Abstract
This experiment investigated the mechanical performance and acid resistance (when subjected to 28 days of exposure to sulfuric and nitric acid of five percent) of ambient-cured geopolymer concrete. Geopolymer concrete (GPC)—which is produced by using industrial by-products, including fly ash and ground granulated blast furnace slag (GGBS)—is a low-carbon and strong substitute of Ordinary Portland Cement (OPC). This experiment examines the mechanical and acid-resisting properties of ambient GPC with different GGBS (10, 30, and 50 percent) contents. The compressive, tensile, and flexural strengths were measured at 7, 14, and 28 days, and durability was measured under an exposure of 5% sulfuric and nitric acids. X-ray diffraction (XRD) and scanning electron microscopy (SEM) showed that gypsum and ettringite were formed by sulfuric acid that weakened the structure, whereas surface decalcification was mostly caused by nitric acid. Mixes with a high fly ash content had more amorphous structures and better acid resistance, whereas those having high GGBS contents had high early strength because of high densities of the C–A–S–H gel. The findings indicate a strength–durability trade-off, which can be used to control the optimized mix design to produce sustainable and long-term infrastructure.
1. Introduction
1.1. Global Context and Motivation
One of the major sources of greenhouse gas emissions and resource consumption in the world is the construction industry. It contributes approximately 40 percent of the total energy consumption and approximately 30 percent of carbon dioxide emissions, mainly because of the manufacturing and consumption of cement [1,2]. Ordinary Portland Cement (OPC), the backbone of modern construction, has a rate of about 0.8–0.9 tons of CO2 per ton of the clinker formed [2,3]. This is attributed to two important processes: the calcification of limestone, which emits a significant quantity of CO2, and the energy consuming kiln processes, which demand temperatures greater than 1400 °C [4]. OPC concrete is extremely vulnerable to decay in cases of intense contact with chemical agents, like in wastewater facilities, sewer systems, and sulfate-containing or nitrate-containing soils. In these environments, calcium hydroxide is washed out of the matrix, causing the formation of gypsum and ettringite, internal microcracking, and a subsequent structural capacity loss [5]. Rebuilding or fixing the worn-out buildings has high economic and environmental expenses [6].
1.2. Geopolymer Concrete as an Alternative
Geopolymer concrete (GPC) has been proposed as a sustainable binder system capable of addressing both carbon emissions and durability issues. First conceptualized by Davidovits in the late 1970s [7], geopolymers are synthesized by activating aluminosilicate-rich precursors such as fly ash, GGBS, and metakaolin with alkaline solutions (e.g., NaOH and Na2SiO3). This reaction forms amorphous to semi-crystalline binding gels—sodium aluminosilicate hydrate (N–A–S–H)—and, when calcium is present, calcium aluminosilicate hydrate (C–A–S–H) [8,9,10]. These gels offer greater microstructure density than OPC hydration products and are less leached in their acidic surroundings. In addition, GPC uses industrial by-products which otherwise could be disposed in landfills, leading to waste management and resource efficiency [11]. In their research, [12] investigated the characteristics of geopolymer concrete (GPC) with the use of fly ash and ground granulated blast furnace slag (GGBS) as binding agents. They discovered that the optimal composition of the GGBS and fly ash was 80% and 20%, respectively, which produced the highest level of consistency and strength in the GPC. Though earlier research found that GGBS was found to be best in some conditions, this research intentionally examined less favorable values (10, 30, and 50%) to determine trade-offs between early strength and acid durability under ambient curing.
1.3. Sustainability Significance
In the environment CO2 can be minimized by 60–80 percent by replacing OPC with fly ash and GGBS [13]. GPC is economically beneficial as it allows industrial symbiosis: in coal-fired electricity plants and the steel industry by-products are used, which minimizes the costs of disposal, saving other natural resources [14]. Policy wise, GPC complies with international climate and infrastructure objectives, such as the United Nations Sustainable Development Goals (SDGs), especially SDG 9 (Industry, Innovation, and Infrastructure), SDG 11 (Sustainable Cities and Communities), and SDG 13 (Climate Action) [14,15]. Life cycle assessments (LCAs) also show that GPC is better than OPC regarding embodied energy, global warming, and end-of-life environmental impacts. In addition to the environmental advantages, higher performance in hostile conditions means a longer service life and lower maintenance expenses, which further reduce impacts on the life cycle [16].
1.4. Acid Resistance in GPC and OPC
In severe service conditions, acid resistance is an important parameter that helps determine the durability of concrete. OPC concretes are especially susceptible to this as a by-product of cement hydration: calcium hydroxide (Ca(OH)2) reacts with acids to form soluble salts and weaken the structure [17]. GPC, on the other hand, does not contain portlandite, and its binding is provided by N–A–S–H gels and C–A–S–H gels, which are overall more chemically stable [18]. Nonetheless, the results of the research vary. The low-calcium GPCs that are produced using fly ash have also been reported to exhibit high acid resistance because of the chemical stability of N–A–S–H gels [19]. On the other hand, GGBS-rich systems with superior mechanical strength are prone to the formation of calcium-containing phases, which can be decalcified and converted to gypsum when strongly attacked by acids [20]. For example, [21] demonstrated that fly ash-based GPC resisted sulfuric acid more effectively than OPC mixes. On the other hand, Wang et al. [22] found that an excessive calcium content in GGBS-rich geopolymers compromised long-term acid resistance. The current studies are focused on the commercialization of industrial solid wastes to produce binders in a sustainable manner. Investigations like [23,24] have shown an increase in the geopolymer strength with multiple industrial wastes’ incorporation, with [25,26]’s evaluations of eco-synergetic interactions between calcium-based and silica-based by-products to stabilize wastes. These observations support geopolymer technology as a sustainable approach to the use of solid waste and the use of low-carbon construction according to the principles of the circular economy. Moreover, [27] the GBFS/recycled steel tire wire synergistic effect facilitates the densification of the matrix and the crack-bridging behavior, resulting in more mechanically sound and compact microstructure composites [28]. The flexural and shear performance of the reinforced geopolymer concrete beam exhibited ductile behavior, minimized crack spacing, and a high load bearing capacity as compared to concrete beams [29]. It was found that the simultaneous application of micro-silica fume and waste steel lathe scraps showed a better improvement in the strength parameters not only in terms of an enhanced matrix density but also a decreased porosity, which is an important revelation in terms of valorizing industrial waste.
1.5. Research Gaps and Objectives
Research Gap: Although significant improvements have been made, there are still lapses in terms of acid resistance mechanisms in the fly ash–GGBS-based GPC. There are few studies that combine microstructural data (XRD and SEM) with mechanical and durability performance and sulfuric and nitric acid environments. The purpose of this study is to determine the mechanical and microstructural properties of fly ash and GGBS geopolymer concretes in aggressive acid conditions in a comprehensive manner.
The objectives of this study are as follows:
- To investigate the mechanical properties of GPC with 10%, 30%, and 50% GGBS replacement levels.
- To determine deterioration during exposure to sulfuric and nitric acids in terms of mass and strength loss indices.
- To conduct microstructural analyses (XRD and SEM) for understanding degradation mechanisms.
- To examine the findings within the broader discourse on sustainable and low-carbon construction.
2. Materials and Methods
2.1. Materials
2.1.1. Fly Ash (Class F)
A locally available Class F fly ash was used as the primary precursor. It contained low amounts of calcium oxide (<5%), high silica (~60%), and alumina (~26%), making it highly pozzolanic [30,31]. Its spherical morphology, confirmed through scanning electron microscopy, enhanced packing density and contributed to workability in fresh mixes. Table 1 show properties of fly ash and GGBS.

Table 1.
Physical properties of fly ash and GGBS.
The GGBS was procured from a steel plant and had a calcium oxide content of approximately 40%, along with significant silica, alumina, and magnesia. The latent hydraulic properties of GGBS facilitated early C–A–S–H gel formation, accelerating strength development compared to fly ash [32]. Table 2 shows the chemical composition of the source materials (fly ash and GGBS).

Table 2.
Chemical composition of source materials.
2.1.2. Alkaline Activator Solution
A binary alkaline solution consisting of sodium hydroxide (NaOH) and sodium silicate (Na2SiO3) was used and their properties are listed in Table 3. Sodium hydroxide pellets were dissolved in distilled water to obtain a 12 M solution, while sodium silicate was blended at a mass ratio of 2.5:1 (Na2SiO3: NaOH). The solution was prepared 24 h prior to mixing to ensure temperature stabilization and complete dissolution, consistent with best practices from prior research [33].

Table 3.
Properties of NaOH and Na2SiO3.
2.1.3. Superplasticizer
A high-range water reducer constructed from sulfonated naphthalene formaldehyde was incorporated at a rate of 2% of the binder’s weight. This admixture improved workability without altering the water-to-binder ratio, thereby avoiding dilution of the geopolymerization process [34].
Fine aggregates: Zone II natural river sand with fineness modulus of 2.6, specific gravity of 2.63, and water absorption of 1.2%.
