Environmental Pollutants, Mucosal Barriers, and Pathogen Susceptibility; The Case for Aflatoxin B1 as a Risk Factor for HIV Transmission and Pathogenesis
Abstract
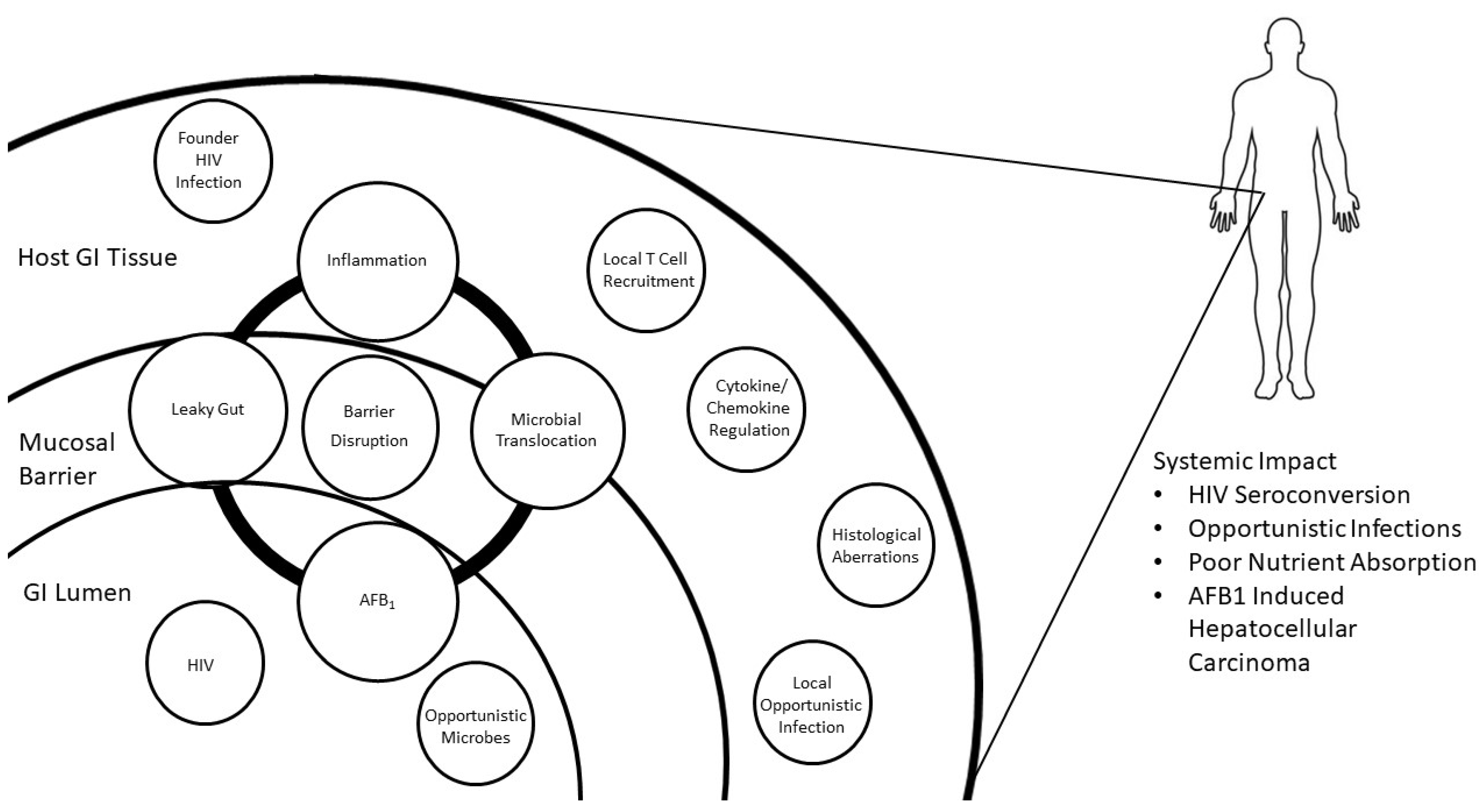
:1. Introduction
2. Effects of AFB1 Exposure on HIV Susceptibility
2.1. HIV Transmission and AFB1 Localization in Distinct Anatomic Tissues
2.1.1. Cervicovaginal HIV Transmission and Localization of AFB1
2.1.2. Vaginal Inflammation and HIV Transmission
2.1.3. STI and HIV
2.1.4. Colorectal HIV Transmission
2.1.5. Colorectal Localization of AFB1 and Pathology
2.1.6. HIV and GI Barrier Function and Immunity
2.2. AFB1 Exposure and HIV Viral Load
2.2.1. HIV Copy Number and Transmission Risk
2.2.2. AFB1 Exposure and HIV Copy Number
2.3. HIV Progression and Inflammation
2.3.1. Inflammation and HIV Replication/Progression
2.3.2. Cytokines Associated with HIV
3. AFB1 and HIV Co-Exposure Research Design Considerations
3.1. Exposure Design
3.2. Accurate Immunological Data Generation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, P.; Borkowf, C.B.; Brooks, J.T.; Lasry, A.; Lansky, A.; Mermin, J. Estimating per-act HIV transmission risk: A systematic review. AIDS 2014, 28, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Maher, J.E.; Peterman, T.A.; Branson, B.M.; Steketee, R.W. Reducing the risk of sexual HIV transmission: Quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex. Transm. Dis. 2002, 29, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Burgener, A.; Mcgowan, I.; Klatt, N.R. HIV and mucosal barrier interactions: Consequences for transmission and pathogenesis. Curr. Opin. Immunol. 2015, 36, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.M.; Cárcamo-Oyarce, G.; Turner, B.S.; Dellos-Nolan, S.; Co, J.Y.; Lehoux, S.; Cummings, R.D.; Wozniak, D.J.; Ribbeck, K. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol. 2019, 4, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jolly, P.E.; Preko, P.; Wang, J.S.; Ellis, W.O.; Phillips, T.D.; Williams, J.H. Aflatoxin-related immune dysfunction in health and in human immunodeficiency virus disease. Clin. Dev. Immunol. 2008, 2008, e790309. [Google Scholar] [CrossRef]
- AVERT HIV and AIDS in East and Southern Africa regional overview. Available online: https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/overview (accessed on 24 September 2019).
