Cognitive Recovery of Young Males in Thermoneutral Indoor Environments: Effects of Sleep Restrictions
Abstract
1. Introduction
2. Materials and Methods
2.1. Brief Information About Participants
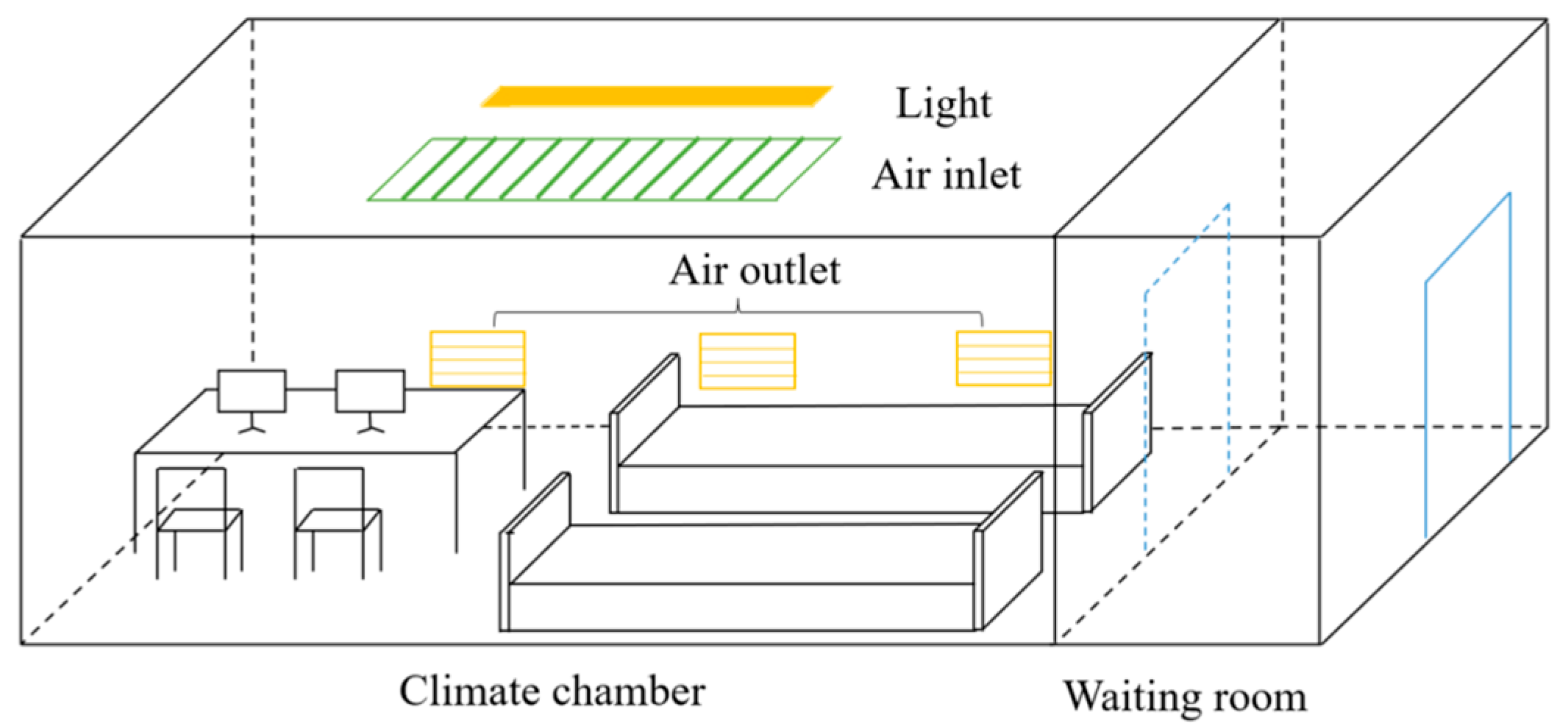
2.2. Experimental Conditions and Materials
2.3. Cognitive Tasks
2.4. Experimental Procedures
2.5. Data Analysis
3. Results
3.1. Results of Subjective Surveys
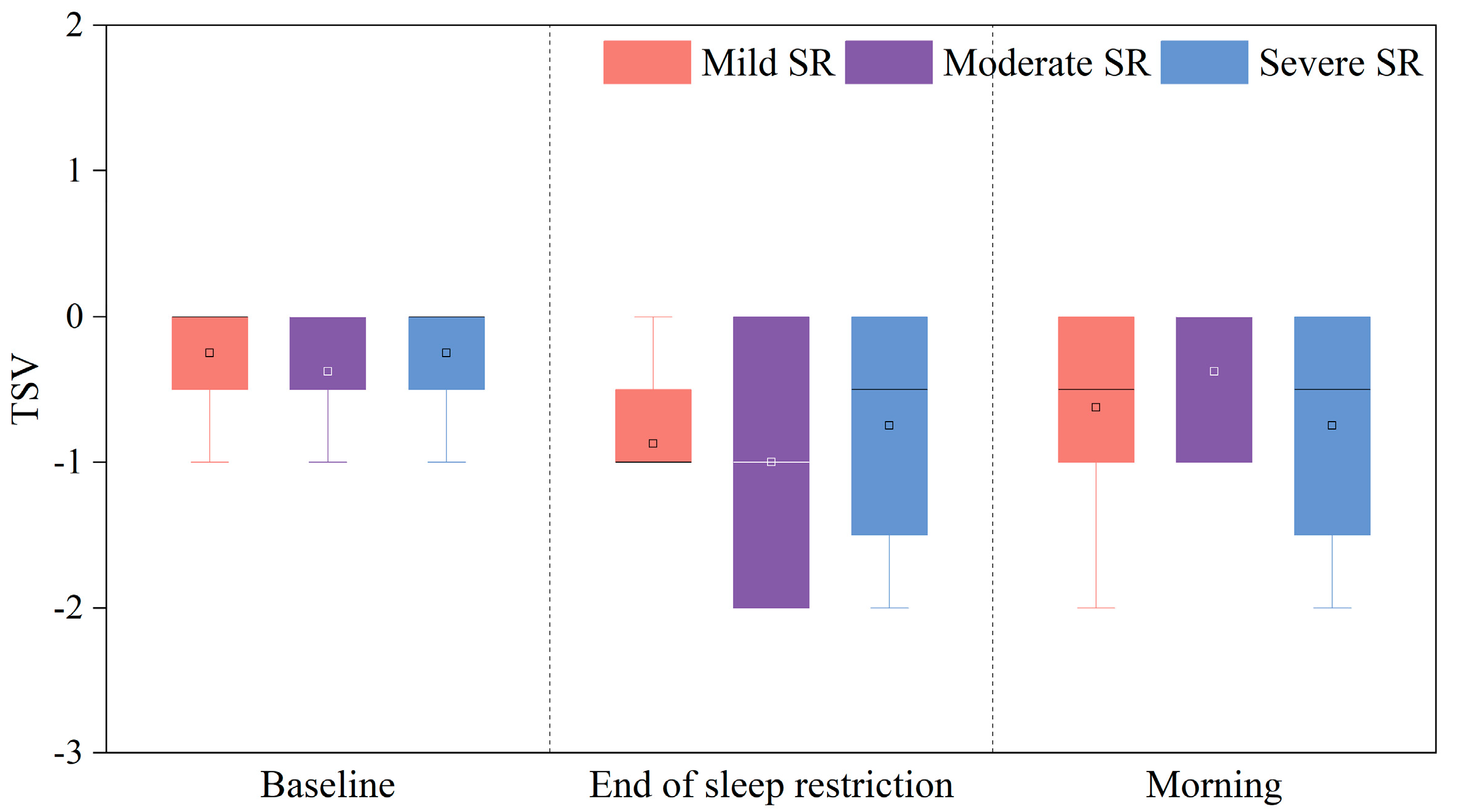
3.1.1. TSV
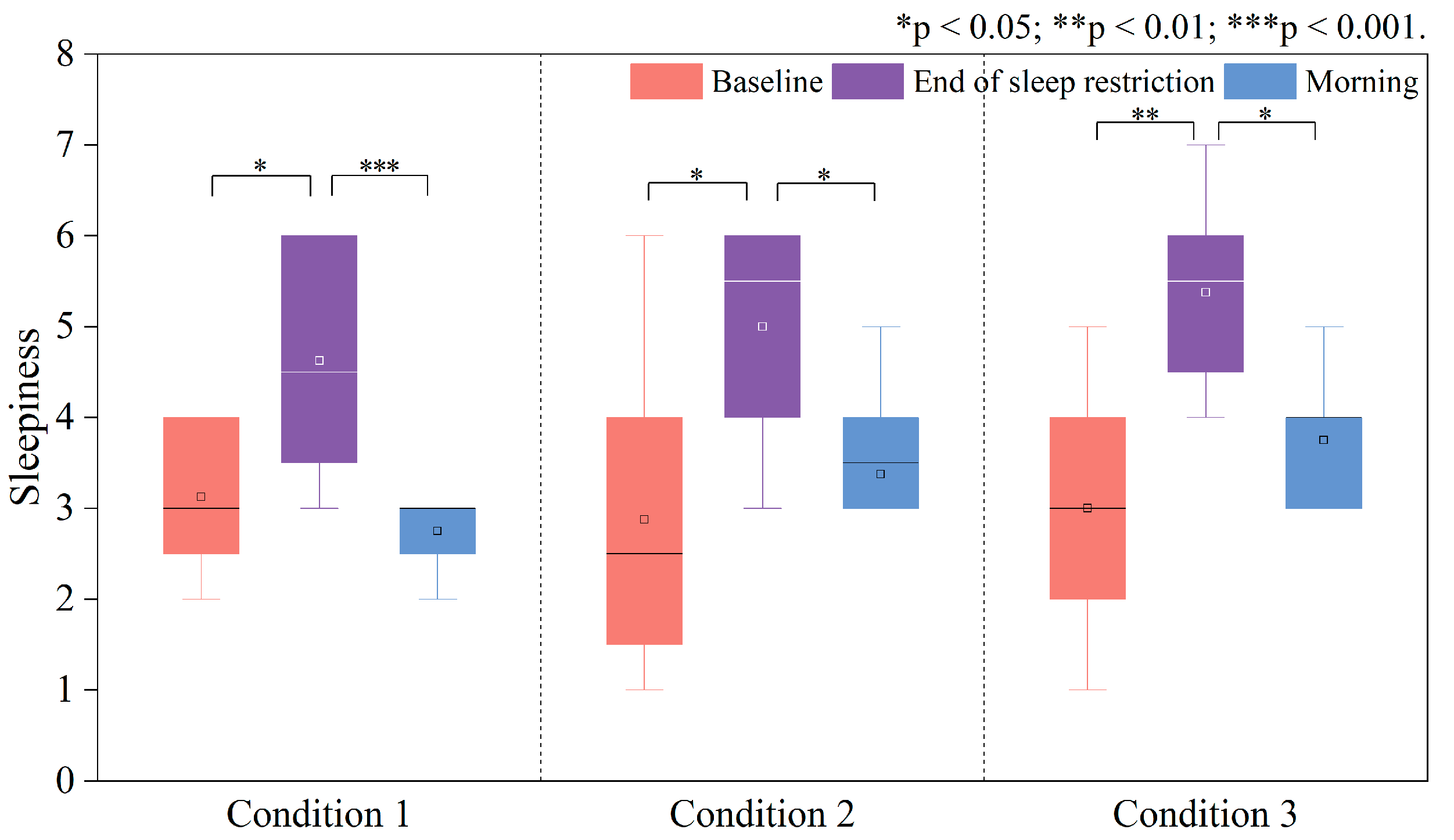
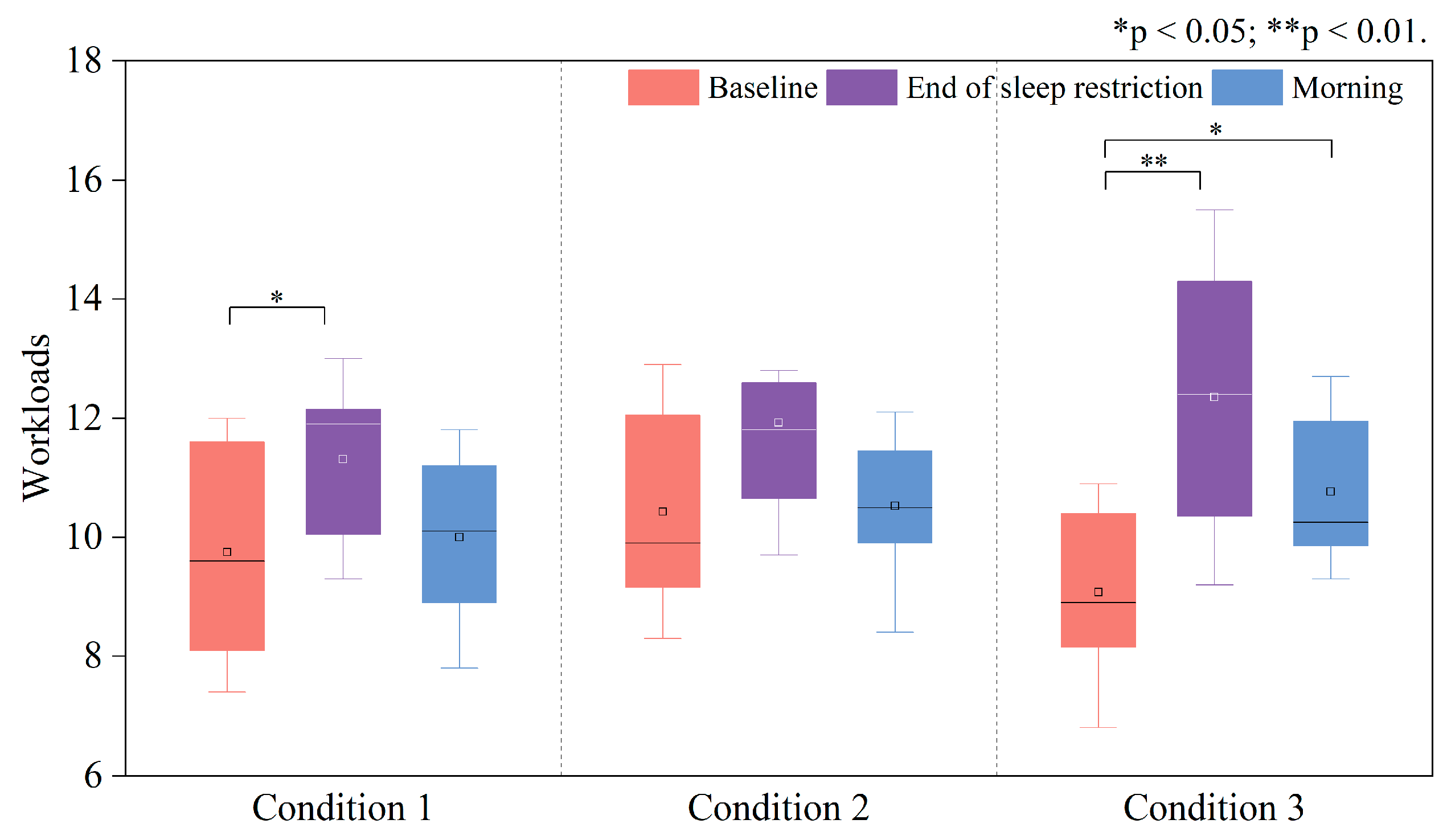
3.1.2. Sleepiness and Perceived Workload
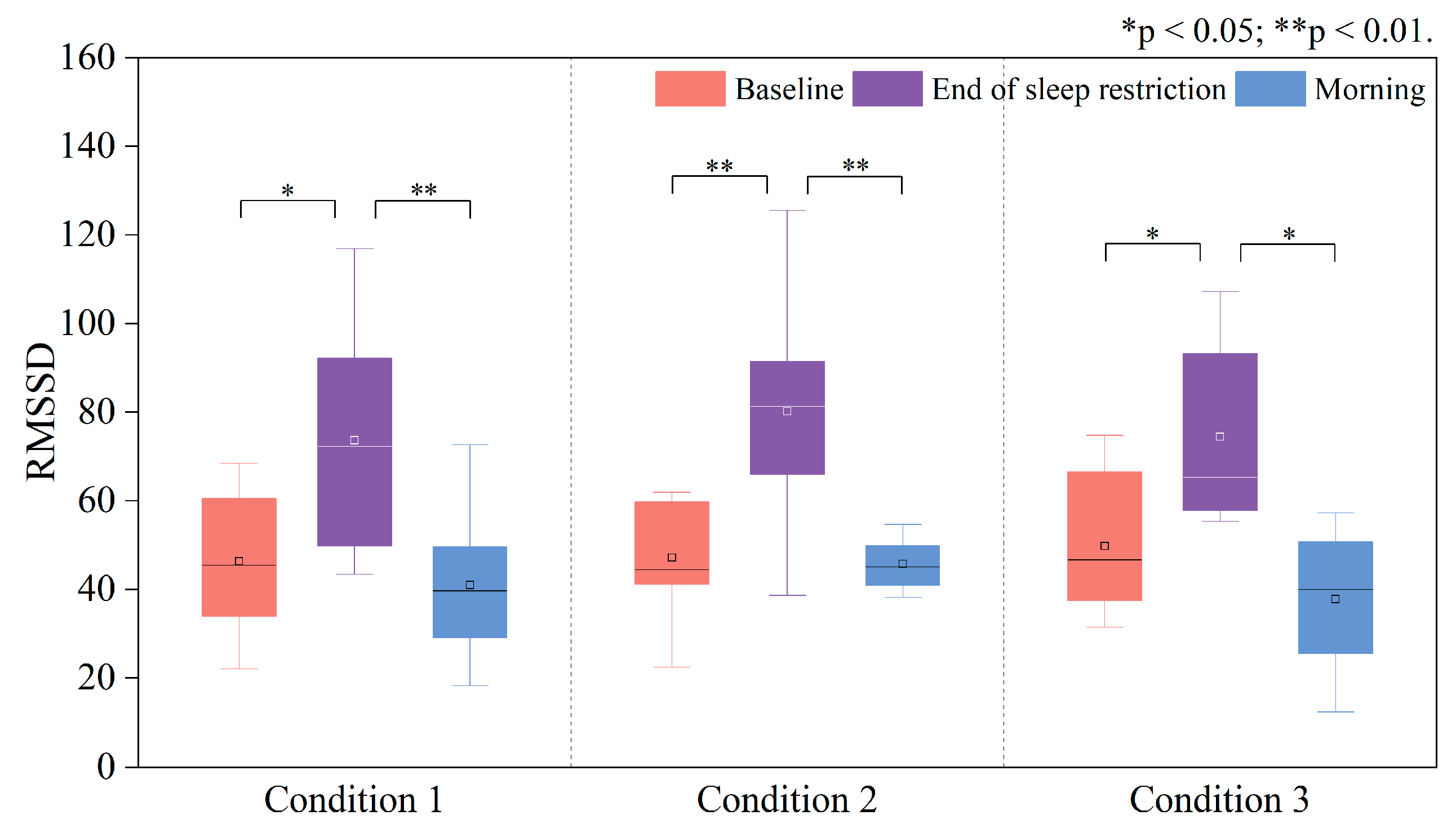
3.2. Changes in HRV Time-Domain Indices
3.3. Variations in Performance Index (PI) Under Three Sleep-Restriction Conditions
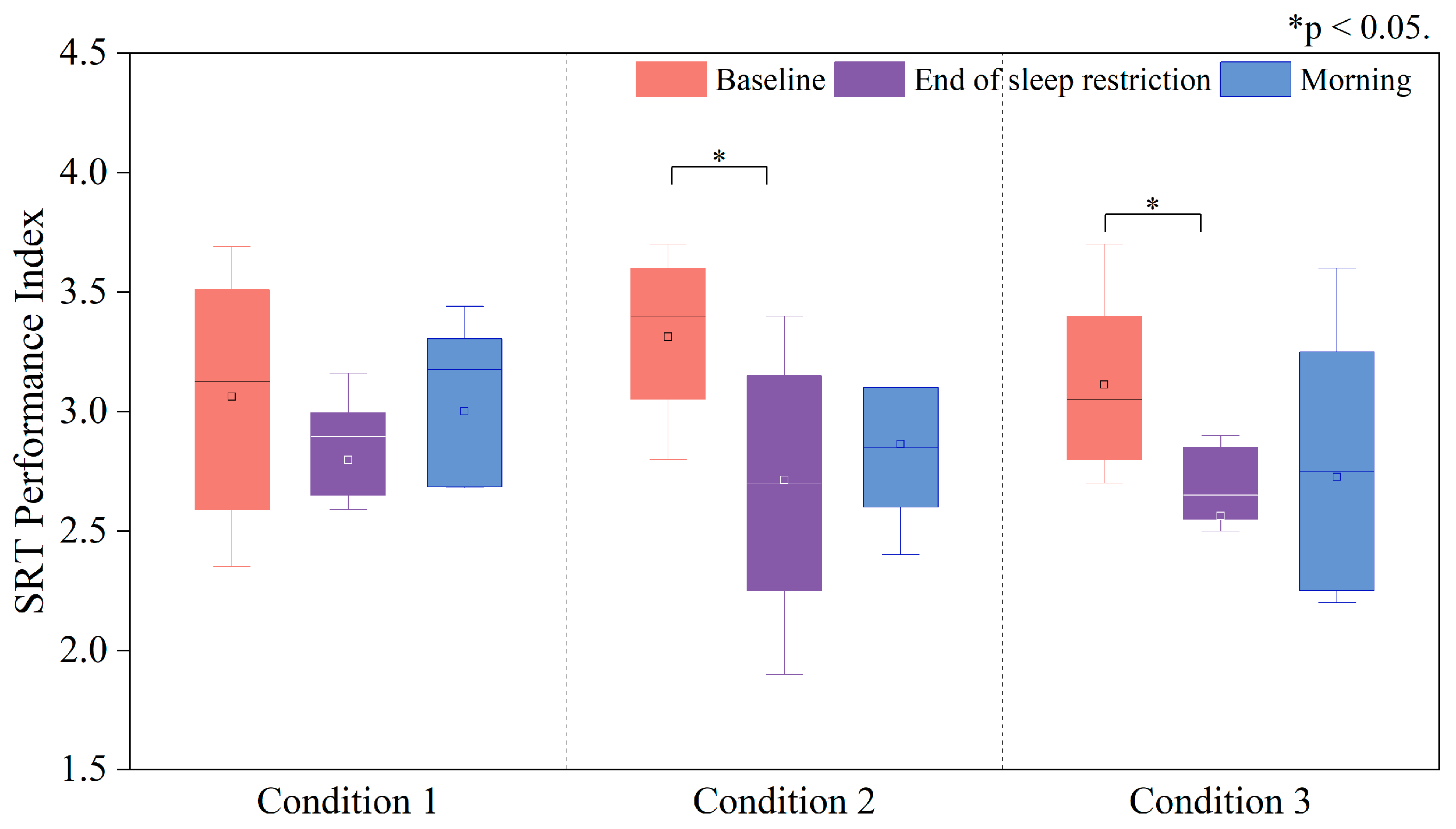
3.3.1. Changes in the PI of the Deary–Liewald Task
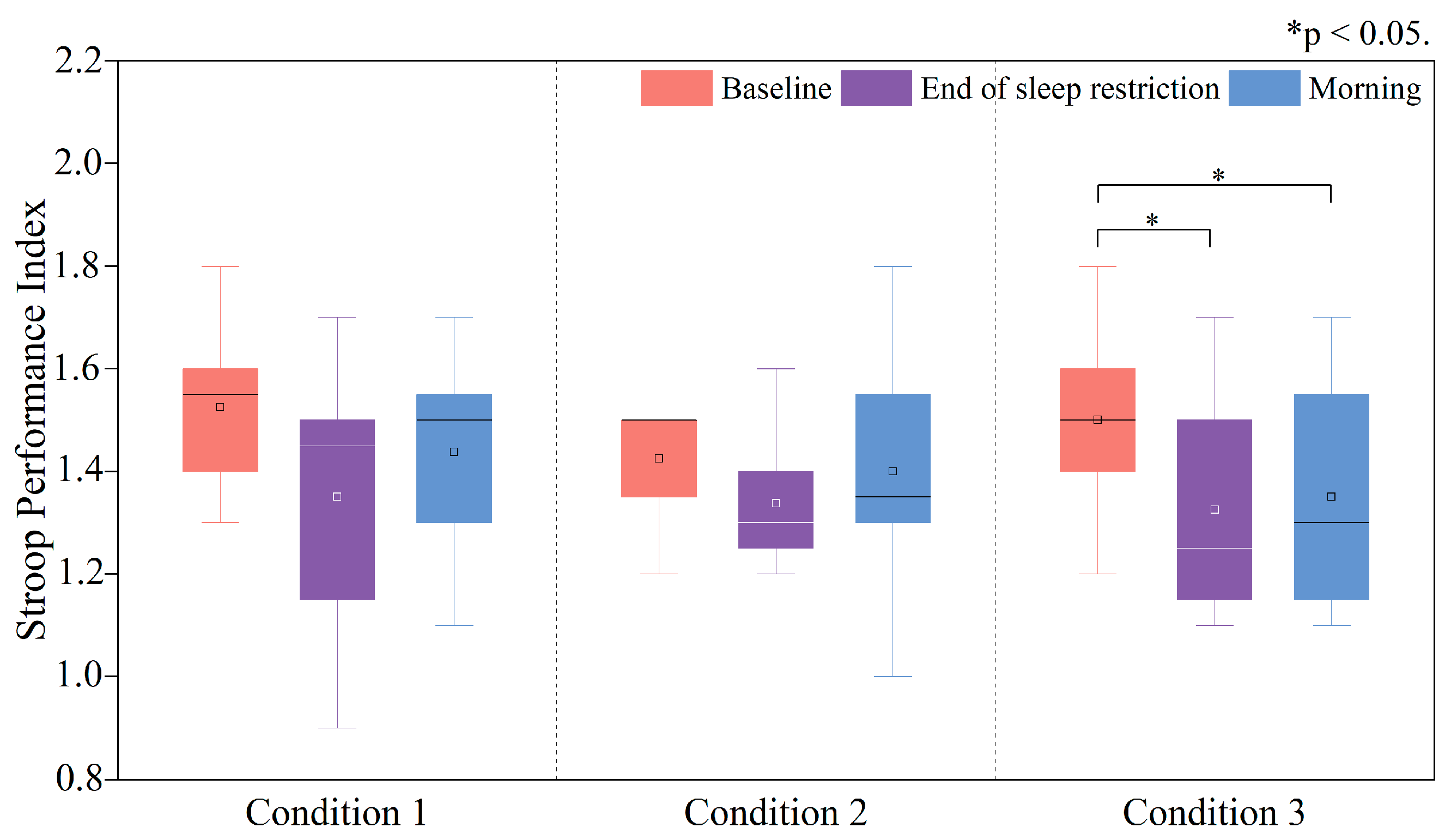
3.3.2. Changes in the PI of the Stroop Task
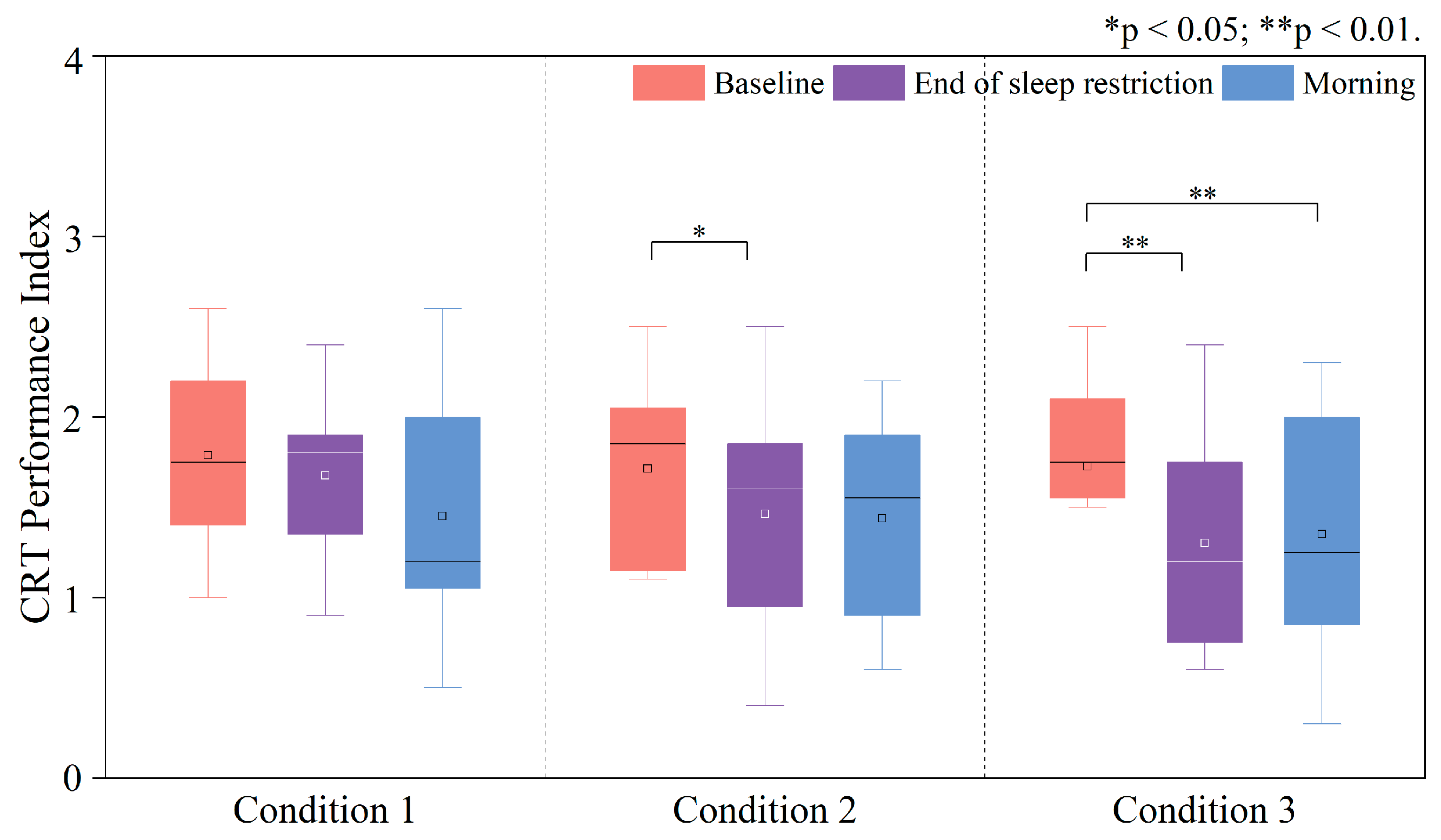
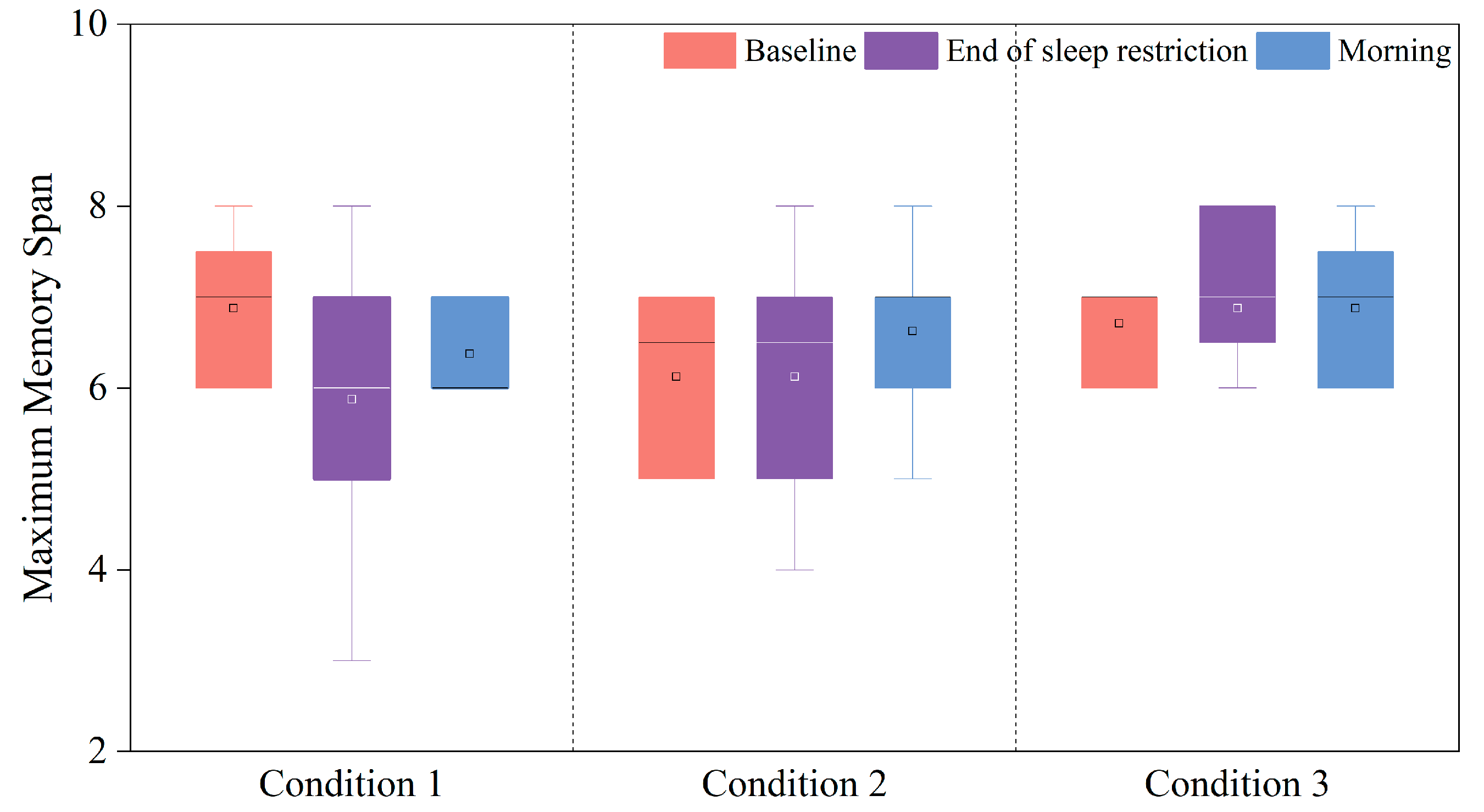
3.3.3. Changes in the PI of the Corsi Block Task
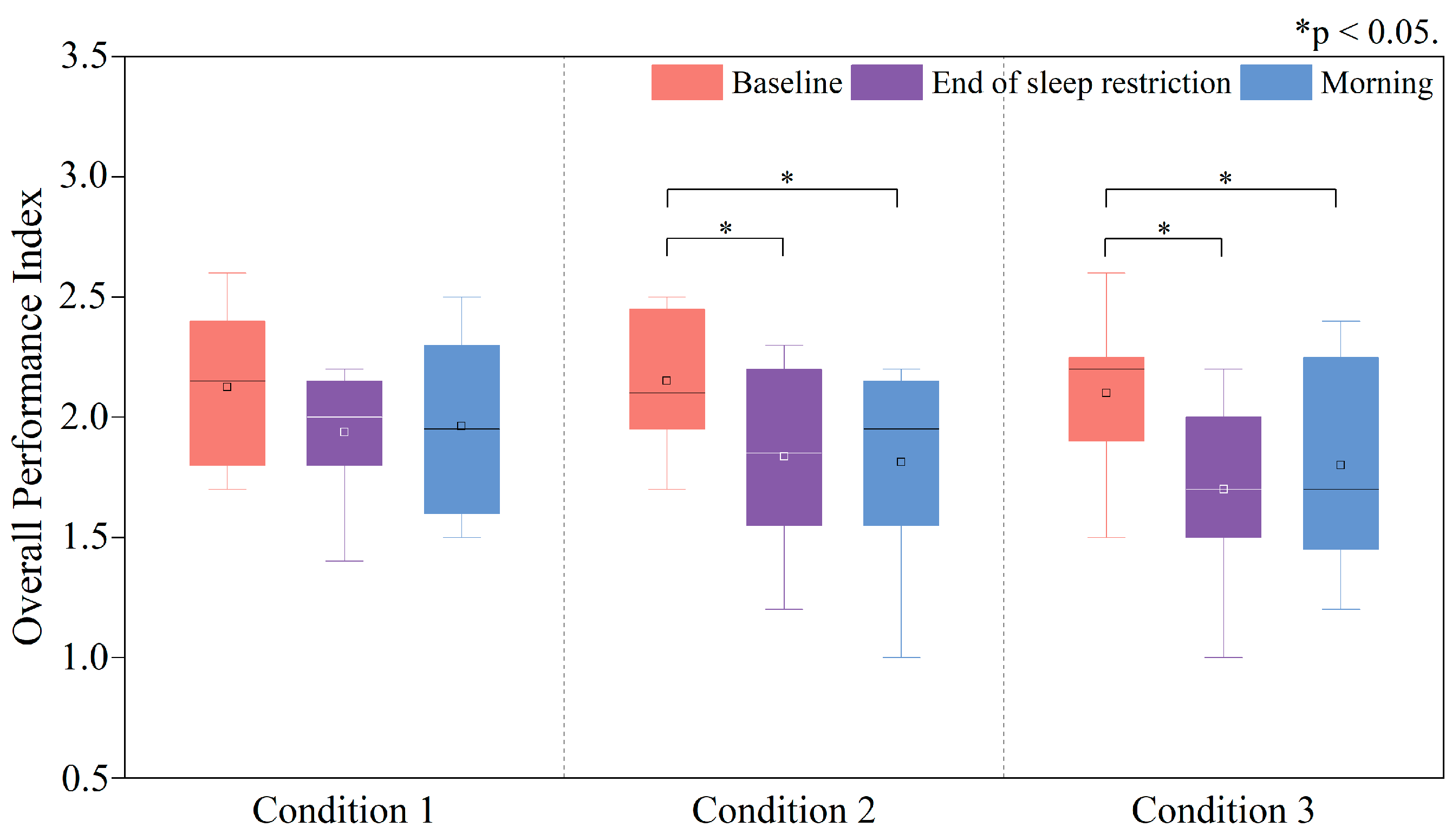
3.3.4. Changes in the Overall Performance Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, J. Research review on overtime issues. Mod. Manag. 2018, 8, 387–394. [Google Scholar] [CrossRef]
- Ministry of Human Resources and Social Security (MoHRSS). China Labor Statistical Yearbook 2019; China Statistics Press: Beijing, China, 2019; p. 140. [Google Scholar]
- Ministry of Health, Labour and Welfare. The 2022 White Paper on Measures to Prevent Karoshi; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2025.