Coarse aggregates: The coarse aggregates utilized are crushed granite, which has a maximum dimension of 20 mm, specific gravity of 2.70, and water absorption rate of 0.5%. These aggregates are in accordance with the requirements of IS 383:2016 [35].
Aggregates were in compliance with IS 383:2016 (Zone II) and the properties are shown in Table 4. Before batching, moisture correction was carried out. In binder mass (fly ash + GGBS), the 2 percent dose of superplasticizer was by mass.

Table 4.
Fine and coarse aggregate properties [35].
2.2. Mix Proportions
Three mixes were designed, varying the GGBS replacement level relative to fly ash: GPC10: 10% GGBS, 90% fly ash; GPC30: 30% GGBS, 70% fly ash; and GPC50: 50% GGBS, 50% fly ash as shown in Table 5.

Table 5.
Mix proportions for GPC10, GPC30, and GPC50.
Each mix had a constant alkaline activator to binder ratio of 0.5 and Na2SiO3-to-NaOH ratio of 2.5. A water-to-binder ratio of 0.35 was maintained, accounting for the water content in sodium silicate.
2.3. Specimen Preparation and Casting
The concrete was prepared in a pan mixer in the following order: dry blending of the precursors (fly ash and GGBS) then aggregates, and then alkaline activator solution and superplasticizer were added. Lubricated steel molds were filled with fresh concrete and pressed with a vibrating table.
The following specimen sizes were prepared:
- ➢
- Compressive strength: Cubes of 150 × 150 × 150 mm.
- ➢
- Split tensile strength: Cylinders of 150 mm diameter × 300 mm height.
- ➢
- Flexural strength: Prisms of 100 × 100 × 500 mm.
2.4. Curing Regime
All specimens were cured at ambient laboratory temperature (27 ± 2 °C). This study did not involve high curing as many past GPC studies did because the aim was to determine the viability of in situ use in real-life construction. This method is indicative of realistic situations, and less energy is required to support sustainability [36].
2.5. Acid Resistance Testing
The more specimens were cured (28 days), the more specimens were put in a problematic environment by putting them into a 5 percent sulfuric acid (H2SO4) and 5 percent nitric acid (HNO3) solution to determine their toughness. To ensure consistency, the PH of solutions was checked and maintained on a weekly basis.
The parameters are established as follows:
Mass loss percentage: This is obtained by weighing the specimen (before the terminal and after the terminal).
Residual strength (%): The compressive strength of the specimens that were both exposed to acid and that of controls, which were measuring the strength of the specimens. The reason why sulfuric and nitric acids were selected for this test design was due to the fact that the sulfuric and nitric acids are frequent aggressive reagents that are present in sewage systems, industrial effluents, and the acidic soils [37].
2.6. Microstructural Analysis
X-ray diffraction (XRD): It was performed at 20 using CuKa radiation at a range of 10–70. Corresponding quartz, mullite, C–A–S–H, and gypsum peaks were observed [38,39].
Scanning electron microscopy (SEM): Gel morphology, microcracking, and secondary crystallization were observed under high-resolution images with magnifications of 1000–5000× frequency [40].
2.7. Testing Arrangements and Test Processes
The mechanical tests were carried out in accordance with the following settings and requirements:
- Compressive Strength: 150 × 150 × 150 mm size cubes were subjected through compressive testing machine (CTM) with a capacity of 2000 kN, according to IS 516:2018. The loading rate was kept at 140 kg/cm2/min until failure.
- Split Tensile Strength: Cylindrical specimens with 150 mm diameter and 300 mm height were tested in accordance with IS 5816:1999. To promote even distribution, the load was placed on the cylinder in a diametrical manner with the help of strips made of steel (3 mm thick).
- Flexural Strength: Beam samples (100 × 100 × 500 mm) were subjected to three-point loading in accordance with IS 516:2018, with a span of 400 mm and loading rate of 180 kg/min.
- Demolding of the samples followed after 24 h, and they were cured at 27 ± 2 °C, RH 60–70, with vibration compaction (30 s) and no bleeding/segregation.
3. Results
Table 6 shows the results for the compressive strength, tensile, and flexural properties for GPC mixes at 7, 14, and 28 days.

Table 6.
Strength results for GPC specimens with fly ash and GBBS.
3.1. Compressive Strength
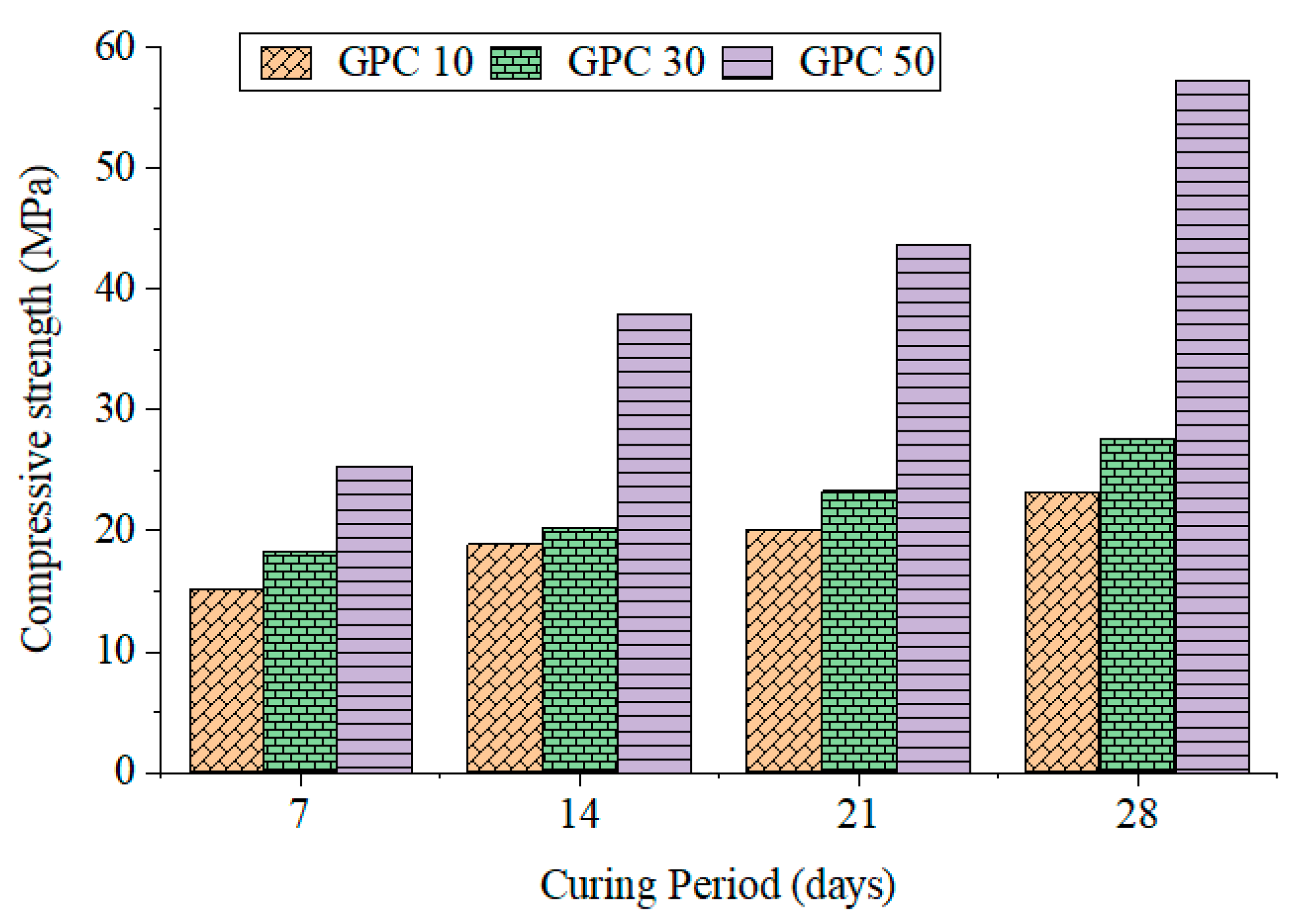
Compressive strength results for GPC10, GPC30, and GPC50 at 7, 14, and 28 days are presented in Table 6 and Figure 1. Results showed that the strength increased with both the curing age and GGBS content. GPC10 reached 23.14 MPa at 28 days, GPC30 reached 27.63 MPa, and GPC50 reached 57.25 MPa. The rate of early strengthening was especially high for GPC50, where the calcium-rich GGBS assisted in the fast development of C–A–S–H gels. Reported values for the strength and mass loss are all mean values of three specimens (n = 3), and variability is reported as the mean. Such observations are consistent with previous findings that GGBS makes the buildup of strength faster in geopolymer binders [41,42]. Bernal et al. [36] demonstrated that the combination of GGBS and fly ash produces hybrid binding systems with complementary N–A–S–H and C–A–S–H gels. Table 6 shows the results for the compressive strength, tensile, and flexural properties for GPC mixes at 7, 14, and 28 days.
