Eco-Friendly Flame-Retardant Construction Composites Based on Bio-Based TPU, Recycled Rice Husk, and Ammonium Polyphosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Materials
2.3. Characterization and Property Test
3. Results and Discussion
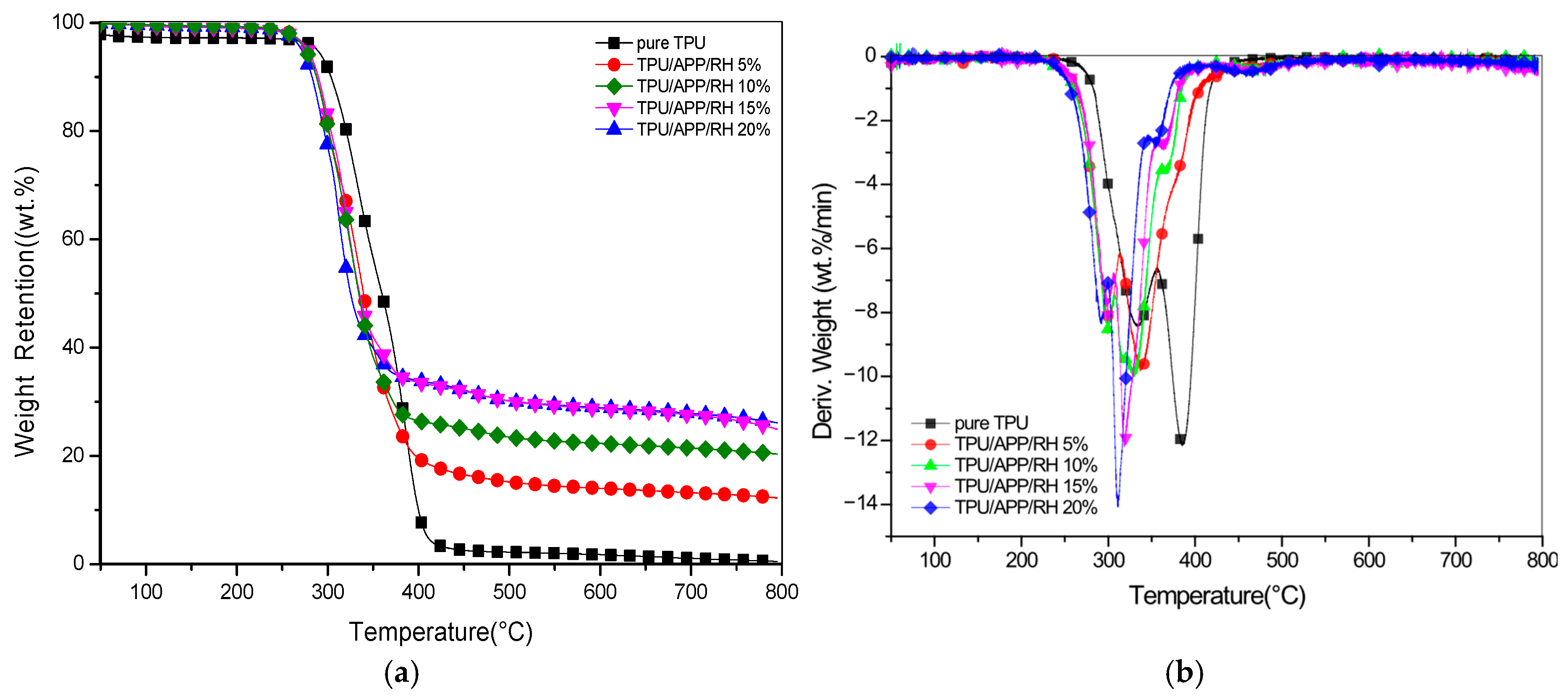
3.1. TGA Analyses
3.2. Toxicity Analyses
Thermogravimetric Analysis–Fourier Transform Infrared Spectroscopy
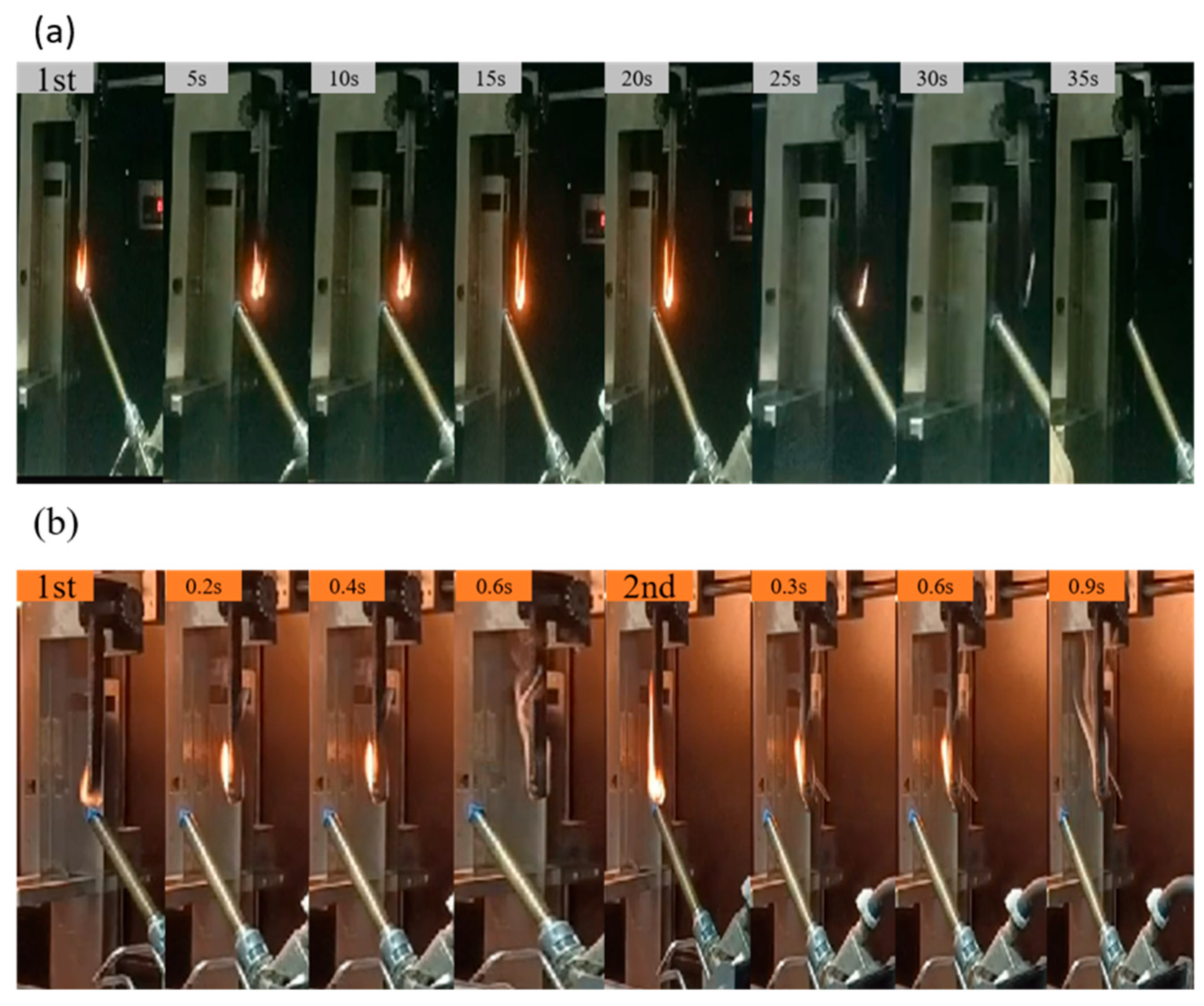
3.3. Flame-Retardant Properties
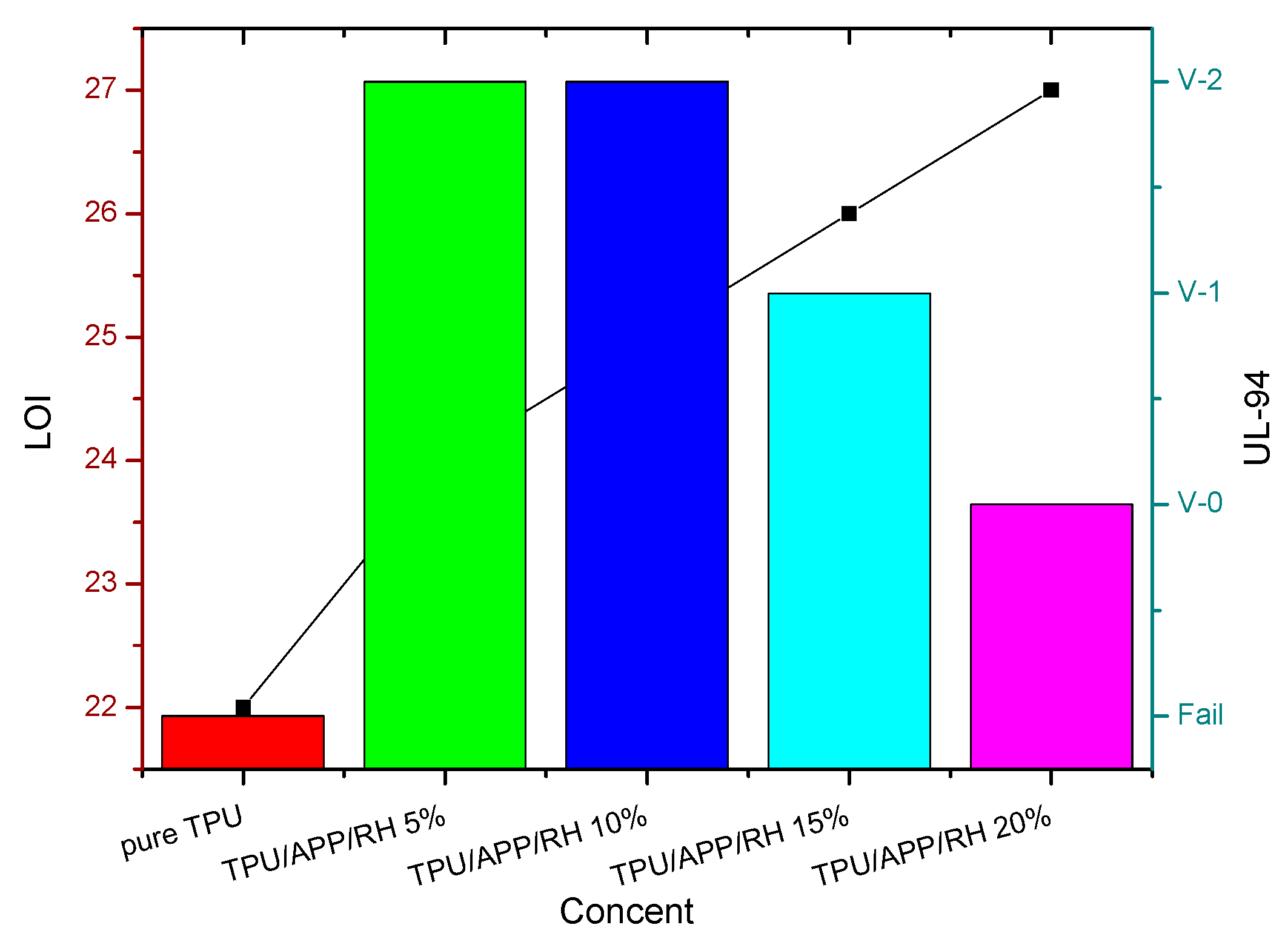
3.3.1. UL-94 and LOI Testing
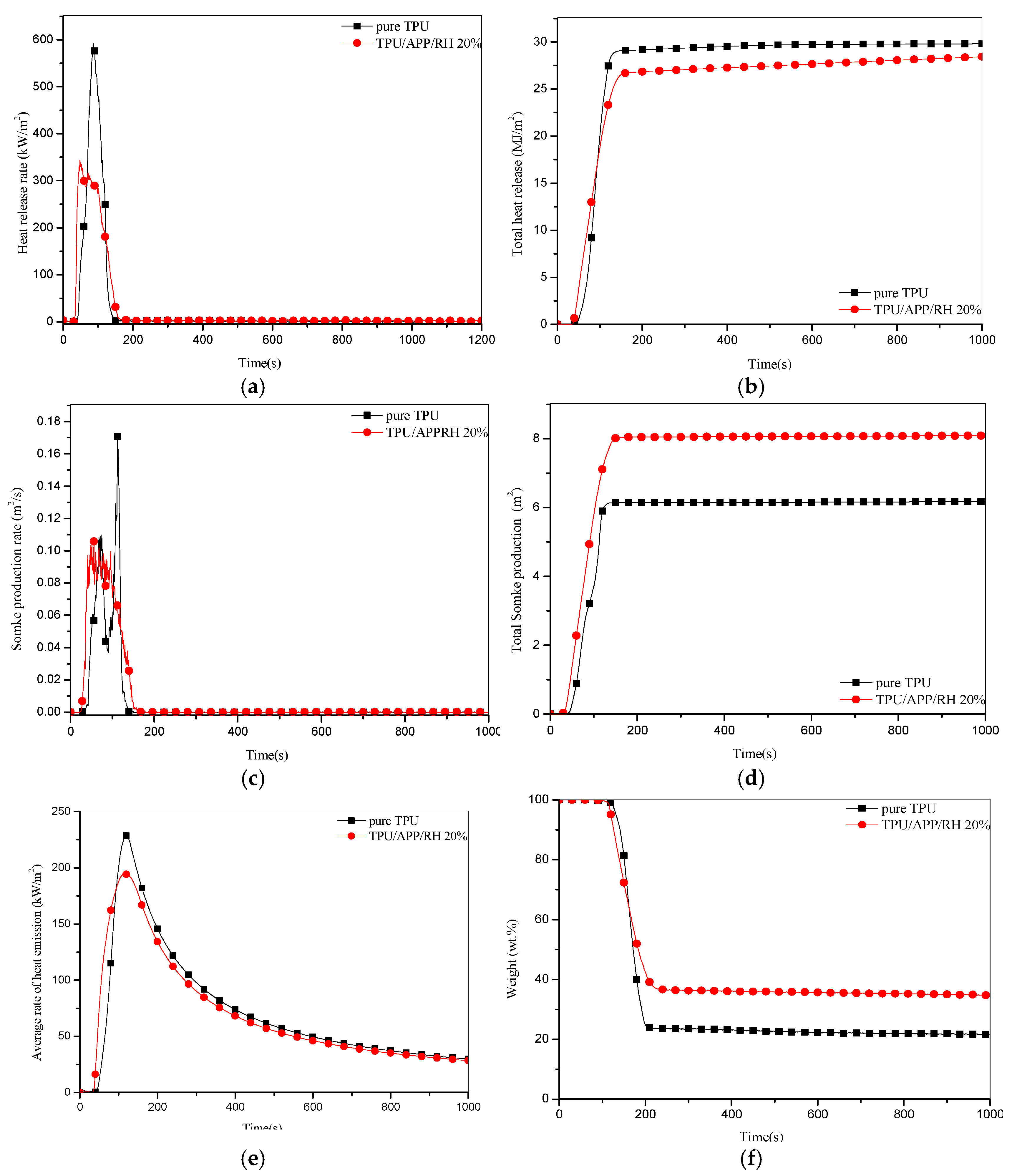
3.3.2. Cone Calorimetry Analysis
3.4. Morphology
3.4.1. SEM
3.4.2. Elemental Analysis Before and After Combustion
3.5. Char Residue Analysis
3.5.1. XPS Analysis of Flame-Retardant Mechanism
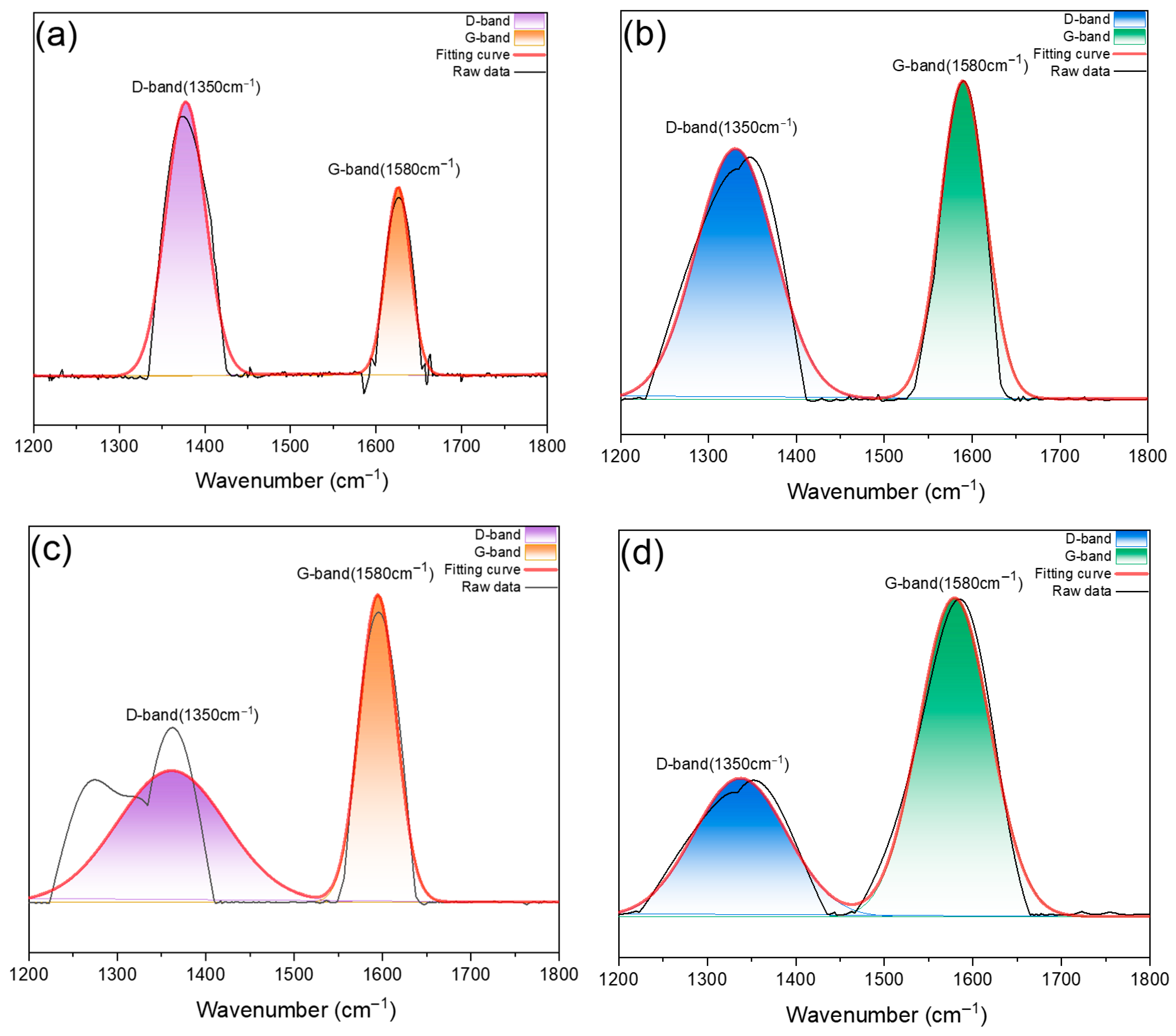
3.5.2. Raman Spectra
3.6. TPU/APP/RH Flame-Retardant Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Liu, C.; Duan, Z.; Yu, B.; Liu, M.; Song, P. Interface engineering of MXene towards super-tough and strong polymer nanocomposites with high ductility and excellent fire safety. Chem. Eng. J. 2020, 399, 125829. [Google Scholar] [CrossRef]
- Parcheta, P.; Głowińska, E.; Datta, J. Effect of bio-based components on the chemical structure, thermal stability and mechanical properties of green thermoplastic polyurethane elastomers. Eur. Polym. J. 2020, 123, 109422. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, H.; Guo, J.; Sun, J.; Li, H.; Zhang, S.; Gu, X. Preparation of cobalt-based metal organic framework and its application as synergistic flame retardant in thermoplastic polyurethane (TPU). Compos. Part B Eng. 2020, 182, 107498. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P.; Liang, K.; Yuan, B. Surface micro-regulation for ammonium polyphosphate: Construction of a fire-safe thermoplastic polyurethane composite based on smoke suppression strategy of char layer reinforcement. Powder Technol. 2025, 464, 121272. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, C.; Ai, Y.; Tang, L.; Cao, K. Phytate-based transparent and waterproof intumescent flame-retardant coating for protection of wood. Mater. Chem. Phys. 2023, 294, 127000. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.; Zhang, W.; Xu, X.; Lin, H.; Wang, W. One-spot synthesis of a benzene-rich triazine-based hyperbranched charring agent and its efficient intumescent flame retardant performance for thermoplastic polyester elastomer. Arab. J. Chem. 2023, 16, 104861. [Google Scholar] [CrossRef]
- Moghaddam, M.R.A.; Hesarinejad, M.A.; Javidi, F. Characterization and optimization of polylactic acid and polybutylene succinate blend/starch/wheat straw biocomposite by optimal custom mixture design. Polym. Test. 2023, 121, 108000. [Google Scholar] [CrossRef]
- Sonseca, A.; McClain, A.; Puskas, J.E.; El Fray, M. Kinetic studies of biocatalyzed copolyesters of poly(butylene succinate) (PBS) containing fully bio-based dilinoleic diol. Eur. Polym. J. 2019, 116, 515–525. [Google Scholar] [CrossRef]
- Phiri, M.J.; Mofokeng, J.P.; Phiri, M.M.; Mngomezulu, M.; Tywabi-Ngeva, Z. Chemical, thermal and morphological properties of polybutylene succinate-waste pineapple leaf fibres composites. Heliyon 2023, 9, e21238. [Google Scholar] [CrossRef]