- Dembe, A.E.; Erickson, J.B.; Delbos, R.G.; Banks, S.M. The impact of overtime and long work hours on occupational injuries and illnesses: New evidence from the United States. Occup. Environ. Med. 2005, 62, 588–597. [Google Scholar] [CrossRef]
- Walker, M.P. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008, 9, S29–S34. [Google Scholar] [CrossRef]
- Christian, M.S.; Ellis, A.P. Examining the effects of sleep deprivation on workplace deviance: A self-regulatory perspective. Acad. Manag. J. 2011, 54, 913–934. [Google Scholar] [CrossRef]
- Hennecke, E.; Lange, D.; Steenbergen, F.; Fronczek-Poncelet, J.; Elmenhorst, D.; Bauer, A.; Aeschbach, D.; Elmenhorst, E.M. Adverse interaction effects of chronic and acute sleep deficits on spatial working memory but not on verbal working memory or declarative memory. J. Sleep Res. 2021, 30, e13225. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.L.; Pires, G.N.; Tufik, S. The Impact of Sleep: From Ancient Rituals to Modern Challenges. Sleep Sci. 2024, 17, e203–e207. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Del Angel, J.; Borrani, J.; Ramirez, C.; Valdez, P. Sleep deprivation effects on basic cognitive processes: Which components of attention, working memory, and executive functions are more susceptible to the lack of sleep? Sleep Sci. 2021, 14, 107. [Google Scholar]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar]