- Simbayi, L.C.; Shisana, O.; Rehle, T.; Onoya, D.; Jooste, S.; Zungu, N.; Zuma, K. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012; Pretoria, South Africa, 2014; Available online: https://repository.hsrc.ac.za/handle/20.500.11910/2490 (accessed on 23 September 2021).
- Boily, M.C.; Baggaley, R.F.; Wang, L.; Masse, B.; White, R.G.; Hayes, R.J.; Alary, M. Heterosexual risk of HIV-1 infection per sexual act: Systematic review and meta-analysis of observational studies. Lancet Infect. Dis. 2009, 9, 118–129. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. IARC monographs on the evaluation of carcinogenic risks to humans. In Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; International Agency for Research on Cancer: Lyon, France, 1992; Volume 82. [Google Scholar]
- Liu, Y.; Wu, F. Global burden of Aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Jolly, P.E.; Ellis, W.O.; Wang, J.-S.; Phillips, T.D.; Williams, J.H. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int. Immunol. 2005, 17, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Su, J.; Liu, Z.; Liu, D.; Gan, F.; Chen, X.; Huang, K. Aflatoxin B1 promotes influenza replication and increases virus related lung damage via activation of tlr4 signaling. Front. Immunol. 2018, 9, 2297. [Google Scholar] [CrossRef] [Green Version]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-Year Odyssey of Mechanistic and Translational Toxicology. Toxicol. Sci. 2011, 120, S28–S48. [Google Scholar] [CrossRef] [Green Version]
- Zhiang, J.; Omenge, E.; Maina, T.; Muthoka, K.; Kiptoo, S.; Groopman, J.; Tong, Y.; Ermel, A.; Loehrer, P.J.; Brown, D. Association of detection of aflatoxin in plasma of Kenyan women with increased detection of oncogenic HPV. J. Clin. Oncol. 2019, 37, 5530. [Google Scholar] [CrossRef]
- Jolly, P.E.; Akinyemiju, T.F.; Jha, M.; Aban, I.; Gonzalez-Falero, A.; Joseph, D. Temporal variation and association of aflatoxin B1 albumin-adduct levels with socio-economic and food consumption factors in HIV positive adults. Toxins 2015, 7, 5129–5140. [Google Scholar] [CrossRef] [Green Version]
- Jolly, P.E.; Inusah, S.; Lu, B.; Ellis, W.O.; Nyarko, A.; Phillips, T.D.; Williams, J.H. Association between high aflatoxin B1 levels and high viral load in HIV-positive people. World Mycotoxin J. 2013, 6, 255–261. [Google Scholar] [CrossRef]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef] [Green Version]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application—A review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO Expert Committee on Food Additives Evaluation of Certain Food Additives. Fifty-Ninth Report of the Joint FAO/WHO Expert Committee on Food Additives.; World Health Organization: Geneva, Switzerland, 2002; Volume 913, ISBN 9241210028. [Google Scholar]
- Guengerich, F.P.; Johnson, W.W.; Shimada, T.; Ueng, Y.-F.; Yamazaki, H.; Langouët, S. Activation and detoxication of aflatoxin B1. Mutat. Res. Mol. Mech. Mutagen. 1998, 402, 121–128. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- Shuaib, F.M.B.; Ehiri, J.; Abdullahi, A.; Williams, J.H.; Jolly, P.E. Reproductive health effects of aflatoxins: A review of the literature. Reprod. Toxicol. 2010, 29, 262–270. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, S. Intestinal absorption and excretion of aflatoxin in rats. Toxicol. Appl. Pharmacol. 1989, 97, 88–97. [Google Scholar] [CrossRef]
- Turner, P.C.; Moore, S.E.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Modification of Immune Function through Exposure to Dietary Aflatoxin in Gambian Children. Environ. Health Perspect. 2003, 111, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Mehrzad, J.; Bahari, A.; Bassami, M.R.; Mahmoudi, M.; Dehghani, H. Immunobiologically relevant level of aflatoxin B1 alters transcription of key functional immune genes, phagocytosis and survival of human dendritic cells. Immunol. Lett. 2018, 197, 44–52. [Google Scholar] [CrossRef]
- Dogi, C.; Cristofolini, A.; Pereyra, M.L.G.; García, G.; Fochesato, A.; Merkis, C.; Dalcero, A.M.; Cavaglieri, L.R. Aflatoxins and Saccharomyces cerevisiae: Yeast modulates the intestinal effect of aflatoxins, while aflatoxin B1 influences yeast ultrastructure. World Mycotoxin J. 2017, 10, 171–181. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Chen, J.; Xiao, A. Response of intestinal bacterial flora to the long-term feeding of aflatoxin B1 (AFB1) in mice. Toxins 2017, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef]
- Pudney, J.; Quayle, A.J.; Anderson, D.J. Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 2005, 73, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Haase, A.T. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010, 464, 217–223. [Google Scholar] [CrossRef]