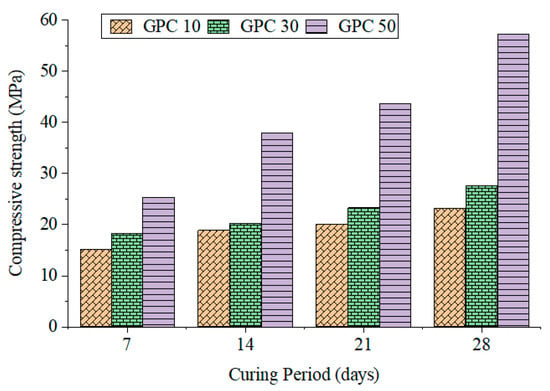
Figure 1.
Compressive strength of GPC specimens.
3.2. Tensile Strength
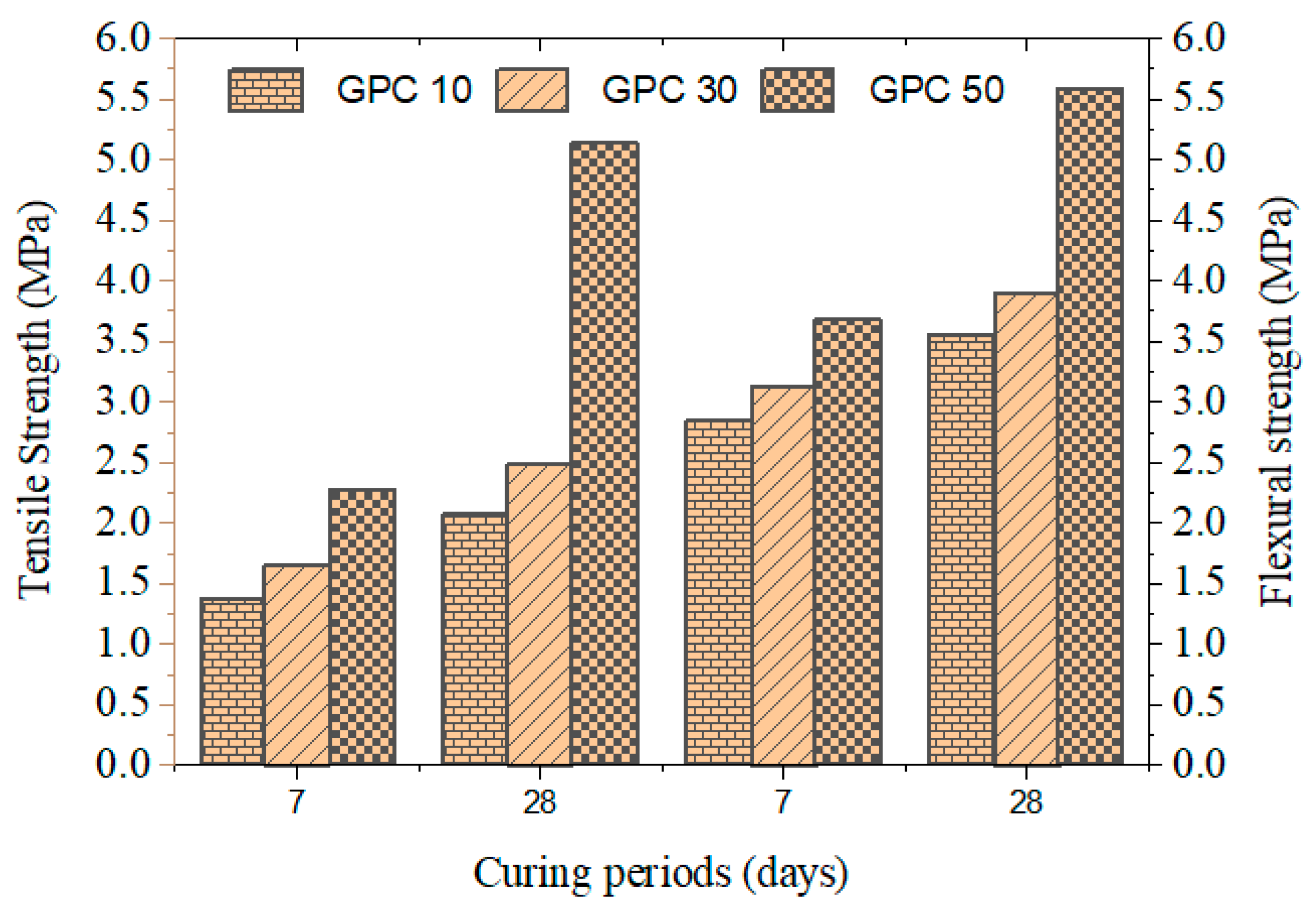
Table 6 and Figure 2 display the tensile strength of the mixes after 28 days. GPC10 registered 2.08 Mpa, GPC30 was 3.62 Mpa, and GPC50 was 5.15 Mpa. The enhancement of the increased GGBS can be explained by the existence of denser microstructures and enhanced interfacial transition zones. These findings are similar to those of [43,44], who reported increased tensile strength in slag-based GPCs, and Law et al. [45], who reported that tensile properties were directly correlated with the calcium content of precursors.
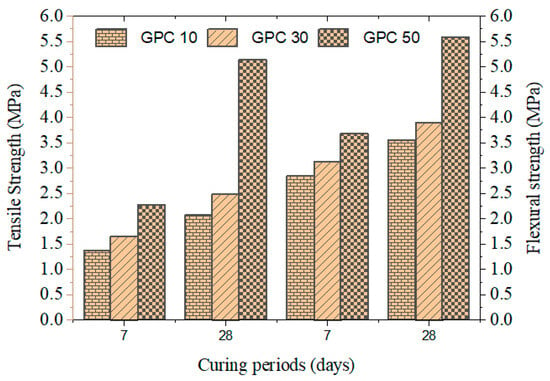
Figure 2.
Tensile and flexural strength of GPC specimens.
3.3. Flexural Strength
Table 6 and Figure 2 present the flexural strength results for 28 days. The value increased to 3.56 Mpa in GPC10 and 5.60 Mpa in GPC50. Although the flexural strength was greatly enhanced with the addition of GGBS, it was still proportionately lower than the compressive strength, which is indicative of the brittle failure characteristic of geopolymer concretes. Ref. [46] pointed to the same tendencies, and reinforcement measures should be taken to balance the brittle materials with fibers or nano-silica.