- Chen, Y.; Pan, L.; Ma, N. Altered effective connectivity of thalamus with vigilance impairments after sleep deprivation. J. Sleep Res. 2022, 31, e13693. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.N.; Van Dongen, H.P.; Honn, K.A. Sleep deprivation, vigilant attention, and brain function: A review. Neuropsychopharmacology 2020, 45, 21–30. [Google Scholar] [CrossRef]
- Banks, S.; Jones, C.W.; McCauley, M.E.; Dorrian, J.; Basner, M.; Maislin, G.; Van Dongen, H.P.; Dinges, D.F. Long-term influence of sleep/wake history on the dynamic neurobehavioural response to sustained sleep restriction. J. Sleep Res. 2024, 33, e14117. [Google Scholar] [CrossRef]
- Albuquerque, P.M.; Franco, C.M.R.; Rocha-Filho, P.A.S. Assessing the impact of sleep restriction on the attention and executive functions of medical students: A prospective cohort study. Acta Neurol. Belg. 2023, 123, 1421–1427. [Google Scholar] [CrossRef]
- Ochab, J.K.; Szwed, J.; Oleś, K.; Bereś, A.; Chialvo, D.R.; Domagalik, A.; Fąfrowicz, M.; Ogińska, H.; Gudowska-Nowak, E.; Marek, T. Observing changes in human functioning during induced sleep deficiency and recovery periods. PLoS ONE 2021, 16, e0255771. [Google Scholar] [CrossRef]
- Skorucak, J.; Arbon, E.L.; Dijk, D.-J.; Achermann, P. Response to chronic sleep restriction, extension, and subsequent total sleep deprivation in humans: Adaptation or preserved sleep homeostasis? Sleep 2018, 41, zsy078. [Google Scholar] [CrossRef]