- Zuo, X.; Zhou, Y.; Hao, K.; Liu, C.; Yu, R.; Huang, A.; Wu, C.; Yang, Y. 3D Printed All-Natural Hydrogels: Flame-Retardant Materials Toward Attaining Green Sustainability. Adv. Sci. 2024, 11, 2306360. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Chang, C.-W.; Hsu, T.-H.; Dai, W.-Y. Application of Sustainable Wood-Plastic Composites in Energy-Efficient Construction. Buildings 2024, 14, 958. [Google Scholar] [CrossRef]
- Wang, L.; Lv, Y.; Wang, T.; Wan, S.; Ye, Y. Assessment of the impacts of the life cycle of construction waste on human health: Lessons from developing countries. Constr. Archit. Manag. 2025, 32, 1348–1369. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Guo, Y.; Ren, Y.; Liu, X. Preparation of flame retardant, smoke suppression and reinforced polyacrylonitrile composite fiber by using fully biomass intumescent flame retardant system and its sustainable recycle application. Compos. Part A Appl. Sci. Manuf. 2023, 173, 107705. [Google Scholar] [CrossRef]
- Khanal, S.; Zhang, W.; Ahmed, S.; Ali, M.; Xu, S. Effects of intumescent flame retardant system consisting of tris(2-hydroxyethyl) isocyanurate and ammonium polyphosphate on the flame retardant properties of high-density polyethylene composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 444–451. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zhang, K.T.; Wang, J.R.; Wang, Y.Z. Biopolymer-Based Flame Retardants and Flame-Retardant Materials. Adv. Mater. 2025, 37, 2414880. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yin, G.-Z.; Yang, Y.; Fu, W.; Palencia, J.L.D.; Zhao, J.; Wang, N.; Jiang, Y.; Wang, D.-Y. Bio-based flame retardants to polymers: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 132–155. [Google Scholar] [CrossRef]