3.4. Acid Resistance—Mass Loss
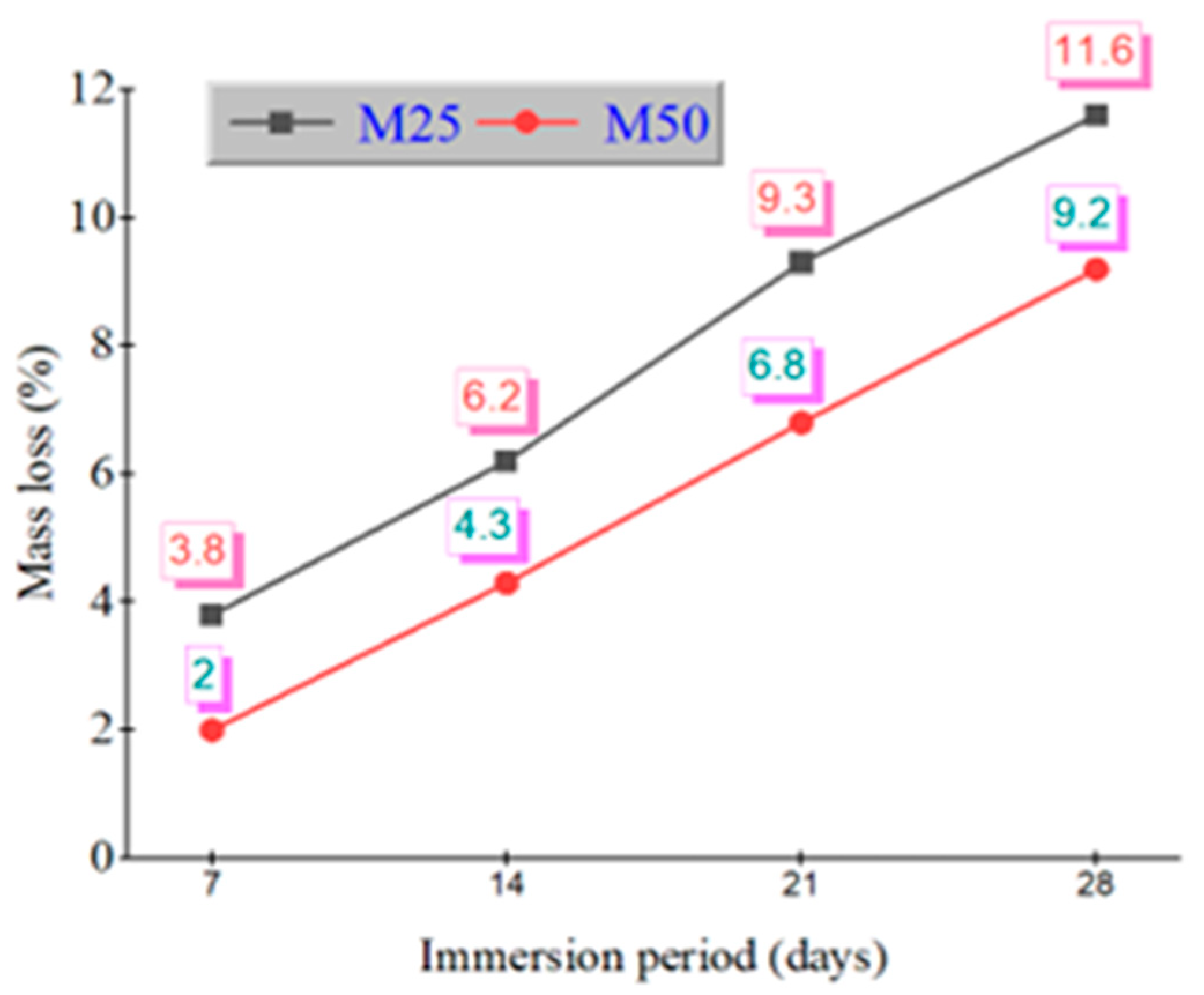
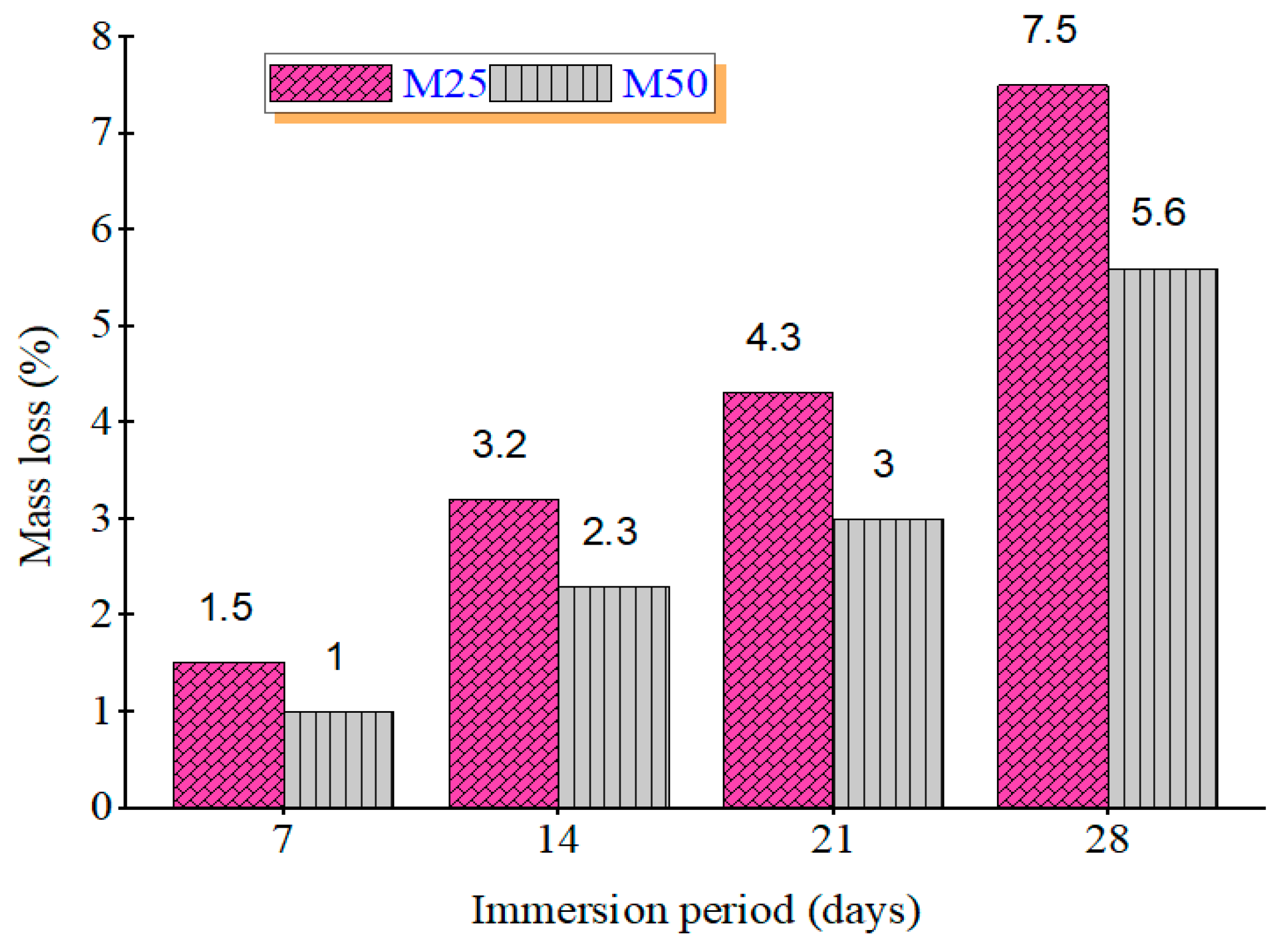
After 28 days of immersion in 5% sulfuric acid and nitric acid, mass loss percentages were recorded. Figure 3 shows the mass loss (%) of GPC mixes under sulfuric and Figure 4 shows nitric acid exposure. In sulfuric acid, GPC10 lost 11.6%, GPC30 lost 10.3%, and GPC50 lost 9.2%. In nitric acid, GPC10 lost 7.5%, GPC30 lost 6.3%, and GPC50 lost 5.6%. Sulfuric acid was more aggressive, primarily due to the gypsum and ettringite formation, which caused expansion and microcracking [47]. Nitric acid induced decalcification but did not produce expansive products, leading to comparatively less deterioration [48].
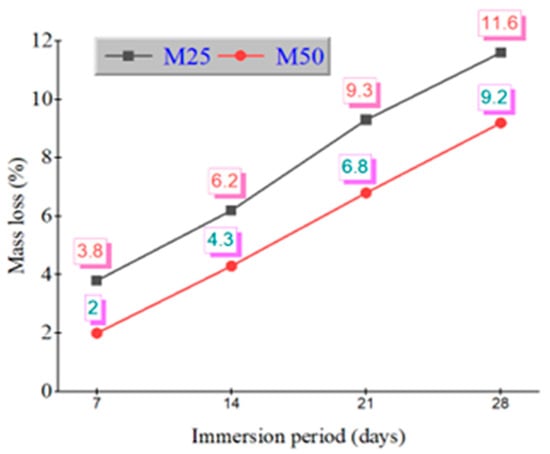
Figure 3.
Mass loss % of M25 and M50 grades during immersion in 5% H2SO4.
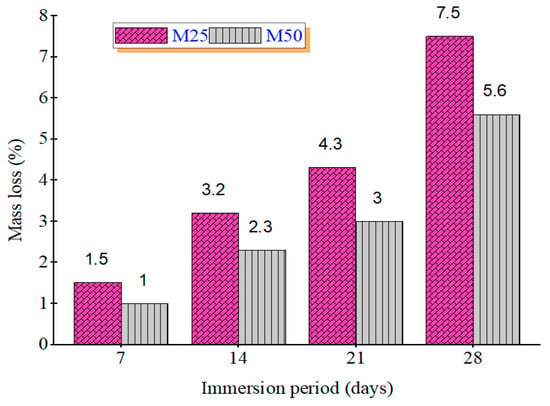
Figure 4.
Mass loss % of M25 and M50 grades during immersion in 5% HNO3.