- Wiggins, E.; Mottarella, M.; Good, K.; Eggleston, S.; Stevens, C. 24-h sleep deprivation impairs early attentional modulation of neural processing: An event-related brain potential study. Neurosci. Lett. 2018, 677, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Tan, J.C.; Parimal, S.; Dinges, D.F.; Chee, M.W. Sleep deprivation impairs object-selective attention: A view from the ventral visual cortex. PLoS ONE 2010, 5, e9087. [Google Scholar] [CrossRef]
- Song, T.; Xu, L.; Peng, Z.; Wang, L.; Dai, C.; Xu, M.; Shao, Y.; Wang, Y.; Li, S. Total sleep deprivation impairs visual selective attention and triggers a compensatory effect: Evidence from event-related potentials. Cogn. Neurodyn. 2023, 17, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.W.; Silva, E.J.; Chang, A.-M.; Ronda, J.M.; Duffy, J.F. One night of sleep deprivation affects reaction time, but not interference or facilitation in a Stroop task. Brain Cogn. 2011, 76, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L.; Hughes, M.E.; Croft, R.J.; Howard, M.E.; Crewther, D.; Kennedy, G.A.; Owens, K.; Pierce, R.J.; O’Donoghue, F.J.; Johnston, P. The effect of sleep deprivation on BOLD activity elicited by a divided attention task. Brain Imaging Behav. 2011, 5, 97–108. [Google Scholar] [CrossRef]
- Chua, E.C.-P.; Fang, E.; Gooley, J.J. Effects of total sleep deprivation on divided attention performance. PLoS ONE 2017, 12, e0187098. [Google Scholar] [CrossRef]
- Buchweitz, A. Models of working memory: Mechanisms of active maintenance and executive control (1999). Ilha Desterro 2002, 43, 193–200. [Google Scholar]
