- Ferreira, S.; Júlio, C.; Queiroz, J.A.; Domingues, F.C.; Oleastro, M. Molecular diagnosis of Arcobacter and Campylobacter in diarrhoeal samples among Portuguese patients. Diagn. Microbiol. Infect. Dis. 2014, 78, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, O.; Dediste, A.; Houf, K.; Ibekwem, S.; Souayah, H.; Cadranel, S.; Douat, N.; Zissis, G.; Butzler, J.-P.; Vandamme, P. Arcobacter species in humans. Emerg. Infect. Dis. 2004, 10, 1864–1867. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Parker, C.T.; Rubenfield, M.; Mendz, G.L.; Wösten, M.M.S.M.; Ussery, D.W.; Stolz, J.F.; Binnewies, T.T.; Hallin, P.F.; Wang, G.; et al. The complete genome sequence and analysis of the epsilonproteobacterium Arcobacter butzleri. PLoS ONE 2007, 2, e1358. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Chen, I.; Dubnau, D. DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2004, 2, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Seitz, P.; Blokesch, M. Cues and regulatory pathways involved in natural competence and transformation in pathogenic and environmental Gram-negative bacteria. FEMS Microbiol. Rev. 2013, 37, 336–363. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.G.; Wackernagel, W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 1994, 58, 563–602. [Google Scholar] [CrossRef]
- Dubnau, D. DNA uptake in bacteria. Annu. Rev. Microbiol. 1999, 53, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Blokesch, M. Natural competence for transformation. Curr. Biol. 2016, 26, R1126–R1130. [Google Scholar] [CrossRef] [PubMed]
- Golz, J.C.; Stingl, K. Natural competence and horizontal gene transfer in Campylobacter. In Current Topics in Microbiology and Immunology; Backert, S., Ed.; Springer International Publishing: Cham, Switzerland, 2021; Volume 431, pp. 265–292. ISBN 9783030654818. [Google Scholar]
- Vegge, C.S.; Brøndsted, L.; Ligowska-Marzeta, M.; Ingmer, H. Natural transformation of Campylobacter jejuni occurs beyond limits of growth. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Taylor, D.E. Natural transformation in Campylobacter species. J. Bacteriol. 1990, 172, 949–955. [Google Scholar] [CrossRef]
- Wilson, D.L.; Bell, J.A.; Young, V.B.; Wilder, S.R.; Mansfield, L.S.; Linz, J.E. Variation of the natural transformation frequency of Campylobacter jejuni in liquid shake culture. Microbiology 2003, 149, 3603–3615. [Google Scholar] [CrossRef][Green Version]
- Isidro, J.; Ferreira, S.; Pinto, M.; Domingues, F.; Oleastro, M.; Gomes, J.P.; Borges, V. Virulence and antibiotic resistance plasticity of Arcobacter butzleri: Insights on the genomic diversity of an emerging human pathogen. Infect. Genet. Evol. 2020, 80, 104213. [Google Scholar] [CrossRef]
- Duarte, A.; Santos, A.; Manageiro, V.; Martins, A.; Fraqueza, M.J.; Caniça, M.; Domingues, F.C.; Oleastro, M. Human, food and animal Campylobacter spp. isolated in Portugal: High genetic diversity and antibiotic resistance rates. Int. J. Antimicrob. Agents 2014, 44, 306–313. [Google Scholar] [CrossRef]
- Gilbreath, J.J.; Cody, W.L.; Merrell, D.S.; Hendrixson, D.R. Change is good: Variations in common biological mechanisms in the Epsilonproteobacterial genera Campylobacter and Helicobacter. Microbiol. Mol. Biol. Rev. 2011, 75, 84–132. [Google Scholar] [CrossRef]