- Harrison, J.C.; Carvajal, M.; Garner, R.C. Does aflatoxin exposure in the United Kingdom constitute a cancer risk? Environ. Health Perspect. 1993, 99, 99–105. [Google Scholar] [CrossRef] [PubMed]
- To, E.E.; Hendrix, C.W.; Bumpus, N.N. Dissimilarities in the metabolism of antiretroviral drugs used in HIV pre-exposure prophylaxis in colon and vagina tissues. Biochem. Pharmacol. 2013, 86, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, M.A.; Vadlamuri, V.; Ghosh, S.; Glover, D.D. Expression and cyclic variability of CYP3A4 and CYP3A7 isoforms in human endometrium and cervix during the menstrual cycle. Drug Metab. Dispos. 2003, 31, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, A.; Moschini, M.; Masoero, F. Aflatoxins absorption in the gastro-intestinal tract and in the vaginal mucosa in lactating dairy cows. Ital. J. Anim. Sci. 2008, 7, 53–63. [Google Scholar] [CrossRef]
- Ibeh, I.N.; Uraih, N.; Ogonar, J.I. Dietary exposure to aflatoxin in human male infertility in Benin City, Nigeria. Int. J. Fertil. Menopausal Stud. 1994, 39, 208–214. [Google Scholar]
- Shen, R.; Richter, H.E.; Smith, P.D. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am. J. Reprod. Immunol. 2011, 65, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Blish, C.A.; McClelland, R.S.; Richardson, B.A.; Jaoko, W.; Mandaliya, K.; Baeten, J.M.; Overbaugh, J. Genital inflammation predicts HIV-1 shedding independent of plasma viral load and systemic inflammation. J. Acquir. Immune Defic. Syndr. 2012, 61, 436–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, L.; Passmore, J.A.S.; Liebenberg, L.J.; Werner, L.; Baxter, C.; Arnold, K.B.; Williamson, C.; Little, F.; Mansoor, L.E.; Naranbhai, V.; et al. Genital Inflammation and the Risk of HIV Acquisition in Women. Clin. Infect. Dis. 2015, 61, 260–269. [Google Scholar] [CrossRef]
- Arnold, K.B.; Burgener, A.; Birse, K.; Romas, L.; Dunphy, L.J.; Shahabi, K.; Abou, M.; Westmacott, G.R.; McCorrister, S.; Kwatampora, J.; et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2016, 9, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Maurya, B.K.; Trigun, S.K. Activation of oxidative stress and inflammatory factors could account for histopathological progression of aflatoxin-B1 induced hepatocarcinogenesis in rat. Mol. Cell. Biochem. 2015, 401, 185–196. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mehrzad, J.; Mahmoudi, M.; Schneider, M. Environmentally relevant level of aflatoxin B1 dysregulates human dendritic cells through signaling on key toll-like receptors. Int. J. Toxicol. 2014, 33, 175–186. [Google Scholar] [CrossRef]
- Watanabe, D.; Uehira, T.; Yonemoto, H.; Bando, H.; Ogawa, Y.; Yajima, K.; Taniguchi, T.; Kasai, D.; Nishida, Y.; Shirasaka, T. Sustained high levels of serum interferon-γ during HIV-1 Infection: A specific trend different from other cytokines. Viral Immunol. 2010, 23, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Meissonnier, G.M.; Pinton, P.; Laffitte, J.; Cossalter, A.-M.; Gong, Y.Y.; Wild, C.P.; Bertin, G.; Galtier, P.; Oswald, I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Peng, X.; Fang, J.; Cui, H.; Yu, Z.; Chen, Z. Effects of Aflatoxin B1 on T-Cell Subsets and mRNA Expression of Cytokines in the Intestine of Broilers. Int. J. Mol. Sci. 2015, 16, 6945–6959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, T.W.; Engel, D.; Mizell, S.B.; Ehler, L.A.; Fauci, A.S. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J. Exp. Med. 1998, 188, 83–91. [Google Scholar] [CrossRef] [Green Version]
- MG, T.; AG, L.; DT, M.; SN, C.; HR, R. Immunobiological effects of AFB1 and AFB1-FB1 mixture in experimental subchronic mycotoxicoses in rats. Toxicology 2003, 186, 159–170. [Google Scholar] [CrossRef]
- Nazli, A.; Chan, O.; Dobson-Belaire, W.N.; Ouellet, M.; Tremblay, M.J.; Gray-Owen, S.D.; Arsenault, A.L.; Kaushic, C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010, 6, e1000852. [Google Scholar] [CrossRef]
- Vandergeeten, C.; Fromentin, R.; Chomont, N. The role of cytokines in the establishment, persistence and eradication of the HIV reservoir. Cytokine Growth Factor Rev. 2012, 23, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korin, Y.D.; Zack, J.A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 1998, 72, 3161–3168. [Google Scholar] [CrossRef] [Green Version]
- Hirbod, T.; Kong, X.; Kigozi, G.; Ndyanabo, A.; Serwadda, D.; Prodger, J.L.; Tobian, A.A.; Nalugoda, F.; Wawer, M.J.; Shahabi, K.; et al. HIV Acquisition is associated with increased antimicrobial peptides and reduced HIV neutralizing IgA in the foreskin prepuce of uncircumcised men. PLoS Pathog. 2014, 10, e1004416. [Google Scholar] [CrossRef] [Green Version]