3.5. Acid Resistance—Residual Strength
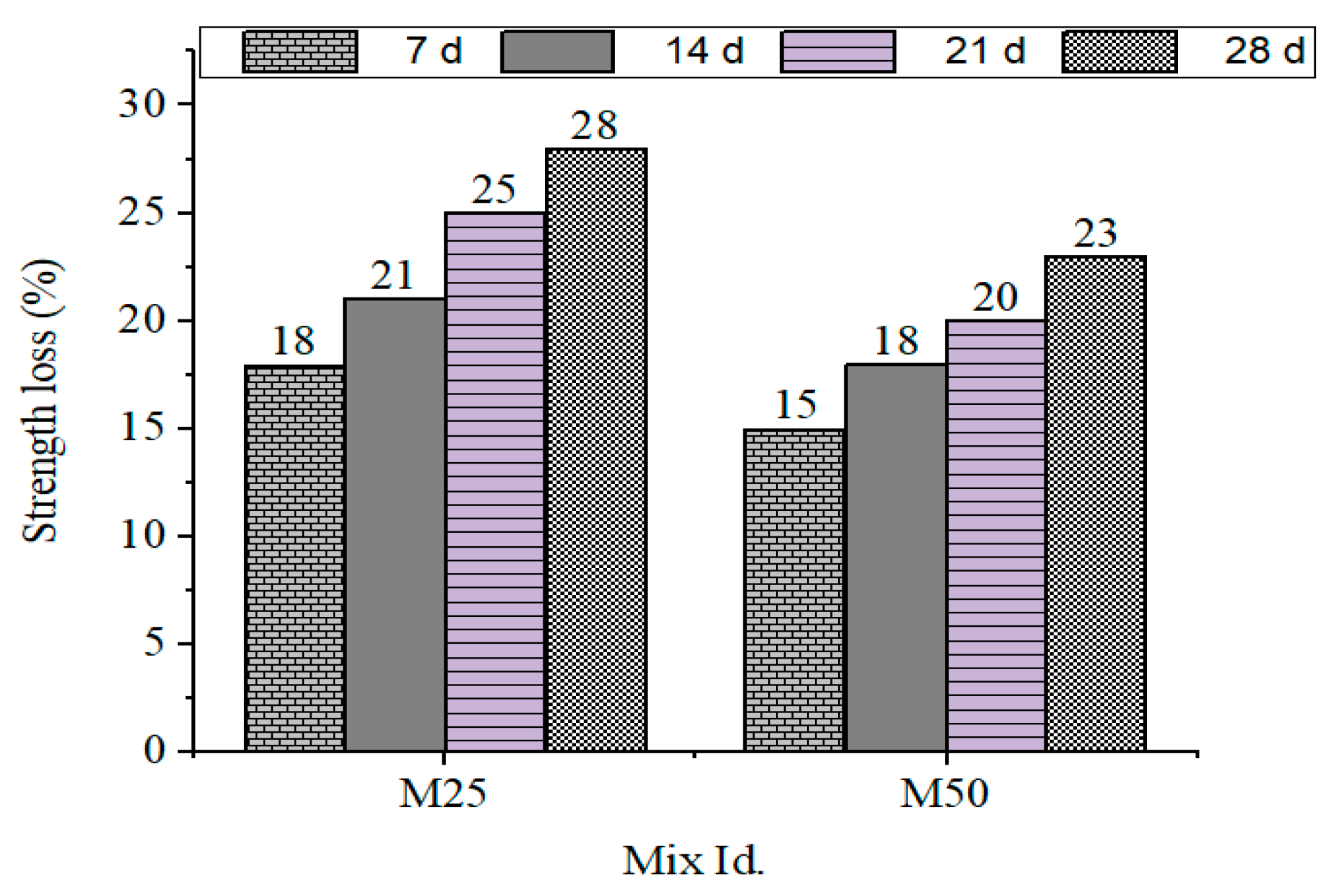
Residual compressive strengths after acid exposure are presented in Figure 5, including the residual strength loss in GPC mixes after sulfuric and nitric acid exposure.
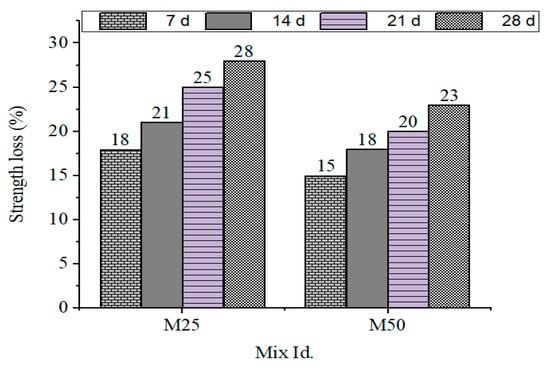
Figure 5.
Strength loss % for M25 and M50 grades during immersion in H2SO4.
- ➢
- Sulfuric acid exposure: The strength loss ranged from 28% (GPC10) to 23% (GPC50).
- ➢
- Nitric acid exposure: The strength loss ranged from 28% (GPC10) to 22% (GPC50).
This shows that the increased GGBS content also improved resistance but caused a susceptibility to gypsum crystallization during the sulfuric acid assault. The same was observed by [49], who observed that systems containing more calcium had better short-term and worse long-term chemical stability in geopolymer systems.
3.6. Microstructural Analysis
3.6.1. XRD Findings
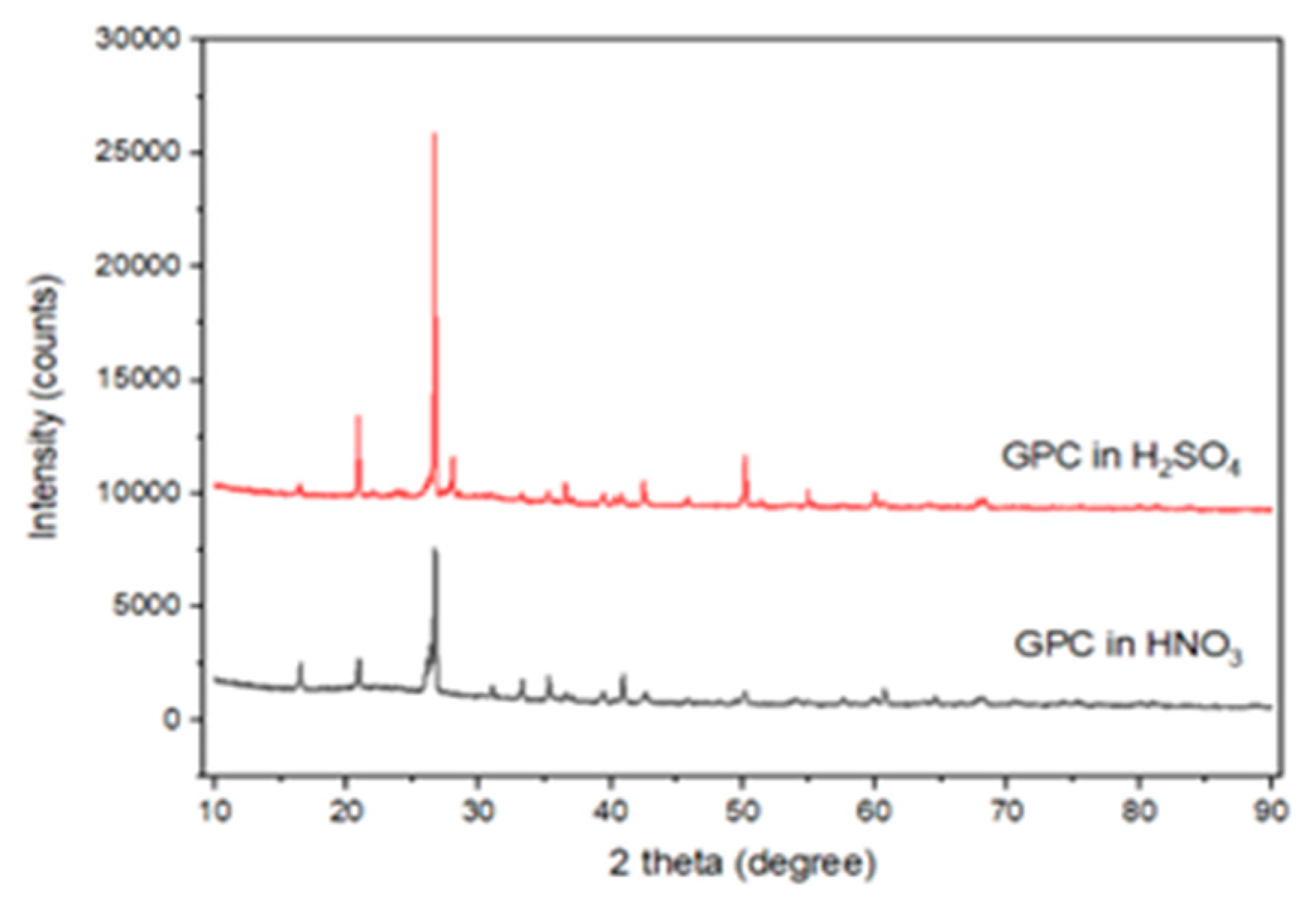
XRD results before and after the acid exposure are shown in Figure 6. Control specimens revealed broad amorphous humps (20–35° 2θ), characteristic of geopolymer gels. After the sulfuric acid attack, distinct gypsum peaks appeared, especially in GPC50.
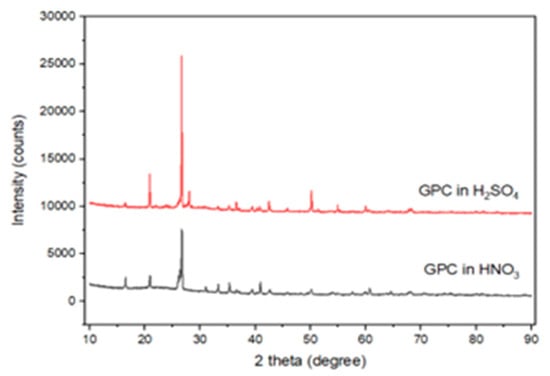
Figure 6.
XRD pattern of GPC specimens immersed in H2SO4 and HNO3.
Gypsum peaks were observed at around 11.6°, 20.7°, and 29.1° (2°), and small peaks that may have been caused by ettringite were noted at 9.1° and 15.8°, which confirmed that the crystallization of sulfates occurred in GGBS-based mixes.