- Wan, M.; Shi, C.; Qian, X.; Qin, Y.; Jing, J.; Che, H.; Ren, F.; Li, J.; Yu, B.; Zhou, K. Design of novel double-layer coated ammonium polyphosphate and its application in flame retardant thermoplastic polyurethanes. Chem. Eng. J. 2023, 459, 141448. [Google Scholar] [CrossRef]
- Shao, Z.B.; Deng, C.; Tan, Y.; Chen, M.J.; Li, C.; Wang, Y.Z. An efficient monocomponent polymeric intumescent flame retardant for polypropylene: Preparation and application. ACS Appl. Mater. Interfaces 2014, 6, 7363–7370. [Google Scholar] [CrossRef]
- Savini, F. Futures of the social metabolism: Degrowth, circular economy and the value of waste. Futures 2023, 150, 103180. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Z.; Li, Y.; Lang, X.; Zong, C.; Cao, L. Synergistic thermodynamic compatibility of polydimethylsiloxane block in thermoplastic polyurethane for flame retardant materials: Super flexible, highly flame retardant and low smoke release. Polymer 2022, 253, 124976. [Google Scholar] [CrossRef]
- Taib, M.N.A.M.; Antov, P.; Savov, V.; Fatriasari, W.; Madyaratri, E.W.; Wirawan, R.; Osvaldová, L.M.; Hua, L.S.; Ghani, M.A.A.; Al Edrus, S.S.A.O. Current progress of biopolymer-based flame retardant. Polym. Degrad. Stab. 2022, 205, 110153. [Google Scholar] [CrossRef]
- Aramwit, P.; Chin Vui Sheng, D.; Krishna Moorthy, G.; Guna, V.; Reddy, N. Rice husk and coir fibers as sustainable and green reinforcements for high performance gypsum composites. Constr. Build. Mater. 2023, 393, 110663. [Google Scholar] [CrossRef]
- ASTM D3801; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM International: West Conshohocken, PA, USA, 2010.