- Levinson, P.; Kaul, R.; Kimani, J.; Ngugi, E.; Moses, S.; MacDonald, K.S.; Broliden, K.; Hirbod, T.; Kibera HIV Study Group. Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009, 23, 309–317. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, S.; Chen, J.; Peng, X.; Wang, F.; Cui, H.; Fang, J. Effects of sodium selenite on the decreased percentage of T cell subsets, contents of serum IL-2 and IFN-γ induced by aflatoxin B1 in broilers. Res. Vet. Sci. 2013, 95, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Luongo, D.; Russo, R.; Balestrieri, A.; Marzocco, S.; Bergamo, P.; Severino, L. In vitro study of AFB1 and AFM1 effects on human lymphoblastoid Jurkat T-cell model. J. Immunotoxicol. 2014, 11, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aujla, S.J.; Dubin, P.J.; Kolls, J.K. Th17 cells and mucosal host defense. Semin. Immunol. 2007, 19, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, A.T.; Hirooka, E.Y.; Alvares e Silva, P.L.; Bracarense, A.P.F.R.L.; Flaiban, K.K.M.; Akagi, C.Y.; Kawamura, O.; Costa, M.C.D.; Itano, E.N. Impact of a Single Oral Acute Dose of Aflatoxin B1 on Liver Function/Cytokines and the Lymphoproliferative Response in C57Bl/6 Mice. Toxins 2017, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Stylianou, E.; Bjerkeli, V.; Yndestad, A.; Heggelund, L.; Wæhre, T.; Damås, J.K.; Aukrust, P.; Frøland, S.S. Raised serum levels of interleukin-18 is associated with disease progression and may contribute to virological treatment failure in HIV-1-infected patients. Clin. Exp. Immunol. 2003, 132, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.E.S.; Allithy, A.N.A.; Abdellatif, N.A.; Anani, M.; Fareed, S.A.; El-Shafei, D.A.; El-Din, E.A.A. Amelioration of pulmonary aflatoxicosis by green tea extract: An in vivo study. Toxicon 2021, 189, 48–55. [Google Scholar] [CrossRef]
- UNAIDS UNAIDS Data. Available online: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf (accessed on 23 September 2021).
- Harrison, A.; Colvin, C.J.; Kuo, C.; Swartz, A.; Lurie, M. Sustained high HIV incidence in young women in southern Africa: Social, behavioral, and structural factors and emerging intervention approaches. Curr. HIV/AIDS Rep. 2015, 12, 207–215. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, L.R.; Karim, Q.A. Factors Driving the HIV Epidemic in Southern Africa. Curr. HIV/AIDS Rep. 2016, 13, 158–169. [Google Scholar] [CrossRef]
- Ward, H.; Rönn, M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr. Opin. HIV AIDS 2010, 5, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Harney, B.L.; Agius, P.A.; El-Hayek, C.; Fairley, C.K.; Chow, E.P.F.; Roth, N.; Tee, B.K.; Leslie, D.; Tachedjian, G.; Hellard, M.; et al. Risk of Subsequent HIV Infection Following Sexually Transmissible Infections among Men Who Have Sex With Men. Open Forum Infect. Dis. 2019, 6, 376. [Google Scholar] [CrossRef]
- Masese, L.; Baeten, J.M.; Richardson, B.A.; Bukusi, E.; John-Stewart, G.; Graham, S.M.; Shafi, J.; Kiarie, J.; Overbaugh, J.; Mcclelland, R.S. Changes in the contribution of genital tract infections to HIV acquisition among Kenyan high-risk women from 1993 to 2012. AIDS 2015, 29, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hladik, F.; Woodward, A.; Klock, A.; Peng, T.; Johnston, C.; Remington, M.; Magaret, A.; Koelle, D.M.; Wald, A.; et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat. Med. 2009, 15, 886–892. [Google Scholar] [CrossRef] [Green Version]
- Abdool Karim, S.S.; Baxter, C.; Passmore, J.A.S.; McKinnon, L.R.; Williams, B.L. The genital tract and rectal microbiomes: Their role in HIV susceptibility and prevention in women. J. Int. AIDS Soc. 2019, 22, e25300. [Google Scholar] [CrossRef] [Green Version]
- McClelland, R.S.; Lingappa, J.R.; Srinivasan, S.; Kinuthia, J.; John-Stewart, G.C.; Jaoko, W.; Richardson, B.A.; Yuhas, K.; Fiedler, T.L.; Mandaliya, K.N.; et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: A nested case-control study. Lancet Infect. Dis. 2018, 18, 554–564. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef] [Green Version]
- Kloos, H.; Mariam, D.H. Some neglected and emerging factors in HIV transmission in Ethiopia. Ethiop. Med. J. 2007, 45, 103–107. [Google Scholar]
- Halperin, D.T. Heterosexual anal intercourse: Prevalence, cultural factors, and HIV infection and other health risks, part I. AIDS Patient Care STDS 1999, 13, 717–730. [Google Scholar] [CrossRef]