- Beauchamp, J.M.; Erfurt, R.S.; Di Rita, V.J. Characterization and localization of the Campylobacter jejuni transformation system proteins CtsE, CtsP, and CtsX. J. Bacteriol. 2015, 197, 636–645. [Google Scholar] [CrossRef]
- Wiesner, R.S.; Hendrixson, D.R.; Di Rita, V.J. Natural transformation of Campylobacter jejuni requires components of a type II secretion system. J. Bacteriol. 2003, 185, 5408–5418. [Google Scholar] [CrossRef][Green Version]
- Jeon, B.; Muraoka, W.; Sahin, O.; Zhang, Q. Role of Cj1211 in natural transformation and transfer of antibiotic resistance determinants in Campylobacter jejuni. Antimicrob. Agents Chemother. 2008, 52, 2699–2708. [Google Scholar] [CrossRef]
- Jeon, B.; Zhang, Q. Cj0011c, a periplasmic single- and double-stranded DNA-binding protein, contributes to natural transformation in Campylobacter jejuni. J. Bacteriol. 2007, 189, 7399–7407. [Google Scholar] [CrossRef]
- Kline, K.A.; Seifert, H.S. Mutation of the priA gene of Neisseria gonorrhoeae affects DNA transformation and DNA repair. J. Bacteriol. 2005, 187, 5347–5355. [Google Scholar] [CrossRef]
- Karudapuram, S.; Zhao, X.; Barcak, G.J. DNA sequence and characterization of Haemophilus influenzae dprA+, a gene required for chromosomal but not plasmid DNA transformation. J. Bacteriol. 1995, 177, 3235–3240. [Google Scholar] [CrossRef][Green Version]
- Takata, T.; Ando, T.; Israel, D.A.; Wassenaar, T.M.; Blaser, M.J. Role of dprA in transformation of Campylobacter jejuni. FEMS Microbiol. Lett. 2005, 252, 161–168. [Google Scholar] [CrossRef]
- Ando, T.; Israel, D.A.; Kusugami, K.; Blaser, M.J. HP0333, a member of the dprA family, is involved in natural transformation in Helicobacter pylori. J. Bacteriol. 1999, 181, 5572–5580. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Longden, L.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Mell, J.C.; Redfield, R.J. Natural competence and the evolution of DNA uptake specificity. J. Bacteriol. 2014, 196, 1471–1483. [Google Scholar] [CrossRef]
- Kim, J.S.; Carver, D.K.; Kathariou, S. Natural transformation-mediated transfer of erythromycin resistance in Campylobacter coli strains from Turkeys and swine. Appl. Environ. Microbiol. 2006, 72, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Maughan, H.; Redfield, R.J. Extensive variation in natural competence in Haemophilus influenzae. Evolution 2009, 63, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, B.M.; Sinha, S.; Boyce, J.D.; Bojesen, A.M.; Mell, J.C.; Redfield, R.J. Natural transformation of Gallibacterium anatis. Appl. Environ. Microbiol. 2012, 78, 4914–4922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Goodman, S.D.; Redfield, R.J.; Chen, C. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J Bacteriol. 2002, 184, 3442–3449. [Google Scholar] [CrossRef] [PubMed]
- Gaasbeek, E.J.; Wagenaar, J.A.; Guilhabert, M.R.; Van Putten, J.P.M.; Parker, C.T.; Van Der Wal, F.J. Nucleases encoded by the integrated elements CJIE2 and CJIE4 inhibit natural transformation of Campylobacter jejuni. J. Bacteriol. 2010, 192, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, J.; Teschner, N.; Wackernagel, W. Highly different levels of natural transformation are associated with genomic subgroups within a local population of Pseudomonas stutzeri from soil. Appl. Environ. Microbiol. 2002, 68, 865–873. [Google Scholar] [CrossRef]