- Arjmandi, R.; Ismail, A.; Hassan, A.; Bakar, A.A. Effects of ammonium polyphosphate content on mechanical, thermal and flammability properties of kenaf/polypropylene and rice husk/polypropylene composites. Constr. Build. Mater. 2017, 152, 484–493. [Google Scholar] [CrossRef]
- Xie, M.; He, J.; Li, X.; Yang, R. Ammonium polyphosphate/montmorillonite nanocomposite with a completely exfoliated structure and charring–foaming agent flame retardant thermoplastic polyurethane. Mater. Sci. Eng. B 2022, 283, 115825. [Google Scholar] [CrossRef]
- Chen, X.; Cai, D.; Yang, Y.; Sun, Y.; Wang, B.; Yao, Z.; Jin, M.; Liu, J.; Reinmöller, M.; Badshah, S.L.; et al. Pyrolysis kinetics of bio-based polyurethane: Evaluating the kinetic parameters, thermodynamic parameters, and complementary product gas analysis using TG/FTIR and TG/GC–MS. Renew. Energy 2023, 205, 490–498. [Google Scholar] [CrossRef]
- Yu, D.; Hu, S.; Liu, W.; Wang, X.; Jiang, H.; Dong, N. Pyrolysis of oleaginous yeast biomass from wastewater treatment: Kinetics analysis and biocrude characterization. Renew. Energy 2020, 150, 831–839. [Google Scholar] [CrossRef]
- Chen, X.; Huo, L.; Jiao, C.; Li, S. TG–FTIR characterization of volatile compounds from flame retardant polyurethane foams materials. J. Anal. Appl. Pyrolysis 2013, 100, 186–191. [Google Scholar] [CrossRef]
- Feng, L.; Wang, W.; Song, B.; Zhu, X.; Wang, L.; Shao, R.; Li, T.; Pei, X.; Wang, L.; Qian, X.; et al. Synthesis of P, N and Si-containing waterborne polyurethane with excellent flame retardant, alkali resistance and flexibility via one-step synthetic approach. Prog. Org. Coat. 2023, 174, 107286. [Google Scholar] [CrossRef]
- Ding, D.; Liu, Y.; Lu, Y.; Liao, Y.; Chen, Y.; Zhang, G.; Zhang, F. Highly effective and durable P–N synergistic flame retardant containing ammonium phosphate and phosphonate for cotton fabrics. Polym. Degrad. Stab. 2022, 200, 109964. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Sun, M.; Deng, G.; Jing, B.; Wang, K.; Shi, Y.; Fu, L.; Feng, Y.; Lv, Y.; et al. Phosphorous–Nitrogen flame retardants engineering MXene towards highly fire safe thermoplastic polyurethane. Compos. Commun. 2022, 29, 101197. [Google Scholar] [CrossRef]
- Qin, Y.; Li, M.; Huang, T.; Shen, C.; Gao, S. A study on the modification of polypropylene by a star-shaped intumescent flame retardant containing phosphorus and nitrogen. Polym. Degrad. Stab. 2022, 195, 109979. [Google Scholar] [CrossRef]