- Baggaley, R.F.; White, R.G.; Boily, M.-C. HIV transmission risk through anal intercourse: Systematic review, meta-analysis and implications for HIV prevention. Int. J. Epidemiol. 2010, 39, 1048–1063. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, A.; DiNenno, E.; Honeycutt, A.; Allaire, B.; Neuwahl, S.; Hicks, K.; Sansom, S. Contribution of anal sex to HIV prevalence among heterosexuals: A modeling analysis. AIDS Behav. 2017, 21, 2895–2903. [Google Scholar] [CrossRef]
- Van Damme, L.; Ramjee, G.; Alary, M.; Vuylsteke, B.; Chandeying, V.; Rees, H.; Sirivongrangson, P.; Mukenge-Tshibaka, L.; Ettiègne-Traoré, V.; Uaheowitchai, C.; et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet 2002, 360, 971–977. [Google Scholar] [CrossRef]
- Bergheim, I.; Bode, C.; Parlesak, A. Distribution of cytochrome P450 2C, 2E1, 3A4, and 3A5 in human colon mucosa. BMC Clin. Pharmacol. 2005, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Dey, D.K.; Kang, S.C. Aflatoxin B1 induces reactive oxygen species-dependent caspase-mediated apoptosis in normal human cells, inhibits Allium cepa root cell division, and triggers inflammatory response in zebrafish larvae. Sci. Total Environ. 2020, 737, 139704. [Google Scholar] [CrossRef]
- Marnett, L.J. Prostaglandin synthase-mediated metabolism of carcinogens and a potential role for peroxyl radicals as reactive intermediates. Environ. Health Perspect. 1990, 88, 5–12. [Google Scholar] [CrossRef]
- Marin, D.E.; Taranu, I. Overview on aflatoxins and oxidative stress. Toxin Rev. 2012, 31, 32–43. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; Angel, J.B. Human immunodeficiency virus and the gastrointestinal immune system: Does highly active antiretroviral therapy restore gut immunity. Mucosal Immunol. 2012, 5, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Akinrinmade, F.J.; Akinrinde, A.S.; Amid, A. Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: Modulatory roles of melatonin and flavonoid-rich fractions from Chromolena odorata. Mycotoxin Res. 2016, 32, 53–60. [Google Scholar] [CrossRef]
- Akinrinde, A.; Ogunbunmi, T.; Akinrinmade, F. Acute aflatoxin B 1-induced gastro-duodenal and hepatic oxidative damage is preceded by time-dependent hyperlactatemia in rats. Mycotoxin Res. 2020, 36, 443–452. [Google Scholar] [CrossRef]
- Robert, H.; Payros, D.; Pinton, P.; Théodorou, V.; Mercier-Bonin, M.; Oswald, I.P. Impact of mycotoxins on the intestine: Are mucus and microbiota new targets? J. Toxicol. Environ. Health Part B 2017, 20, 249–275. [Google Scholar] [CrossRef]
- Voth-Gaeddert, L.E.; Torres, O.; Maldonado, J.; Krajmalnik-Brown, R.; Rittmann, B.E.; Oerther, D.B. Aflatoxin Exposure, Child Stunting, and Dysbiosis in the Intestinal Microbiome Among Children in Guatemala. Environ. Eng. Sci. 2019, 36, 958–968. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Glenn, T.C.; Wang, J.-S. Aflatoxin B1 Induced Compositional Changes in Gut Microbial Communities of Male F344 Rats. Toxicol. Sci. 2016, 150, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Reed, K.M.; Mendoza, K.M.; Coulombe, R.A. Differential Transcriptome Responses to Aflatoxin B1 in the Cecal Tonsil of Susceptible and Resistant Turkeys. Toxins 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful Effects and Control Strategies of Aflatoxin B1 Produced by Aspergillus flavus and Aspergillus parasiticus Strains on Poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratz, S.; Mykkänen, H.; Ouwehand, A.C.; Juvonen, R.; Salminen, S.; El-Nezami, H. Intestinal mucus alters the ability of probiotic bacteria to bind aflatoxin B1 in vitro. Appl. Environ. Microbiol. 2004, 70, 6306–6308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatt, N.R.; Chomont, N.; Douek, D.C.; Deeks, S.G. Immune activation and HIV persistence: Implications for curative approaches to HIV infection. Immunol. Rev. 2013, 254, 326–342. [Google Scholar] [CrossRef] [Green Version]
- Lederman, M.M.; Funderburg, N.T.; Sekaly, R.P.; Klatt, N.R.; Hunt, P.W. Residual immune dysregulation syndrome in treated HIV infection. Adv. Immunol. 2013, 119, 51–83. [Google Scholar] [CrossRef] [Green Version]
- Mehandru, S.; Poles, M.A.; Tenner-Racz, K.; Horowitz, A.; Hurley, A.; Hogan, C.; Boden, D.; Racz, P.; Markowitz, M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004, 200, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; MacE, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkurst, C.N.; Muratet, M.; et al. A validated regulatory network for Th17 cell specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014, 10, e1004543. [Google Scholar] [CrossRef] [Green Version]