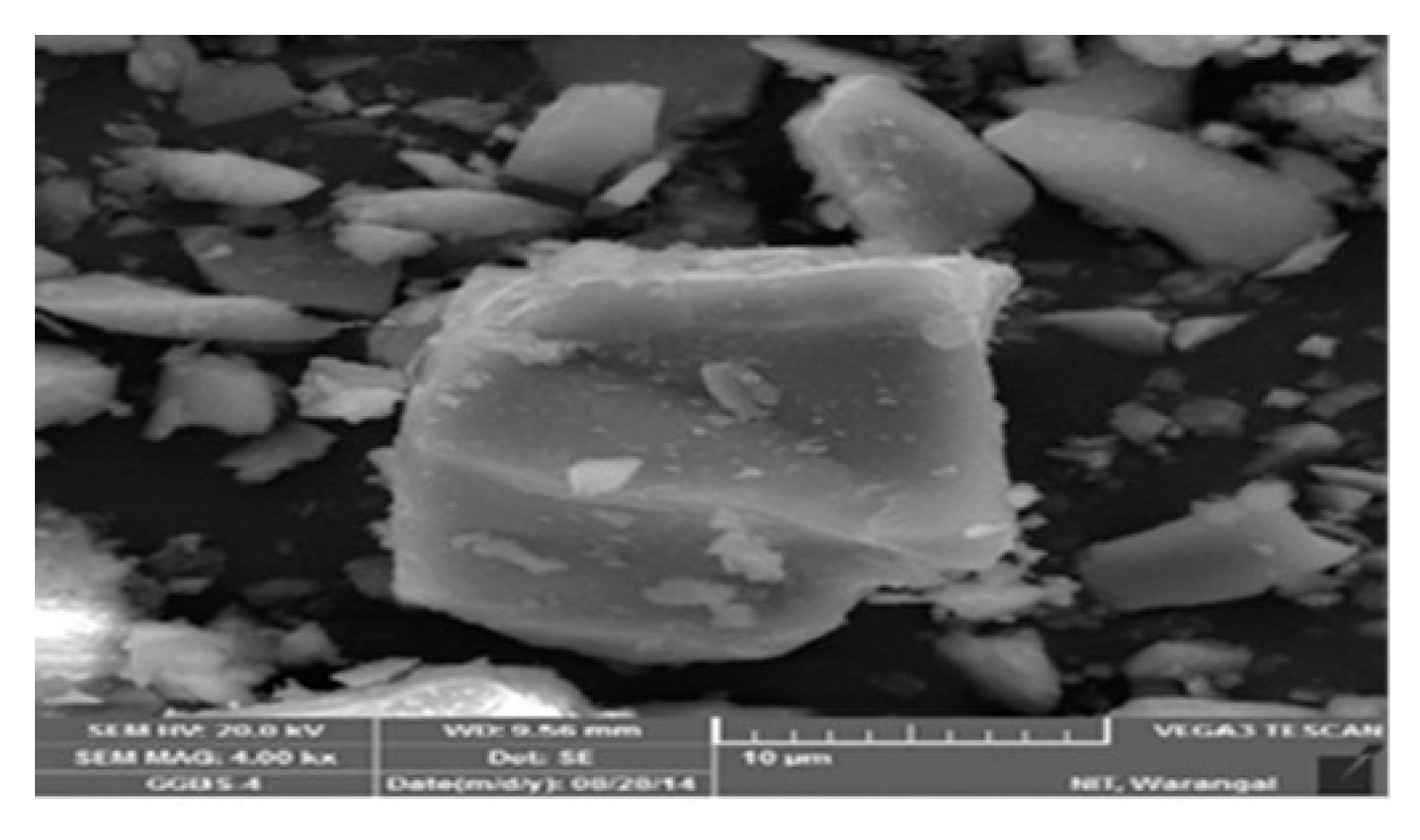
Fly ash-rich mixes retained more of their amorphous structure, indicating chemical stability. These results are consistent with [46,50,51], who reported that fly ash-based systems-maintained N–A–S–H stability even after acid exposure. Figure 7 depicts the micrograph of the SEM of the fly ash-based GPC10 mix, which indicates a comparatively porous matrix with unreacted spherical particles and micropores. The poor gel structure and diffused holes are a sign of incomplete geopolymerization during ambient curing. The GPC50 mix shown in Figure 8 has a GGBS-based structure that was significantly denser and more compact; this indicates the existence of continuous C–A–S–H gel networks. Upon immersion in sulfuric acid, gypsum crystals were also found in the pores, and the secondary reaction products were found to be responsible for the expansion and degradation of the surface. The comparative analysis of both figures shows that the fly ash-rich mix remained structurally intact by being slightly dissolved on the surface, as compared to the slag-rich mix, which exhibited crystalline deposition, as was the case with the XRD. These findings reiterate that calcium-enriched systems though stronger are more susceptible to chemical degradation in the presence of sulfuric acid.

Figure 7.
Scanned electron microscope image of fly ash.
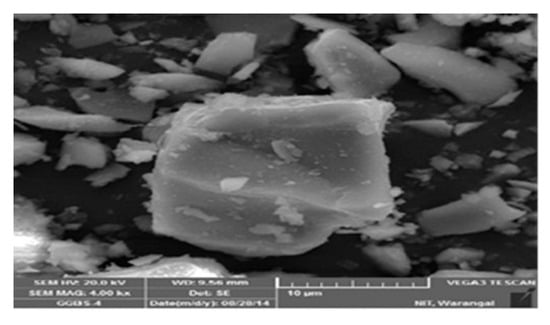
Figure 8.
Scanned electron microscope image of GGBS.
3.6.2. SEM Findings
From the SEM images shown in Figure 7 and Figure 8, GPC10 exhibited significant microcracking and leaching voids. GPC50 displayed denser microstructures initially, but after the sulfuric acid immersion, gypsum crystals were evident within pores. The nitric acid exposure produced surface roughening and minor microcracks but without expansive products. These findings align with [52], who found that microcracks propagate in slag-rich systems exposed to sulfuric acid, whereas fly ash-based systems retain their integrity longer.
4. Discussion
In this research paper, there is an ecologically realistic and practical evaluation of ambient-cured geopolymer concrete, which is a significant step away from the high curing used in earlier studies. This work compares the action of sulfuric and nitric acids in a systematic manner and presents the various mechanisms of the degradation of the gel using the calcium contents and gel chemistry. The findings provide original empirical information on the trade-off between the early-age strength (which should be dominated by the C–A–S–H formation under the influence of GGBS) and the acid resistance (which should be governed by the stability of N–A–S–H in the fly ash-based systems). These results are important sources in the existing literature about the relationship between field-feasible curing, microstructural evolution, and sustainability implications in a single context.
4.1. Mechanical Properties of Fly Ash–GGBS Geopolymer Concretes
The precursor geopolymer concrete (GPC) chemistry and reactivity have a strong effect on the mechanical performance of geopolymers. The use of GGBS in this paper has shown that compressive, tensile, and flexural strengths were significantly improved over the low-slag counterparts, as was previously reported; slag promotes geopolymerization because of its high calcium level [27,28]. Calcium in GGBS enhances the fast formation of calcium aluminosilicate hydrate (C–A–S–H) gels that complement sodium aluminosilicate hydrate (N–A–S–H) networks formed by fly ash at 28 days. This two-gel system explains why the GPC50 mix experienced high gains in strength relative to the GPC10 mix, which was over two times stronger at 28 days.
The increase in tensile and flexural strength is also an indicator of the densification of the matrix and a better interfacial transition zone (ITZ) between the binder and aggregates. The same findings were achieved by [16], who found that the incorporation of GGBS refined the pore structure as well as increased crack resistance in geopolymer composites. Other researchers warn, however, that extremely high GGBS contents (>60%) can cause autogenous shrinkage and microcracking, which can negate the strength gains [53]. Thus, while GGBS is clearly beneficial, an optimal proportion must be identified for structural applications.
4.2. Acid Resistance and Durability
The durability results highlight a critical trade-off: although GGBS-rich mixes achieved higher strengths, they were more vulnerable to the acid attack. Sulfuric acid caused a significantly higher mass loss and strength reduction than nitric acid, which aligns with the findings of [54]. The sulfate attack on slag-rich mixes is caused by the large formation of gypsum and ettringite, which is responsible for the extreme degradation. Conversely, nitric acid mostly causes the loss of alkalis and the gradual dissolution of the matrices and thus causes less devastating damage [55,56,57].
Fly ash-rich (e.g., GPC10) mixes had a better performance in hostile acidic conditions despite being weaker mechanically. This comparative stability can be explained by the fact that the proportion of N–A–S–H gels is less reactive with the sulfate ions than C–A–S–H phases [12]. An example is that fly ash-dominant binders could be useful in infrastructure in acidic soils and wastewater systems, whereas higher GGBS contents could be useful in precast structural elements that need high early strength.