- Frenda, S.J.; Fenn, K.M. Sleep less, think worse: The effect of sleep deprivation on working memory. J. Appl. Res. Mem. Cogn. 2016, 5, 463–469. [Google Scholar] [CrossRef]
- Gohar, A.; Adams, A.; Gertner, E.; Sackett-Lundeen, L.; Heitz, R.; Engle, R.; Haus, E.; Bijwadia, J. Working memory capacity is decreased in sleep-deprived internal medicine residents. J. Clin. Sleep Med. 2009, 5, 191–197. [Google Scholar] [CrossRef]
- Mathew, G.; Strayer, S.; Ness, K.; Buxton, O.; Chang, A. Vulnerability to sleep restriction is associated with decreased working memory performance. Sleep 2020, 43 (Suppl. 1), A34–A35. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Ou, S.; Jiang, T.; Wang, L.; Ma, N. The effect of total sleep deprivation on working memory: Evidence from diffusion model. Sleep 2024, 47, zsae006. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P. The nature and organization of individual differences in executive functions: Four general conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- Latibeaudiere, A.; Butler, S.; Owens, M. Decision-making and performance in the Iowa Gambling Task: Recent ERP findings and clinical implications. Front. Psychol. 2025, 16, 1492471. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Renlai, Z. Effect of 72 h of sleep deprivation on the Iowa Gambling Task. Nöropsikiyatri Arş. 2016, 53, 357. [Google Scholar]
- Salfi, F.; Lauriola, M.; Tempesta, D.; Calanna, P.; Socci, V.; De Gennaro, L.; Ferrara, M. Effects of total and partial sleep deprivation on reflection impulsivity and risk-taking in deliberative decision-making. Nat. Sci. Sleep 2020, 12, 309–324. [Google Scholar] [CrossRef]