- Somsouk, M.; Estes, J.D.; Deleage, C.; Dunham, R.M.; Albright, R.; Inadomi, J.M.; Martin, J.N.; Deeks, S.G.; McCune, J.M.; Hunt, P.W. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS 2015, 29, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Wacleche, V.S.; Landay, A.; Routy, J.P.; Ancuta, P. The Th17 lineage: From barrier surfaces homeostasis to autoimmunity, cancer, and HIV-1 pathogenesis. Viruses 2017, 9, 303. [Google Scholar] [CrossRef]
- Lautenbach, E.; Lichtenstein, G.R. Human immunodeficiency virus infection and Crohn’s disease: The role of the CD4 cell in inflammatory bowel disease. J. Clin. Gastroenterol. 1997, 25, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.B.; Gelb, A.; Tabanda-Lichauco, R. Crohn’s ileitis in a patient with longstanding HIV infection. Am. J. Gastroenterol. 1994, 89, 937–939. [Google Scholar] [PubMed]
- Kalichman, S.C.; Di Berto, G.; Eaton, L. Human immunodeficiency virus viral load in blood plasma and semen: Review and implications of empirical findings. Sex. Transm. Dis. 2008, 35, 55–60. [Google Scholar] [CrossRef]
- Rodger, A.J.; Cambiano, V.; Phillips, A.N.; Bruun, T.; Raben, D.; Lundgren, J.; Vernazza, P.; Collins, S.; Degen, O.; Corbelli, G.M.; et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet 2019, 393, 2428–2438. [Google Scholar] [CrossRef] [Green Version]
- Quinn, T.C.; Wawer, M.J.; Sewankambo, N.; Serwadda, D.; Li, C.; Wabwire-Mangen, F.; Meehan, M.O.; Lutalo, T.; Gray, R.H. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 2000, 342, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; Van Lunzen, J.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016, 316, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Granfors, M.T.; Wang, J.S.; Kajosaari, L.I.; Laitila, J.; Neuvonen, P.J.; Backman, J.T. Differential inhibition of cytochrome P450 3A4, 3A5 and 3A7 by five human immunodeficiency virus (HIV) protease inhibitors in vitro. Basic Clin. Pharmacol. Toxicol. 2006, 98, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.B.; Owen, S.M.; Wesolowski, L.G.; Bennett, B.; Werner, B.G.; Wroblewski, K.E.; Pentella, M.A. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations; Atlanta, GA, USA, 2014. Available online: https://stacks.cdc.gov/view/cdc/23447 (accessed on 23 September 2021).
- Politch, J.A.; Mayer, K.H.; Welles, S.L.; O’Brien, W.X.; Xu, C.; Bowman, F.P.; Anderson, D.J. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS 2012, 26, 1535–1543. [Google Scholar] [CrossRef] [Green Version]
- Lambert-Niclot, S.; Tubiana, R.; Beaudoux, C.; Lefebvre, G.; Caby, F.; Bonmarchand, M.; Naouri, M.; Schubert, B.; Dommergues, M.; Calvez, V.; et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma on a 2002–2011 survey. AIDS 2012, 26, 971–975. [Google Scholar] [CrossRef]
- Jiao, Y.-M.; Chen, G.-L.; Zhu, W.-J.; Huang, H.; Fu, J.; Chen, W.; Shi, M.; Zhang, T.; Wu, H.; Wang, F.-S. Higher viral load and genetic diversity of HIV-1 in seminal compartments than in blood of seven Chinese men who have sex with men and have early HIV-1 infection. Microbiol. Immunol. 2017, 61, 239–246. [Google Scholar] [CrossRef]
- Kaul, R.; Prodger, J.; Joag, V.; Shannon, B.; Yegorov, S.; Galiwango, R.; McKinnon, L. Inflammation and HIV transmission in sub-Saharan Africa. Curr. HIV/AIDS Rep. 2015, 12, 216–222. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Sheth, P.M.; Yi, T.J.; Kovacs, C.; Kemal, K.S.; Jones, R.B.; Osborne, B.; Pilon, R.; la Porte, C.; Ostrowski, M.; Mazzulli, T.; et al. Mucosal correlates of isolated HIV semen shedding during effective antiretroviral therapy. Mucosal Immunol. 2012, 5, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Pícha, J.; Cerovský, J.; Píchová, D. Fluctuation in the concentration of sex steroids and aflatoxin B1 in the seminal plasma of boars and its relation to sperm production. Vet. Med. 1986, 31, 347–357. [Google Scholar]
- Wild, C.P.; Jiang, Y.Z.; Allen, S.J.; Jansen, L.A.M.; Hall, A.J.; Montesano, R. Aflatoxin-albumin adducts in human sera from different regions of the world. Carcinogenesis 1990, 11, 2271–2274. [Google Scholar] [CrossRef] [PubMed]
- Scholl, P.F.; Groopman, J.D. Long-term stability of human aflatoxin B1 albumin adducts assessed by isotope dilution mass spectrometry and high-performance liquid chromatography-fluorescence. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1436–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPhee, E.; Grabowski, M.K.; Gray, R.H.; Ndyanabo, A.; Ssekasanvu, J.; Kigozi, G.; Makumbi, F.; Serwadda, D.; Quinn, T.C.; Laeyendecker, O. Short Communication: The interaction of HIV set point viral load and subtype on disease progression. AIDS Res. Hum. Retrovir. 2019, 35, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Chomont, N.; Eller, L.A.; Kroon, E.; Tovanabutra, S.; Bose, M.; Nau, M.; Fletcher, J.L.K.; Tipsuk, S.; Vandergeeten, C.; et al. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine 2016, 11, 68–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkosky, J.; Nunnari, G.; Otero, M.; Calarota, S.; Dornadula, G.; Zhang, H.; Malin, A.; Sullivan, J.; Xu, Y.; DeSimone, J.; et al. Intensification and stimulation therapy for Human Immunodeficiency Virus Type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J. Infect. Dis. 2002, 186, 1403–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, D.M. Immunotoxicity of Aflatoxin B1 in Rats: Effects on Lymphocytes and the Inflammatory Response in a Chronic Intermittent Dosing Study. Toxicol. Sci. 2003, 73, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef] [Green Version]
- Rahier, J.F.; Ben-Horin, S.; Chowers, Y.; Conlon, C.; De Munter, P.; D’Haens, G.; Domènech, E.; Eliakim, R.; Eser, A.; Frater, J.; et al. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohn’s Colitis 2009, 3, 47–91. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Brown, C.J.; Abdo, Z.; Davis, C.C.; Hansmann, M.A.; Joyce, P.; Foster, J.A.; Forney, L.J. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007, 1, 121–133. [Google Scholar] [CrossRef]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Bammler, T.K.; Slone, D.H.; Eaton, D.L. Effects of dietary oltipraz and ethoxyquin on aflatoxin B1 biotransformation in non-human primates. Toxicol. Sci. 2000, 54, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, Y.; Beatty, C.; Biradar, S.; Castronova, I.; Ho, S.; Melody, K.; Turkle Bility, M. Moving beyond the mousetrap: Current and emerging humanized mouse and rat models for investigating prevention and cure strategies against HIV infection and associated pathologies. Retrovirology 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buss, P.; Caviezel, M.; Lutz, W.K. Linear dose-response relationship for dna adducts in rat liver from chronic exposure to aflatoxin b1. Carcinogenesis 1990, 11, 2133–2135. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Wu, S.; Yue, Y.; Wang, S.; Wang, Y.; Tao, L.; Tian, H.; Xie, J.; Ding, H. A high-throughput method for the simultaneous determination of multiple mycotoxins in human and laboratory animal biological fluids and tissues by PLE and HPLC-MS/MS. J. Chromatogr. 2013, 942–943, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Groopman, J.D.; Egner, P.A.; Schulze, K.J.; Wu, L.S.F.; Merrill, R.; Mehra, S.; Shamim, A.A.; Ali, H.; Shaikh, S.; Gernand, A.; et al. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B1-lysine albumin biomarkers. Food Chem. Toxicol. 2014, 74, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castle, P.E.; Hildesheim, A.; Bowman, F.P.; Strickler, H.D.; Walker, J.L.; Pustilnik, T.; Edwards, R.P.; Crowley-Nowick, P.A. Cervical concentrations of interleukin-10 and interleukin-12 do not correlate with plasma levels. J. Clin. Immunol. 2002, 22, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Juno, J.; Burgener, A.; Rahman, S.; Mogk, K.; Wachihi, C.; Mwanjewe, J.; Plummer, F.A.; Kimani, J.; Ball, T.B.; et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol. 2012, 5, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N. Guiding the vaginal microbicide trials with biomarkers of inflammation. J. Acquir. Immune Defic. Syndr. 2004, 37, S184–S193. [Google Scholar] [CrossRef]
- Freeman, T.C.; Ivens, A.; Baillie, J.K.; Beraldi, D.; Barnett, M.W.; Dorward, D.; Downing, A.; Fairbairn, L.; Kapetanovic, R.; Raza, S.; et al. A gene expression atlas of the domestic pig. BMC Biol. 2012, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zheng, N.; Liu, J.; Li, F.D.; Li, S.L.; Wang, J.Q. Aflatoxin B1 and aflatoxin M1 induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells. Food Chem. Toxicol. 2015, 83, 54–60. [Google Scholar] [CrossRef]
- Coombes, J.L.; Powrie, F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 2008, 8, 435–446. [Google Scholar] [CrossRef]
- Rhodes, J.W.; Tong, O.; Harman, A.N.; Turville, S.G. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front. Immunol. 2019, 10, e1088. [Google Scholar] [CrossRef] [Green Version]
- Masson, L.; Mlisana, K.; Little, F.; Werner, L.; Mkhize, N.N.; Ronacher, K.; Gamieldien, H.; Williamson, C.; Mckinnon, L.R.; Walzl, G.; et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: A cross-sectional study. Sex. Transm. Infect. 2014, 90, 580–587. [Google Scholar] [CrossRef]
- UNAIDS Joint United Nations Programme on HIV/AIDS. Global AIDS Update 2020: Siezing The Moment, Tackling Entrenched Inequalities to End Epidemics; Geneva, Switzerland, 2020; Available online: https://www.unaids.org/en/resources/documents/2020/global-aids-report (accessed on 23 September 2021).