The degradation mechanism under acidic exposure is under the control of dissolution–precipitation kinetics. The calcium ions of GGBS react with the sulfate ions to create gypsum and ettringite, which expand in the matrix and thus causes cracking. The thermodynamic benefit of this reaction is that CaSO4 2H2O has a lower solubility at room temperature and is hence volatile. On the other hand, nitric acid is primarily a decalcifying agent, and the salts of nitrate are more soluble and do not precipitate, and thus the effect of the deterioration is slower.
The fly ash-rich systems are thermodynamically stable due to the lower Gibbs free energy change in the N–A–S–H gels in the presence of acids, hence they dissolve less than the calcium-bearing C–A–S–H phases. These findings are in line with the earlier studies of acid–gel interactions by Bakharev (2005) [31], who also found an identical stability sequence of the amorphous aluminosilicate gels in geopolymer binders.
4.3. Microstructural Insights
X-ray diffraction (XRD) and scanning electron microscopy (SEM) findings can be useful in terms of supporting the existing performance patterns. Slag-rich mixes’ XRD patterns indicated crystalline peaks of C–A–S–H, whereas those of the acid-exposed samples had an additional peak of gypsum and ettringite, which proved the degradation caused by sulfates. Other microstructural changes were also observed by [27,38], who demonstrated that the calcium–silica ratio is critical in determining the stability of geopolymer binders in acidic environments. SEM micrographs also revealed that the denser the matrices were, the higher the content of GGBS was, and this is the reason why GPC30 and GPC50 had better strengths. Nevertheless, SEM after the acid addition showed drastic surface cracking and the deposition of gypsum crystals in slag-laden mixes and more porous yet chemically stable mixes in fly ash-laden mixes. This is aligned with reports by [46], who found that microstructural compactness enhances strength but also promotes expansive stress development when exposed to aggressive acids.
The microstructural tendencies observed during the current work are consistent with the model of degradation suggested by Fernández-Jiménez and Palomo (2005) [47], according to which decalcification caused by acids weakens the C–A–S–H phases and does not affect the silica–alumina network of N–A–S–H gels. This model is further developed by the existing results to include dual-acid exposure environments and ambient curing environments, which provide a more realistic assessment of the behavior of geopolymer concretes in the field of service.
4.4. Sustainability and Environmental Concerns
The replacement of OPC by geopolymer binders is very beneficial to the environment. Around the world, cement manufacturing accounts for approximately 7–8 percent of CO2 gases [11]. Embodied carbon may be cut by 40–80 percent using geopolymers based on fly ash and GGBS, depending on the mix production and transportation distances [58]. In addition, the valorization of industrial by-products will reduce waste disposal in landfills and promote circular economic programs. However, it is not possible to evaluate sustainability using embodied carbon alone. Durability is also a critical factor since high-GGBS-level mixes will require protection (e.g., surface coating and altered activator chemistry) to maintain long-term stability in acidic conditions. It is necessary, therefore, to have a life cycle viewpoint that is a combination of embodied carbon analysis and durability-motivated service life predictions [31,59].
5. Conclusions
The mechanical performance, acid resistance, and microstructural behavior of geopolymer concretes using different proportions of fly ash and ground-granulated blast furnace slag (GGBS) were the focus of the experimental studies. Depending on the findings, it is possible to draw several conclusions:
5.1. Summary of Key Findings
5.1.1. Strength Development
The compressive, tensile, and flexural strength increased with the increase in the GGBS content. The highest strength of GPC50 (50% GGBS) was recorded at 57.25 Mpa (28 days), which was a lot higher than GPC10 (23.14 Mpa). The strength gain in the slag-laden mixes was explained by the fact that the C–A–S–H gel formed rapidly, which counterbalanced the slow geopolymerization of the fly ash.
5.1.2. Acid Resistance
All mixes degenerated in the presence of both sulfuric and nitric acids, but those in the presence of sulfuric acid were more severely damaged. The mass loss was between 11.6 (GPC10 in H2SO4) and 5.6 (GPC50 in HNO3). The loss of strength in the remains of the residue was of the same order; sulfuric acid produced expanding gypsum and ettringite, whereas nitric acid produced decalcification but no expanding products.
5.1.3. Microstructural Insights
XRD showed that GGBS-rich specimens that were exposed to sulfuric acid exhibited gypsum peaks, whereas amorphous phases were retained in fly ash-rich mixes. SEM established microcracking and leaching pores in fly ash blends and gypsum crystallization in GGBS mixes.
5.1.4. Durability Trade-Offs
The GGBS improved the strength of the material in the early days and at the expense of long-term performance in the acid-prone gypsum formation. The fly ash benefited the material in regard to long-term chemical stability but at the cost of decreased early strength.
5.1.5. Sustainability Contributions
According to the literature, the CO2 reduction potential of geopolymers of fly ash GGBS is 40–70% (A1–A3 scope, 1 m3 functional unit). Further LCA validation is proposed. The promotion of fly ash and GGBS is in line with the concept of a circle economy and minimizes landfills. Better life in aggressive conditions increases service life and life cycle costs, and environmental effects are minimized. On the whole, the findings affirm the fact that the fly ash–GGBS ratios should be optimized to achieve mechanical performance, durability, and sustainability in geopolymer concrete.
5.1.6. Practical Guidance
The general recommendation for sewer environments should be less than 30% GGBS, and for precasts it should be more than 50%. Key parameters include the following: GGBS%, NaOH M, Na2SiO3:NaOH ratio, and curing RH.
5.2. The Implications for Construction Practice
The findings provide good guidance for practical applications. GGBS-rich mixes might be of benefit where high strength is required, e.g., precast elements, high-rise constructions or pavements, as these cannot develop strength very quickly, and microstructures are dense. Sewer networks, chemical plants, or even wastewater treatment systems, which require acidic hazardous environments, ought to prefer fly ash-dominant mixes because of their better acid resistance. General-purpose construction facilitates better options (such as GPC30) and can offer an ideal balance between strength and durability. Procedural curing, especially the one that was embraced in this research, also makes the case of practical adoption stronger, as longer curing is usually not practical in the field.
5.3. Future Research Directions
Notwithstanding the informative results, there are yet a number of limitations: The exposure to acid was not long-term (28 days), and long-term (up to 1 year) studies need to confirm durability. This study only investigated sulfuric and nitric acids; in reality the exposures are usually mixed (chlorides, carbonation, and freeze thaw). One concentration of the activators (12 M NaOH) was tested; properly looking at other molarities and combinations of the activators may enhance the performance further. To avoid premature reactions, the NaOH solution was allowed to cool at 25 °C for 24 h before mixing. Sustainability claims require life cycle assessments (LCAs) with field-scale trials to be completely validated. Future studies would also consider nanomaterial additives (e.g., nano-silica and graphene oxide) that would improve mechanical as well as durability properties without leading to sustainability losses [60,61].