- Qin, R.; Song, Y.; Niu, M.; Xue, B.; Liu, L. Construction of flame retardant coating on polyester fabric with ammonium polyphosphate and carbon microspheres. Polym. Degrad. Stab. 2020, 171, 109074. [Google Scholar] [CrossRef]
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame retardancy index for thermoplastic composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef]
- Baath, Y.S.; Nikrityuk, P.A.; Gupta, R. Experimental and numerical verifications of biochar gasification kinetics using TGA. Renew. Energy 2022, 185, 717–733. [Google Scholar] [CrossRef]
- Xu, P.; Luo, Y.; Zhang, P. Interfacial architecting of organic–inorganic hybrid toward mechanically reinforced, fire-resistant and smoke-suppressed polyurethane composites. J. Colloid Interface Sci. 2022, 621, 385–397. [Google Scholar] [CrossRef]
- Guo, K.Y.; Wu, Q.; Mao, M.; Chen, H.; Zhang, G.D.; Zhao, L.; Gao, J.F.; Song, P.; Tang, L.C. Water-based hybrid coatings toward mechanically flexible, super-hydrophobic and flame-retardant polyurethane foam nanocomposites with high-efficiency and reliable fire alarm response. Compos. Part B Eng. 2020, 193, 108121. [Google Scholar] [CrossRef]
- Zhang, P.; Fan, H.; Hu, K.; Gu, Y.; Chen, Y.; Yan, J.; Tian, S.; He, Y. Solvent-free two-component polyurethane conjugated with crosslinkable hydroxyl-functionalized ammonium polyphosphate: Curing behaviors, flammability and mechanical properties. Prog. Org. Coat. 2018, 120, 88–99. [Google Scholar] [CrossRef]
- Xue, B.; Yang, S.; Qin, R.; Deng, S.; Niu, M.; Zhang, L. Effect of a graphene-APP composite aerogel coating on the polyester fabric for outstanding flammability. Prog. Org. Coat. 2022, 172, 107346. [Google Scholar] [CrossRef]
- Sun, H.; Chen, K.; Liu, Y.; Wang, Q. Improving flame retardant and smoke suppression function of ethylene vinyl acetate by combining the piperazine pyrophosphate, expandable graphite and melamine phosphate. Eur. Polym. J. 2023, 194, 112408. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.J.; Li, P.; Zhang, F.Q.; Liu, Y.; Zhu, P. Flame-retardant polyester/cotton blend with phosphorus/nitrogen/silicon-containing nano-coating by layer-by-layer assembly. Appl. Surf. Sci. 2020, 509, 145323. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Tarani, E.; Ainali, N.M.; Chrissafis, K.; Wurzer, C.; Mašek, O.; Bikiaris, D.N. The Effect of Biochar Addition on Thermal Stability and Decomposition Mechanism of Poly(butylene succinate) Bionanocomposites. Molecules 2023, 28, 5330. [Google Scholar] [CrossRef] [PubMed]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Gengembre, L. XPS study of an intumescent coating: II. Application to the ammonium polyphosphate/pentaerythritol/ethylenic terpolymer fire retardant system with and without synergistic agent. Appl. Surf. Sci. 1997, 120, 15–29. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Z.; Wang, J.; Wang, H.; Wei, H.; Wang, Q.; Wang, C.; Shen, Q. Constructing magnetic iron-based core-shell structure and dielectric nitrogen-doped reduced graphene oxide nanocomposite for enhanced microwave absorption performance. Appl. Surf. Sci. 2023, 607, 155013. [Google Scholar] [CrossRef]
- Gu, H.; Liu, C.; Liu, Q.; Jia, H.; Qiao, Y.; Zhao, W.; Chen, Y.; Jian, X. High- and low-temperature resistant and intrinsically flame retardant poly(bisphthalazinone thioether sulfone ketone)s: Synthesis, structures and properties. Chem. Eng. J. 2023, 471, 144480. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, G.; Zhong, F.; Dong, S.; Yang, Z.; Wang, M.; Zou, R. Silicon oxide encapsulated ZIF-8 loaded on reduced graphene oxide to improve flame retardancy of waterborne epoxy coatings. Prog. Org. Coat. 2022, 163, 106683. [Google Scholar] [CrossRef]
- Pan, M.; Mei, C.; Du, J.; Li, G. Synergistic effect of nano silicon dioxide and ammonium polyphosphate on flame retardancy of wood fiber–polyethylene composites. Compos. Part A Appl. Sci. Manuf. 2014, 66, 128–134. [Google Scholar] [CrossRef]
- Wu, T.; Yang, F.; Tao, J.; Zhao, H.B.; Yu, C.; Rao, W. Design of P-decorated POSS towards flame-retardant, mechanically-strong, tough and transparent epoxy resins. J. Colloid Interface Sci. 2023, 640, 864–876. [Google Scholar] [CrossRef] [PubMed]



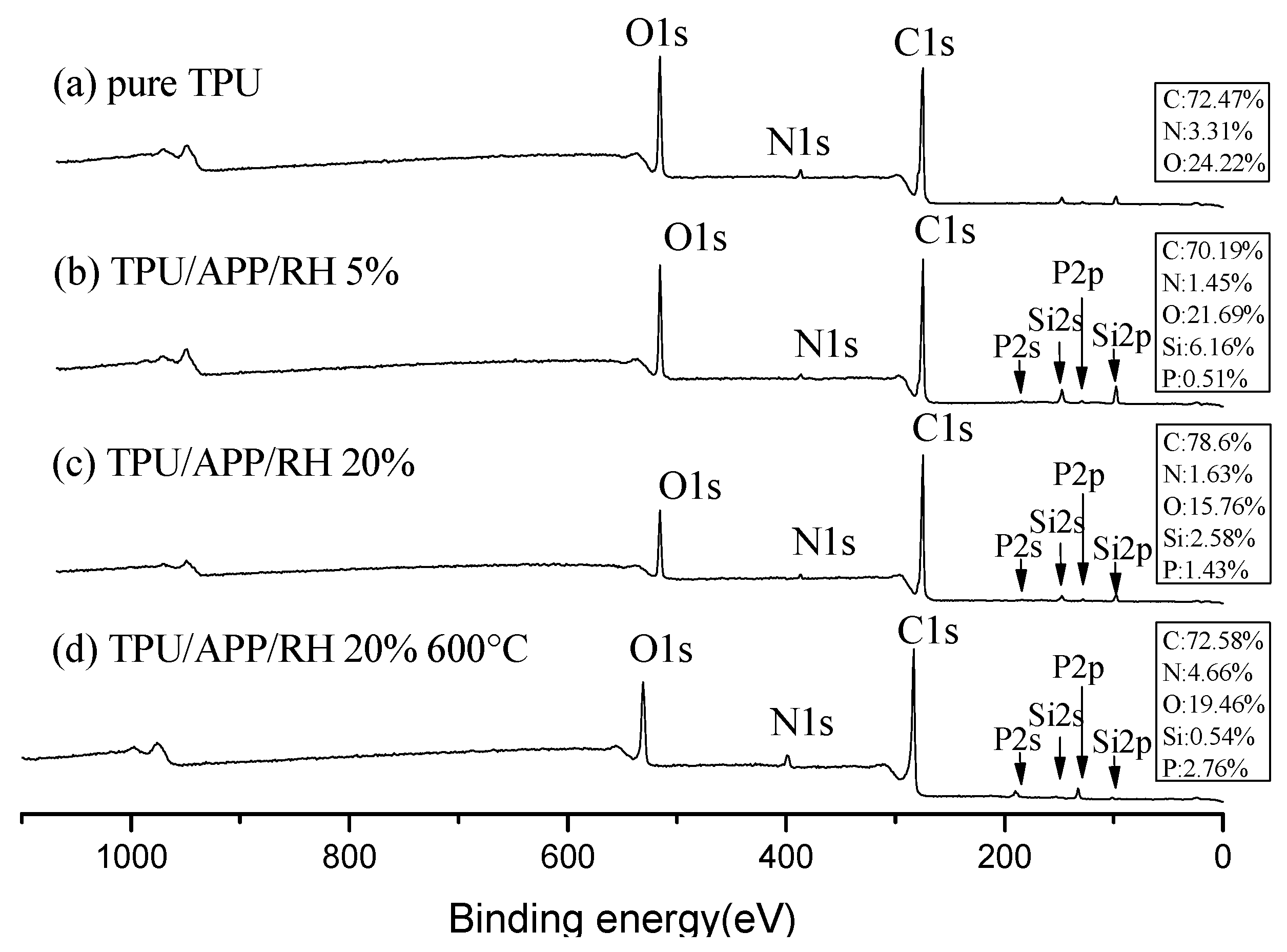
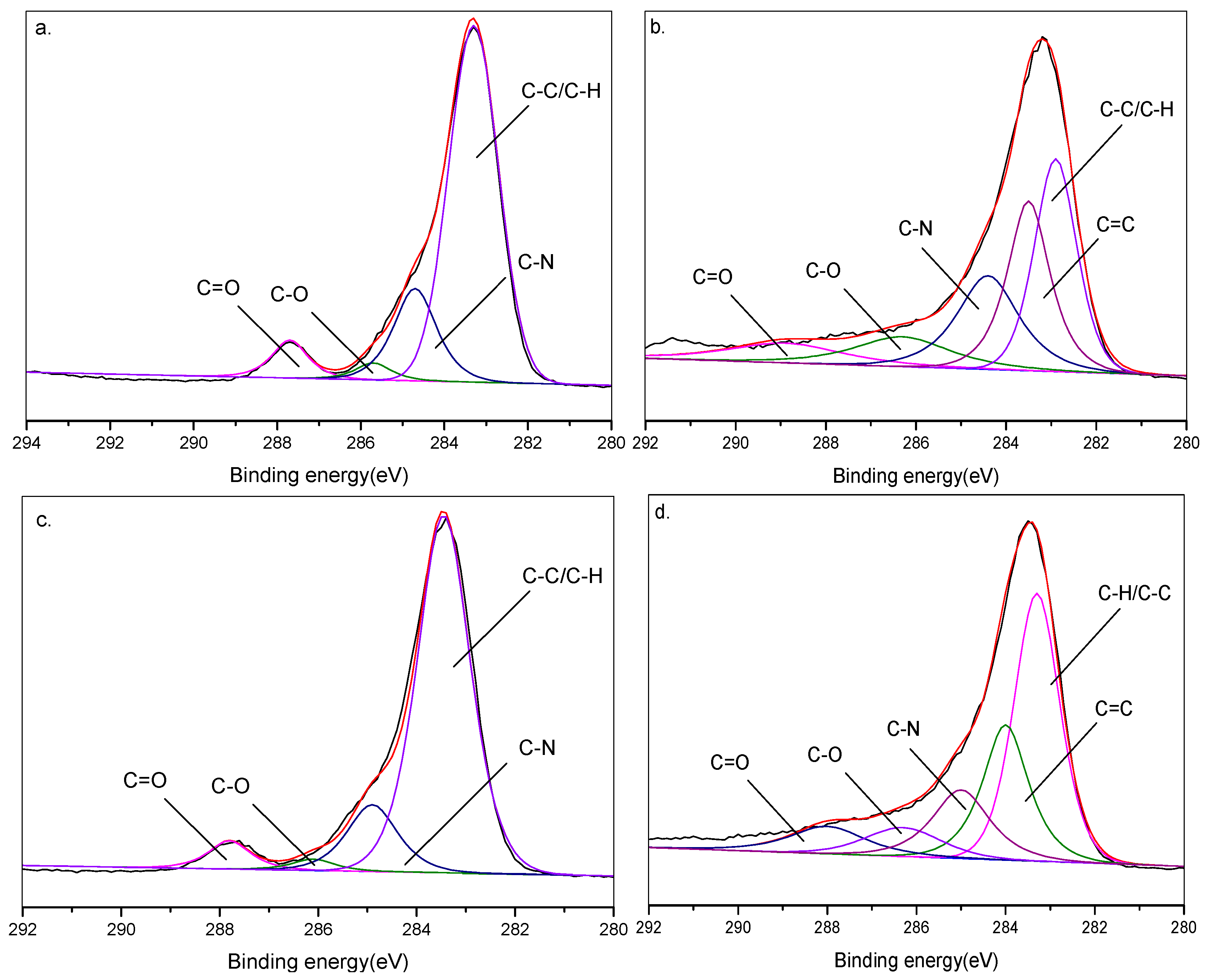
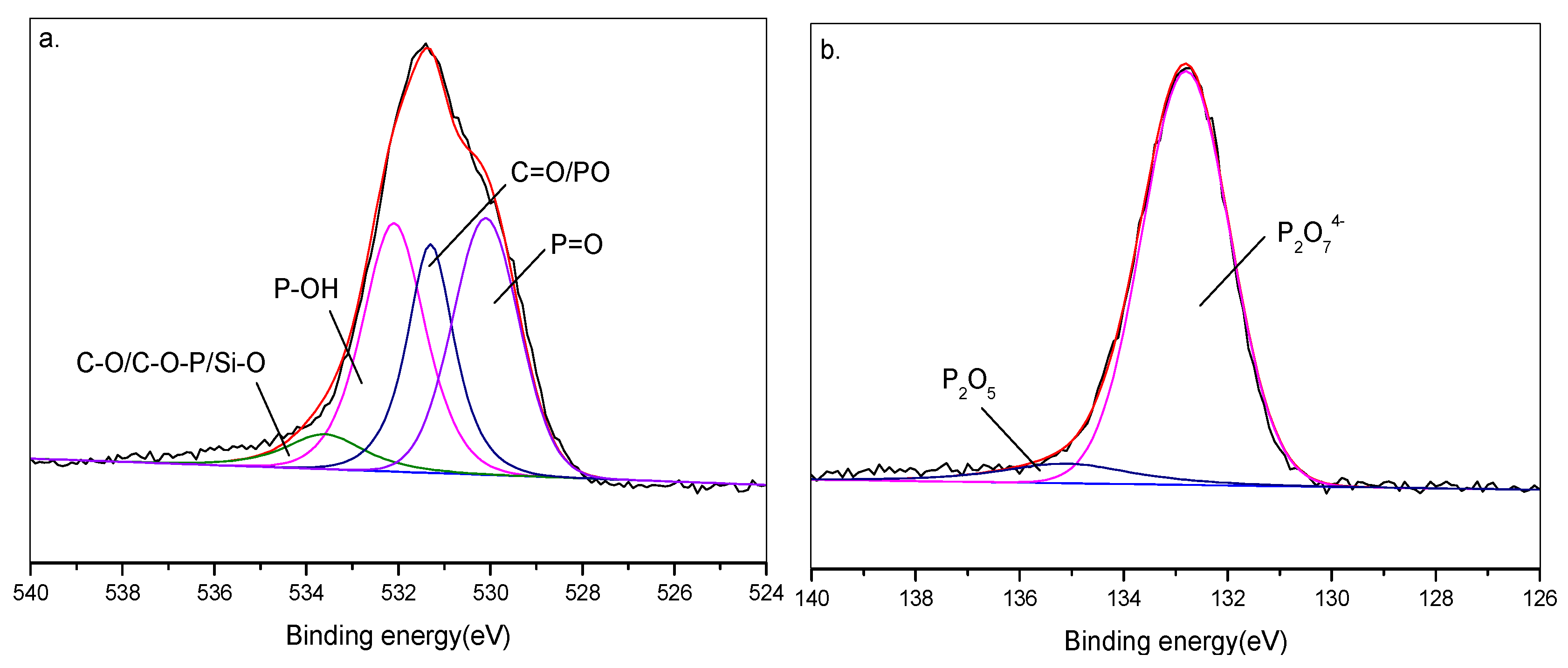
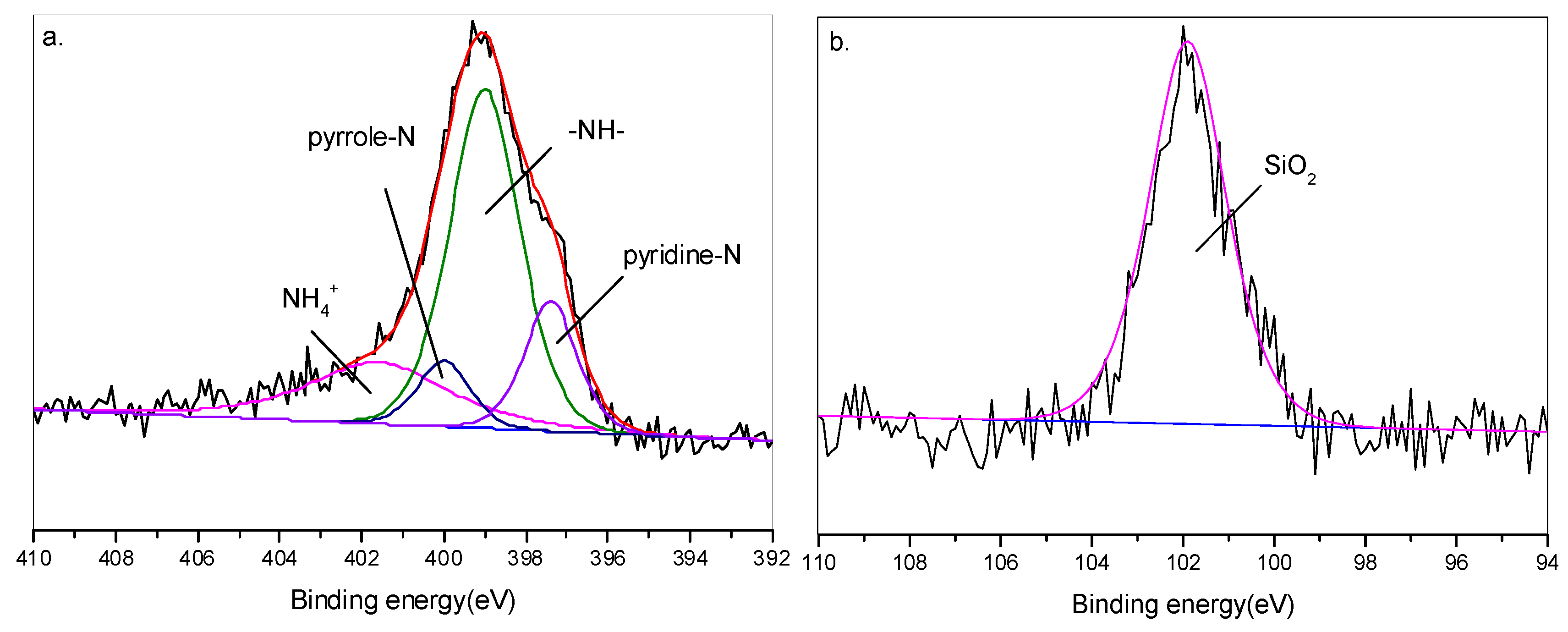

| Sample | TPU (wt%) | APP (wt%) | RH (wt%) |
|---|---|---|---|
| Pure TPU | 100 | 0 | 0 |
| TPU/APP/RH 5% | 95 | 3.33 | 1.67 |
| TPU/APP/RH 10% | 90 | 6.67 | 3.33 |
| TPU/APP/RH 15% | 85 | 10 | 5 |
| TPU/APP/RH 20% | 80 | 13.33 | 6.67 |
| Sample | Td5 a (°C) | Tmax b (°C) | Rmax c (wt%/min) | C.Y. (wt%) |
|---|---|---|---|---|
| Neat TPU | 294 | 385 | −12.16 | 0.51 |
| TPU/APP/RH 5% | 277 | 337 | −9.72 | 12.2 |
| TPU/APP/RH 10% | 276 | 328 | −9.86 | 20.4 |
| TPU/APP/RH 15% | 278 | 320 | −12.09 | 24.9 |
| TPU/APP/RH 20% | 272 | 312 | −13.96 | 26.1 |
| Sample | UL-94 | LOI (%) | |||
|---|---|---|---|---|---|
| t1 (s) | t2 (s) | Dripping | Ranking | ||
| Pure TPU | BC a | - | Yes | Fail | 22 |
| TPU/APP/RH 5% | 3.8 ± 0.1 | 1.6 ± 0.2 | Yes | V-2 | 24 |