- Sussan, T.E.; Gajghate, S.; Thimmulappa, R.K.; Ma, J.; Kim, J.H.; Sudini, K.; Consolini, N.; Cormier, S.A.; Lomnicki, S.; Hasan, F.; et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS ONE 2015, 10, e0116861. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef] [Green Version]
- Clay, K.; Lewis, J.; Severnini, E. Pollution, infectious disease, and mortality: Evidence from the 1918 Spanish influenza pandemic. J. Econ. Hist. 2018, 78, 1179–1209. [Google Scholar] [CrossRef] [Green Version]
- Kumari, N.; Sharma, S. Modeling the Dynamics of Infectious Disease Under the Influence of Environmental Pollution. Int. J. Appl. Comput. Math. 2018, 4, 84. [Google Scholar] [CrossRef]
- Bashir, M.F.; Jiang, B.; Komal, B.; Bashir, M.A.; Farooq, T.H.; Iqbal, N.; Bashir, M. Correlation between environmental pollution indicators and COVID-19 pandemic: A brief study in Californian context. Environ. Res. 2020, 187, 109652. [Google Scholar] [CrossRef] [PubMed]
- Suk, W.A.; Ahanchian, H.; Asante, K.A.; Carpenter, D.O.; Diaz-Barriga, F.; Ha, E.H.; Huo, X.; King, M.; Ruchirawat, M.; da Silva, E.R.; et al. Environmental pollution: An underrecognized threat to children’s health, especially in low- and middle-income countries. Environ. Health Perspect. 2016, 124, A43–A45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020, 261, 114465. [Google Scholar] [CrossRef] [PubMed]
| HIV | AFB1 Exposure | ||||||
|---|---|---|---|---|---|---|---|
| Reg ₸. | Outcome | Tissue | Ref. | Reg ₸. | Model, Tissue | Ref. | |
| IL-1α | U | risk factor | vaginal mucosa | [42] | U | rat, liver | [44] |
| IL-1β | U | risk factor | vaginal mucosa | [42] | U | dendritic cells | [45] |
| IL-2 | U | susceptibility | resting T cells | [46,47] | D | chicken, GI | [48] |
| U | replication | resting CD4+ T cells | [49] | D | rat, SMC * | [50] | |
| U | disrupted epithelial tight junctions/barrier | intestinal mucosa | [51] | ||||
| IL-4 | U | susceptibility | resting T cells | [52,53] | U | rat, SMC * | [50] |
| IL-6 | U U U U | incr. replication during infection activates replication disrupted epithelial tight junctions/barrier | vaginal mucosa intestinal cell model resting CD4+ T cells intestinal mucosa | [3,54,55] [51] [49,52] [51] | U D | dendritic cells chicken, ileum | [45] [56] |
| IL-7 | U | incr. susceptibility | resting T cells | [52,53] | |||
| IL-8 | U | risk factor | vaginal mucosa | [42] | U | Jurkat T cells | [57] |
| IL-12 | U U | during replication incr. local CD4+ T cells | vaginal mucosa intestinal mucosa | [54,55] [3] [46,52] | |||
| IL-15 | U | incr. susceptibility | Resting T cells | [52,53] | |||
| IL-17 | D | microbial translocation | intestinal mucosa | [52,58] | U D | mouse, liver chicken, GI | [59] [48] |
| IL-18 | U U U U | replication ART failure incr. replication incr. local CD4+ T cells | vaginal mucosa blood plasma monocytes and T cells intestinal mucosa | [3,54,55] [52,60] [52,60] [46,52] | |||
| IL-22 | D | microbial translocation | intestinal mucosa | [52,58] | |||
| TNF-α | U U U U U | during replication risk factor during infection activates replication disrupted epithelial tight junctions/barrier | vaginal mucosa vaginal mucosa intestinal cell model resting CD4+ T cells intestinal mucosa | [3,54,55] [42] [51] [49,52] [51] | U D | rat, serum chicken, GI | [61] [48,56] |
| IFN-Υ | U | during replication | vaginal mucosa | [3,54,55] | U U | pig, spleen mouse, liver | [47] [59] |
| MIP-1a | U | risk factor | vaginal mucosa | [42] | |||
| MIP-1b | U | risk factor | vaginal mucosa | [42] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeen, E.P.; Maldarelli, F.; Groopman, J.D. Environmental Pollutants, Mucosal Barriers, and Pathogen Susceptibility; The Case for Aflatoxin B1 as a Risk Factor for HIV Transmission and Pathogenesis. Pathogens 2021, 10, 1229. https://doi.org/10.3390/pathogens10101229
Madeen EP, Maldarelli F, Groopman JD. Environmental Pollutants, Mucosal Barriers, and Pathogen Susceptibility; The Case for Aflatoxin B1 as a Risk Factor for HIV Transmission and Pathogenesis. Pathogens. 2021; 10(10):1229. https://doi.org/10.3390/pathogens10101229
Chicago/Turabian StyleMadeen, Erin P., Frank Maldarelli, and John D. Groopman. 2021. "Environmental Pollutants, Mucosal Barriers, and Pathogen Susceptibility; The Case for Aflatoxin B1 as a Risk Factor for HIV Transmission and Pathogenesis" Pathogens 10, no. 10: 1229. https://doi.org/10.3390/pathogens10101229
APA StyleMadeen, E. P., Maldarelli, F., & Groopman, J. D. (2021). Environmental Pollutants, Mucosal Barriers, and Pathogen Susceptibility; The Case for Aflatoxin B1 as a Risk Factor for HIV Transmission and Pathogenesis. Pathogens, 10(10), 1229. https://doi.org/10.3390/pathogens10101229