- Herscovitch, J.; Stuss, D.; Broughton, R. Changes in cognitive processing following short-term cumulative partial sleep deprivation and recovery oversleeping. J. Clin. Exp. Neuropsychol. 1980, 2, 301–319. [Google Scholar] [CrossRef]
- Khazaie, H.; Tahmasian, M.; Ghadami, M.R.; Safaei, H.; Ekhtiari, H.; Samadzadeh, S.; Schwebel, D.C.; Russo, M.B. The effects of chronic partial sleep deprivation on cognitive functions of medical residents. Iran. J. Psychiatry 2010, 5, 74–79. [Google Scholar] [PubMed]
- Couyoumdjian, A.; Sdoia, S.; Tempesta, D.; Curcio, G.; Rastellini, E.; De Gennaro, L.; Ferrara, M. The effects of sleep and sleep deprivation on task-switching performance. J. Sleep Res. 2010, 19, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Finelli, L.; Baumann, H.; Borbély, A.; Achermann, P. Dual electroencephalogram markers of human sleep homeostasis: Correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience 2000, 101, 523–529. [Google Scholar] [CrossRef]
- Habeck, C.; Rakitin, B.C.; Moeller, J.; Scarmeas, N.; Zarahn, E.; Brown, T.; Stern, Y. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Cogn. Brain Res. 2004, 18, 306–321. [Google Scholar] [CrossRef]
- Harrison, Y.; Horne, J.A. The impact of sleep deprivation on decision making: A review. J. Exp. Psychol. Appl. 2000, 6, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Harrison, Y.; Horne, J.A. One night of sleep loss impairs innovative thinking and flexible decision making. Organ. Behav. Hum. Decis. Process. 