| TPU/APP/RH 10% | 0.9 ± 0.1 | 1.9 ± 0.1 | Yes | V-2 | 25 |
| TPU/APP/RH 15% | 0.8 ± 0.1 | 0.7 ± 0.1 | Yes | V-1 | 26 |
| TPU/APP/RH 20% | 0.6 ± 0.1 | 0.9 ± 0.1 | NO | V-0 | 27 |
| Sample | Pure TPU | TPU/APP/RH 20% |
|---|---|---|
| TTI (s) | 39 | 34 |
| pHRR (kw/m2) | 593 | 344 |
| tpHRR (s) | 86 | 48 |
| THR (MJ/m2) | 29.3 | 27 |
| EHC (MJ/Kg) | 20.3 | 16.6 |
| FRI | - | 1.6 |
| pSPR (m2/s2) | 0.171 | 0.106 |
| TSP (m2) | 6.1 | 7.9 |
| MARHE (kw/m2) | 228.8 | 194.4 |
| Weight residue (wt%) | 22.6 | 34.8 |
| FPI (m2s/kw) | 0.066 | 0.099 |
| FGI (kw/m2s) | 6.9 | 7.2 |
| Sample | Elements (wt%) | ||||
|---|---|---|---|---|---|
| C | O | N | Si | P | |
| TPU/APP/RH 5% (before burning) | 59.62 | 34.54 | 4.91 | 0.06 | 0.88 |
| TPU/APP/RH 5% (after burning) | 58.06 | 35.01 | 4.66 | 0.14 | 2.14 |
| TPU/APP/RH 20%(before burning) | 53.63 | 33.77 | 8.01 | 0.25 | 4.33 |
| TPU/APP/RH 20% (after burning) | 52.06 | 38.33 | 5.17 | 0.20 | 4.24 |
| Sample | Temperature | |
|---|---|---|
| RT | 600 °C | |
| TPU/APP/RH 5% | 0.69 | 0.57 |
| TPU/APP/RH 20% | 0.70 | 0.53 |
| Sample | D-Band | G-Band | ID/IG | |
|---|---|---|---|---|
| (1350 cm−1) | (1580 cm−1) | |||
| TPU/APP/RH 5% | 1min | 3249.4 | 1385.0 | 2.35 |
| 5 min | 78,841.4 | 58,192.2 | 1.35 | |
| TPU/APP/RH 20% | 1min | 79,809.1 | 63,712.4 | 1.25 |
| 5 min | 105,413.1 | 185,994.7 | 0.57 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuan, C.-F.; Yang, C.-Y.; Kuan, H.-C.; Chung, M.-C.; Shih, Y.-F. Eco-Friendly Flame-Retardant Construction Composites Based on Bio-Based TPU, Recycled Rice Husk, and Ammonium Polyphosphate. Buildings 2025, 15, 3420. https://doi.org/10.3390/buildings15183420
Kuan C-F, Yang C-Y, Kuan H-C, Chung M-C, Shih Y-F. Eco-Friendly Flame-Retardant Construction Composites Based on Bio-Based TPU, Recycled Rice Husk, and Ammonium Polyphosphate. Buildings. 2025; 15(18):3420. https://doi.org/10.3390/buildings15183420
Chicago/Turabian StyleKuan, Chen-Feng, Chane-Yuan Yang, Hsu-Chiang Kuan, Min-Chin Chung, and Yeng-Fong Shih. 2025. "Eco-Friendly Flame-Retardant Construction Composites Based on Bio-Based TPU, Recycled Rice Husk, and Ammonium Polyphosphate" Buildings 15, no. 18: 3420. https://doi.org/10.3390/buildings15183420
APA StyleKuan, C.-F., Yang, C.-Y., Kuan, H.-C., Chung, M.-C., & Shih, Y.-F. (2025). Eco-Friendly Flame-Retardant Construction Composites Based on Bio-Based TPU, Recycled Rice Husk, and Ammonium Polyphosphate. Buildings, 15(18), 3420. https://doi.org/10.3390/buildings15183420