- Huddleston, J.R.; Brokaw, J.M.; Zak, J.C.; Jeter, R.M. Natural transformation as a mechanism of horizontal gene transfer among environmental Aeromonas species. Syst. Appl. Microbiol. 2013, 36, 224–234. [Google Scholar] [CrossRef]
- Israel, D.A.; Lou, A.S.; Blaser, M.J. Characteristics of Helicobacter pylori natural transformation. FEMS Microbiol. Lett. 2000, 188, 275–280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dai, K.; He, L.; Chang, Y.F.; Cao, S.; Zhao, Q.; Huang, X.; Wu, R.; Huang, Y.; Yan, Q.; Han, X.; et al. Basic characterization of natural transformation in a highly transformable Haemophilus parasuis strain SC1401. Front. Cell. Infect. Microbiol. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Liu, M.F.; Zhang, L.; Huang, L.; Biville, F.; Zhu, D.K.; Wang, M.S.; Jia, R.Y.; Chen, S.; Sun, K.F.; Yang, Q.; et al. Use of natural transformation to establish an easy knockout method in Riemerella anatipestifer. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Vesel, N.; Blokesch, M. Pilus production in Acinetobacter baumannii is growth phase dependent and essential for natural transformation. J. Bacteriol. 2021, 203. [Google Scholar] [CrossRef]
- De Vries, J.; Meier, P.; Wackernagel, W. The natural transformation of the soil bacteria Pseudomonas stutzeri and Acinetobacter sp. by transgenic plant DNA strictly depends on homologous sequences in the recipient cells. FEMS Microbiol. Ecol. 2001, 195, 211–215. [Google Scholar] [CrossRef]
- Palmen, R.; Buijsman, P.; Hellingwerf, K.J. Physiological regulation of competence induction for natural transformation in Acinetobacter calcoaceticus. Arch. Microbiol. 1994, 162, 344–351. [Google Scholar] [CrossRef]
- Van Hoek, A.H.A.M.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J.M. Acquired antibiotic resistance genes: An overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Martin, B.; Fichant, G.; Polard, P.; Claverys, J.P. Bacterial transformation: Distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 2014, 12, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Silva, A.L.; Tomás, J.; Mateus, C.; Domingues, F.; Oleastro, M. Characterization of AreABC, an RND-type efflux system involved in antimicrobial resistance of Aliarcobacter butzleri. Antimicrob. Agents Chemother. 2021. accepted for publication. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Hwang, C.Y.; Cho, B.C. Arcobacter marinus sp. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 531–536. [Google Scholar] [CrossRef]
- Abdelbaqi, K.; Ménard, A.; Prouzet-Mauleon, V.; Bringaud, F.; Lehours, P.; Mégraud, F. Nucleotide sequence of the gyrA gene of Arcobacter species and characterization of human ciprofloxacin-resistant clinical isolates. FEMS Immunol. Med. Microbiol. 2007, 49, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.L.; Lo Cascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]

| Recipient A. butzleri Strain | Origin/Year of Isolation | ST a | Resistance Profile a | Agar Transformation Method | Biphasic System Method |
|---|---|---|---|---|---|
| 1426_2003 | Diarrheic human stool/2003 | 47 | NAL, CTA b | + + - | - - - |
| Ab_1711 | Poultry slaughterhouse equipment surface/2011 | ST_new1 | LEV, CIP, NAL, CTA | - - - | - - - |
| Ab_2211 | Slaughterhouse surface/2011 | 460 | AMP, NAL, CTA | - - - | - - - |
| Ab_2811 | Poultry carcass neck skin/2011 | 107 | AMP, ERY, LEV, CIP, NAL, CTA | - + + | + - - |