Author Contributions
Conceptualization, K.K.P. and M.R.; Methodology, M.R.; Investigation, K.K.P., Z.A.A. and A.N.H.; Writing—original draft, S.A.; Writing—review & editing, K.K.P. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential, economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Poloju, K.K. Geopolymer Concrete: Principles, Applications and Testing Methods, 1st ed.; Series ISSN 2191-530X, Springer Briefs in Applied Sciences and Technology; Springer: Singapore, 2025; ISBN 978-981-96-2478-2. [Google Scholar]
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Chen, Y.; Gao, J.; Tang, L.; Li, X. Resistance of concrete against combined attack of chloride and sulfate under drying–wetting cycles. Constr. Build. Mater. 2016, 106, 650–658. [Google Scholar] [CrossRef]
- Alexander, M.; Bertron, A.; De Belie, N. (Eds.) Performance of Cement-Based Materials in Aggressive Aqueous Environments; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Poloju, K.K. Advanced Materials and Sustainability in Civil Engineering, 1st ed.; Series ISSN 2191-530X, Springer Briefs in Applied Sciences and Technology; Springer: Singapore, 2023; ISBN 978-981-16-5949-2. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. (Eds.) Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Wang, S.-D.; Scrivener, K.L.; Pratt, P.L. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Jawahar, J.; Mounika, G. Strength properties of fly ash and GGBS based geo polymer concrete. Asian J. Civ. Eng. 2016, 17, 127–135. [Google Scholar]
- Habert, G.; D’Espinose de Lacaillerie, J.-B.; Roussel, N. An environmental evaluation of geopolymer concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Poloju, K.K.; Annadurai, S.; Manchiryal, R.K.; Goriparthi, M.R.; Baskar, P.; Prabakaran, M.; Kim, J. Analysis of Rheological Characteristic Studies of Fly-Ash-Based Geopolymer Concrete. Buildings 2023, 13, 811. [Google Scholar] [CrossRef]
- Poloju, K.K.; Manchiryal, R.K.; Al Balushi, Y.A.A.; Al Banna, W.N.M. Variation of sodium hydroxide concentration impact on rheological properties of geopolymer paste. Int. J. Adv. Appl. Sci. 2023, 10, 62–68. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Nirmala Kumar Parshwanath, R. Quantities of sodium hydroxide solids and water to prepare sodium hydroxide solution of given molarity for Geopolymer Concrete mixes. Indian Concr. J. 2016, 90, 68–79. [Google Scholar]
- Wallah, S.E.; Rangan, B.V. Low-Calcium Fly Ash-Based Geopolymer Concrete: Long-Term Properties (Research Report GC 2); Curtin University of Technology: Perth, Australia, 2006. [Google Scholar]
- Rashad, A.M. A comprehensive overview about the influence of different additives on the properties of alkali-activated slag–A guide for Civil Engineer. Constr. Build. Mater. 2015, 87, 29–55. [Google Scholar] [CrossRef]
- Kathirvel, P.; Kaliyaperumal, S.R.M. Performance of alkali activated slag concrete under aggressive environment. Sci. Iran. Trans. A Civ. Eng. 2018, 25, 2451–2460. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Ou, Z. Durability of alkali-activated materials in aggressive environments: A review on recent studies. Constr. Build. Mater. 2017, 152, 598–613. [Google Scholar] [CrossRef]
- Ralli, Z.G.; Pantazopoulou, S.J. State of the art on geopolymer concrete. Int. J. Struct. Integr. 2021, 12, 511–533. [Google Scholar] [CrossRef]
- Wang, H.; Zentar, R.; Wang, D.; Dong, L.; Sun, D. Recycling single use surgical face mask waste for reinforcing cement-treated/untreated dredged marine sediments: Strength, deformation and micro-mechanisms analysis. Constr. Build. Mater. 2024, 449, 138450. [Google Scholar] [CrossRef]
- Poloju, K.; Anil, V.; Manchiryal, R.K. Impact of nano-silica on strength and durability properties of self-compacting concrete. Int. J. Adv. Appl. Sci. 2017, 4, 120–126. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wang, D.; Zentar, R.; Lang, L.; Sun, D.; Dong, L. Bibliometric analysis of research on recycling dredged sediment as construction material during the past three decades: Trends and focal points. J. Soils Sediments 2025, 25, 2126–2151. [Google Scholar] [CrossRef]
- Çelik, A.; Karalar, M.; Aksoylu, C.; Mydin, M.; Althaqafi, E.; Yılmaz, F.; Umiye, O.; Özkılıç, Y. Effect of GBFS Ratio and Recycled Steel Tire Wire on the Mechanical and Microstructural Properties of Geopolymer Concrete under Ambient and Oven Curing Conditions. Case Stud. Constr. Mater. 2024, 21, e03890. [Google Scholar] [CrossRef]
- Ozkılıç, Y.O.; Çelik, A.İ.; Aksoylu, C.; Karalar, M.; Mydin, M.A.O.; Althaqafi, E.; Yılmaz, F.; Umiye, O.A. Shear and flexural performance of reinforced geopolymer concrete beams cured under ambient and oven conditions with environmentally friendly waste steel tire wire additives. Sci. Rep. 2025, 15, 22765. [Google Scholar] [CrossRef] [PubMed]
- Çelik, A.İ.; Özkılıç, Y.O.; Bahrami, A.; Hakeem, I.Y. Mechanical performance of geopolymer concrete with micro silica fume and waste steel lathe scraps. Case Stud. Constr. Mater. 2023, 19, e02548. [Google Scholar] [CrossRef]
- Li, J.; Tay, B.W.Y.; Lei, J.; Yang, E.-H. Experimental investigation of Seebeck effect in metakaolin-based geopolymer. Constr. Build. Mater. 2021, 272, 121615. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterization of fly ashes. Potential reactivity as alkaline cement. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Mallikarjuna Rao, G.; Gunneswara Rao, T.D. A quantitative method of approach in designing the mix proportions of fly ash and GGBS-based geopolymer concrete. Aust. J. Civ. Eng. 2018, 16, 53–63. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Mandal, S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Constr. Build. Mater. 2014, 70, 453–459. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Mechanical and durability properties of fly ash geopolymer concrete containing recycled coarse aggregates. Int. J. Sustain. Built Environ. 2016, 5, 277–287. [Google Scholar] [CrossRef]
- Poloju, K.K.; Srinivasu, K. Influence of GGBS and concentration of sodium hydroxide on strength behavior of geopolymer mortar. Mater. Today Proc. 2022, 65, 702–706. [Google Scholar] [CrossRef]
- Poloju, K.K.; Srinivasu, K. Impact of GGBS and strength ratio on mechanical properties of geopolymer concrete under ambient curing and oven curing. Mater. Today Proc. 2021, 42, 962–968. [Google Scholar] [CrossRef]
- Rathanasalam, V.; Perumalsami, J.; Jayakumar, K. Effect of ultrafine ground granulated blast-furnace slag (UFGGBFS) and copper slag on ambient cured geopolymer concrete. Ann. Chim.-Sci. Matériaux 2019, 43, 377–382. [Google Scholar] [CrossRef]
- Patankar, S.V.; Jamkar, S.S.; Ghugal, Y.M. Effect of concentration of sodium hydroxide and degree of heat curing on fly ash-based geopolymer mortar. Indian J. Mater. Sci. 2014, 2014, 938789. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutiérrez, R. Evolution of binder structure in sodium silicate-activated slag–metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Use of OPC to improve setting and early strength properties of low-calcium fly ash geopolymer concrete cured at room temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Patankar, S.V.; Ghugal, Y.M.; Jamkar, S.S. Mix Design of Fly Ash Based Geopolymer Concrete. In Advances in Structural Engineering; Matsagar, V., Ed.; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Shaikh, F.; Supit, S. Mechanical and durability properties of high volume fly ash (HVFA) concrete containing calcium carbonate (CaCO3) nanoparticles. Constr. Build. Mater. 2014, 70, 309–321. [Google Scholar] [CrossRef]
- Adam, A.; Horianto, X.X.X. The Effect of Temperature and Duration of Curing on the Strength of Fly Ash Based Geopolymer Mortar. Procedia Eng. 2014, 95, 410–414. [Google Scholar] [CrossRef]
- Law, D.; Adam, A.; Molyneaux, T.; Patnaikuni, I.; Wardhono, A. Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 2014, 48, 721–731. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T. Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 2017, 86, 9–18. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Poloju, K.K.; Al Banna, W.N. Microstructure Studies and Strength Determination of Different Binder Contents of Geopolymer Concrete. In Advancements in Science and Technology for Healthcare, Agriculture, and Environmental Sustainability; Karras, D.A., Thakur, S., Oruganti, S.K., Eds.; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-032-70832-4. [Google Scholar]
- Al Banna, W.N.; Poloju, K.K. Study on Characteristics of Geopolymer Concrete. In Recent Advances in Civil Engineering; Kumar, P.G., Subramaniam, K.V.L., Santhakumar, S.M., Satyam, D.N., Eds.; Lecture Notes in Civil Engineering; Springer: Singapore, 2022; Volume 233, pp. 539–551. ISSN 2366-2557. [Google Scholar]
- Wong, L.S. Durability Performance of Geopolymer Concrete: A Review. Polymers 2022, 14, 868. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Shi, X.; Feng, Y.; Zhang, Y.; Su, Y. A comprehensive investigation on sulphate resistance of geopolymer recycled concrete: Macro and micro properties. Constr. Build. Mater. 2023, 403, 133052. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L. Experimental Study of Geopolymer Concrete Produced from Waste Concrete. J. Mater. Civ. Eng. 2019, 31, 04019114. [Google Scholar] [CrossRef]
- Vora, P.R.; Dave, U.V. Parametric studies on compressive strength of geopolymer concrete. Procedia Eng. 2013, 51, 210–219. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Patankar, S.; Jamkar, S.; Ghugal, Y. Effect of Water-to-Geopolymer Binder Ratio on the Production of Fly ash Based Geopolymer Concrete. Int. J. Adv. Technol. Civ. Eng. 2013, 2, 79–83. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F. Synthesis of heat and ambient cured one-part geopolymer mixes with different Grades of sodium silicate. Ceram. Int. 2015, 41, 5696–5704. [Google Scholar] [CrossRef]
- Olayinka, R.; Jafari, R.; Fiset, M. Shrinkage Characteristics of Geopolymer Concrete: A Comprehensive Review. Materials 2025, 18, 4528. [Google Scholar] [CrossRef]
- Olivia, M.; Nikraz, H. Properties of fly ash geopolymer concrete designed by Taguchi method. Mater. Des. 2011, 36, 191–198. [Google Scholar] [CrossRef]
- Salas, D.; Ramirez, A.; Ulloa, N.; Baykara, H.; Boero, A. Life cycle assessment of geopolymer concrete. Constr. Build. Mater. 2018, 190, 170–177. [Google Scholar] [CrossRef]
- Vasudevareddy, P.; Reddy, K. Effect of graphene oxide and nano silica on mechanical and durability properties of cement mortar. Mater. Today Proc. 2022, 60, 1042–1050. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).