1999, 78, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Harrison, Y.; Horne, J. Sleep loss impairs short and novel language tasks having a prefrontal focus. J. Sleep Res. 1998, 7, 95–100. [Google Scholar] [CrossRef]
- Massar, S.A.; Lim, J.; Huettel, S.A. Sleep deprivation, effort allocation and performance. Prog. Brain Res. 2019, 246, 1–26. [Google Scholar]
- Tucker, A.M.; Whitney, P.; Belenky, G.; Hinson, J.M.; Van Dongen, H.P. Effects of sleep deprivation on dissociated components of executive functioning. Sleep 2010, 33, 47–57. [Google Scholar] [CrossRef]
- Kheloui, S.; Jacmin-Park, S.; Larocque, O.; Kerr, P.; Rossi, M.; Cartier, L.; Juster, R.P. Sex/gender differences in cognitive abilities. Neurosci. Biobehav. Rev. 2023, 152, 105333. [Google Scholar] [CrossRef]
- ISO 7730:2005; Ergonomics of the Thermal Environment—Analytical Determination and Interpretation of Thermal Comfort Using Calculation of the PMV and PPD Indices and Local Thermal Comfort Criteria. ISO: Geneva, Switzerland, 2005.
- ISO/CIE 8995-1:2025; Light and Lighting—Lighting of Work Places—Part 1: Indoor. ISO: Geneva, Switzerland, 2025.
- ANSI/ASHRAE Standard 55-2023; Thermal Environmental Conditions for Human Occupancy. ASHRAE: Atlanta, GA, USA, 2023.
- ISO 17772-1:2017; Energy Performance of Buildings—Indoor Environmental Quality—Part 1: Indoor Environmental Input Parameters for the Design and Assessment of Energy Performance of Buildings. ISO: Geneva, Switzerland, 2017.