| Ab_4211 | Poultry carcass drippings/2011 | ST_new2 | AMP, LEV, CIP, NAL, CTA | + + + | - + + |
| Ab_4511 | Poultry carcass drippings/2011 | 510 | AMP, NAL, CTA | + + - | + + + |
| CR424 | Poultry meat/2015 | ST_new3 | AMP, ERY, NAL, CTA | - - - | - - - |
| CR502 | Poultry meat/2015 | ST_new4 | ERY, LEV, CIP, NAL, CTA | - - - | - - - |
| CR604 | Beef meat/2015 | ST_new5 | NAL, CTA | + + + | + + + |
| CR641 | Poultry meat/2015 | 108 | ERY, NAL, CTA | + - - | - - - |
| CR891 | Poultry meat/2016 | 94 | NAL, CTA | - - - | - - - |
| CR892 | Poultry meat/2016 | ST_new6 | AMP, LEV, CIP, NAL, CTA | + + + | - + - |
| CR1132 | Ready-to-eat vegetables/2016 | ST_new7 | NAL, CTA | + + + | + + + |
| CR1143 | Poultry meat/2016 | ST_new8 | AMP, LEV, CIP, NAL, CTA | + + + | + - + |
| DQ20dA1 | Goat milk/2015 | ST_new9 | AMP, LEV, CIP, NAL, CTA | - - - | - - - |
| DQ31A1 | Sheep milk/2015 | 172 | AMP, NAL, CTA | + + + | - - - |
| DQ40A1 | Dairy plant equipment surface/2015 | ST_new10 | NAL, CTA | + + + | + + + |
| Donor DNA (Nature of DNA/Strain) | Resistance Marker | Length of the PCR Fragment | Transformants/CFU |
|---|---|---|---|
| gDNA/Campylobacter coli 873 | aphA-3 | - | NT |
| gyrA PCR fragment/Ab_2811 strain | C254T in gyrA gene | 344 bp | (6.70 ± 2.72) × 10−9 |
| gyrA PCR fragment/Ab_2811 strain | C254T in gyrA gene | 1410 pb | (7.85 ± 1.13) × 10−8 |
| gDNA/CR1132ΔareB::kanR | aphA-3 (KanR cassette from pUC18-K2) | - | (2.59 ± 0.51) × 10−7 |
| PCR fragment/of DQ40A1ΔareB::kanR | aphA-3 (KanR cassette from pUC18-K2), flanked by upstream and downstream regions of about 400 bp | 1638 bp | (3.96 ± 3.54) × 10−7 |
| gDNA/Ab_2811 strain | C254T in gyrA gene | - | (6.92 ± 7.67) × 10−7 |
| gDNA/DQ40A1ΔareB::kanR | aphA-3 (KanR cassette from pUC18-K2) | - | (7.65 ± 2.25) × 10−6 |
| Primers | Sequence | Size of Amplified Fragment (bp) | Reference |
|---|---|---|---|
| gyrA_Fw | TGGACGTGCATTACCAGATG | 1410 | [50] |
| gyrA_Rv | GCAACTTTTCCTTTTCCACCT | ||
| F-QRDR | TGGATTAAAGCCAGTTCATAGAAG | 344 | [51] |
| R2-QRDR | TCATMGWATCATCATAATTTGGWAC | ||
| areB_A1 | TTGAAATAAGGGCTACTTACTCAGG | 1638 | [49] |
| areB_B2 | GTTCGCTCTGGCTTGCAAAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonifácio, M.; Mateus, C.; Alves, A.R.; Maldonado, E.; Duarte, A.P.; Domingues, F.; Oleastro, M.; Ferreira, S. Natural Transformation as a Mechanism of Horizontal Gene Transfer in Aliarcobacter butzleri. Pathogens 2021, 10, 909. https://doi.org/10.3390/pathogens10070909
Bonifácio M, Mateus C, Alves AR, Maldonado E, Duarte AP, Domingues F, Oleastro M, Ferreira S. Natural Transformation as a Mechanism of Horizontal Gene Transfer in Aliarcobacter butzleri. Pathogens. 2021; 10(7):909. https://doi.org/10.3390/pathogens10070909
Chicago/Turabian StyleBonifácio, Marina, Cristiana Mateus, Ana R. Alves, Emanuel Maldonado, Ana P. Duarte, Fernanda Domingues, Mónica Oleastro, and Susana Ferreira. 2021. "Natural Transformation as a Mechanism of Horizontal Gene Transfer in Aliarcobacter butzleri" Pathogens 10, no. 7: 909. https://doi.org/10.3390/pathogens10070909
APA StyleBonifácio, M., Mateus, C., Alves, A. R., Maldonado, E., Duarte, A. P., Domingues, F., Oleastro, M., & Ferreira, S. (2021). Natural Transformation as a Mechanism of Horizontal Gene Transfer in Aliarcobacter butzleri. Pathogens, 10(7), 909. https://doi.org/10.3390/pathogens10070909