- Stoet, G. PsyToolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach. Psychol. 2017, 44, 24–31. [Google Scholar] [CrossRef]
- Koelblen, B.; Psikuta, A.; Bogdan, A.; Annaheim, S.; Rossi, R. Thermal sensation models: A systematic comparison. Indoor Air 2017, 27, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Hoddes, E.; Zarcone, V.; Smythe, H.; Phillips, R.; Dement, W.C. Quantification of sleepiness: A new approach. Psychophysiology 1973, 10, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of empirical and theoretical research. In Advances in Psychology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 52, pp. 139–183. [Google Scholar]
- Herlambang, M.B.; Cnossen, F.; Taatgen, N.A. The effects of intrinsic motivation on mental fatigue. PLoS ONE 2021, 16, e0243754. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Hsu, H.-T. Mental fatigue measurement using EEG. In Risk Management Trends; IntechOpen: London, UK, 2011. [Google Scholar]
- Forte, G.; Favieri, F.; Casagrande, M. Heart rate variability and cognitive function: A systematic review. Front. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef]
- Deary, I.J.; Liewald, D.; Nissan, J. A free, easy-to-use, computer-based simple and four-choice reaction time programme: The Deary-Liewald reaction time task. Behav. Res. Methods 2011, 43, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Golden, C. Stroop, Test de Colores y Palabras; Tea Ediciones: Madrid, Spain, 1994. [Google Scholar]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain; McGill University: Montreal, QC, Canada, 1972. [Google Scholar]
- Whitney, P.; Hinson, J.M.; Satterfield, B.C.; Grant, D.A.; Honn, K.A.; Van Dongen, H.P. Sleep deprivation diminishes attentional control effectiveness and impairs flexible adaptation to changing conditions. Sci. Rep. 2017, 7, 16020. [Google Scholar] [CrossRef]
- Lan, L.; Wargocki, P.; Lian, Z. Quantitative measurement of productivity loss due to thermal discomfort. Energy Build. 2011, 43, 1057–1062. [Google Scholar] [CrossRef]
- Paech, G.M.; Ferguson, S.A.; Sargent, C.; Kennaway, D.J.; Roach, G.D. The Relative Contributions of the Homeostatic and Circadian Processes to Sleep Regulation under Conditions of Severe Sleep Restriction. Sleep 2012, 35, 941–948. [Google Scholar] [CrossRef]
- Kontou, T.G.; Roach, G.D.; Sargent, C. Mild to moderate sleep restriction does not affect the cortisol awakening response in healthy adult males. Clocks Sleep 2022, 4, 722–734. [Google Scholar] [CrossRef]
- Wickens, C.D. Multiple resources and performance prediction. Theor. Issues Ergon. Sci. 2002, 3, 159–177. [Google Scholar] [CrossRef]
- Middleton, E.L.; Schwartz, M.F.; Dell, G.S.; Brecher, A. Learning from errors: Exploration of the monitoring learning effect. Cognition 2022, 224, 105057. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar]
- Chua, E.C.; Tan, W.Q.; Yeo, S.C.; Lau, P.; Lee, I.; Mien, I.H.; Puvanendran, K.; Gooley, J.J. Heart rate variability can be used to estimate sleepiness-related decrements in psychomotor vigilance during total sleep deprivation. Sleep 2012, 35, 325–334. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Azarbarzin, A.; Ostrowski, M.; Hanly, P.; Younes, M. Relationship between arousal intensity and heart rate response to arousal. Sleep 2014, 37, 645–653. [Google Scholar] [CrossRef]
- Srinivasan, A.G.; Smith, S.S.; Pattinson, A.L.; Mann, D.; Sullivan, K.; Salmon, P.; Soleimanlooa, S.S. Heart rate variability as an indicator of fatigue: A structural equation model approach. Transp. Res. Part F Psychol. Behaiour 2024, 103, 420–429. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Liao, Q.; Ukai, M.; Zhang, F.; Hu, S. Cognitive performances during sleep restrictions in thermoneutral indoor environments. Energy Build. 2025, 346, 116163. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Wang, Y.; Su, H.; Yu, C.W. Cognitive performance in the heat: A prediction with heart rate variability (HRV) time-domain indices. Indoor Built Environ. 2025, 34, 319–329. [Google Scholar] [CrossRef]
- Hick, W.E. On the rate of gain of information. Q. J. Exp. Psychol. 1952, 4, 11–26. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Dinges, D.F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 2010, 136, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.W.; Choo, W.C. Functional imaging of working memory after 24 hr of total sleep deprivation. J. Neurosci. 2004, 24, 4560–4567. [Google Scholar] [CrossRef] [PubMed]


| Metric | Physiological/Psychological Significance | Reason(s) | |
|---|---|---|---|
| TSV | Reflects subjective perception of environmental temperature | Continuous monitoring of thermal sensation to simulate the physical environment during real overtime work during the night [48] | |
| SSS | Quantifies momentary sleepiness levels (1–7 scale) | Validated for sleep-deprivation studies to capture transitions from alertness to near-sleep states [49] | |
| NASA-TLX | Multi-dimensional assessment of perceived workload (six weighted dimensions) | Evaluates mental, physical, and temporal demands to quantify the perceived workload [50,51,52] | |
| HRV | Heart Rate RMSSD | Indicates the dynamic balance of the autonomic nervous system | Links sympathetic–parasympathetic modulation to cognitive functions (e.g., working memory, sustained attention) [53] |
| Task Type | Cognitive Functions | Duration | |
|---|---|---|---|
| Deary–Liewald Task [54] | Simple Response Time task | Sustained Attention | 3.5 min |
| Choice Response Time task | Selective Attention | ||
| Stroop Task [55] | Inhibitory Control, Working memory, Executive function | 3 min | |
| Corsi Block Task [56] | Spatial working memory, Short-term Memory | 3.5 min | |
| Cognitive Functions | Mild SR (Before 1:20 a.m.) | Moderate SR (Before 2:30 a.m.) | Severe SR (Before 3:40 a.m.) | |||
|---|---|---|---|---|---|---|
| End of SR | Morning | End of SR | Morning | End of SR | Morning | |
| Sustained attention | → | → | ↓* | ↓ | ↓* | ↓ |
| Selective attention | → | ↓ | ↓* | ↓ | ↓* | ↓* |
| Working memory, Executive function, Inhibitory control | ↓ | → | ↓ | → | ↓* | ↓* |
| Short-term memory | ↓ | → | → | ↑ | → | → |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Yang, D.; Liao, Q.; Yuan, D.; Zhang, F.; Ukai, M.; Ma, L. Cognitive Recovery of Young Males in Thermoneutral Indoor Environments: Effects of Sleep Restrictions. Buildings 2025, 15, 3021. https://doi.org/10.3390/buildings15173021
Zhu H, Yang D, Liao Q, Yuan D, Zhang F, Ukai M, Ma L. Cognitive Recovery of Young Males in Thermoneutral Indoor Environments: Effects of Sleep Restrictions. Buildings. 2025; 15(17):3021. https://doi.org/10.3390/buildings15173021
Chicago/Turabian StyleZhu, Hui, Duo Yang, Quanna Liao, Da Yuan, Fan Zhang, Masanari Ukai, and Le Ma. 2025. "Cognitive Recovery of Young Males in Thermoneutral Indoor Environments: Effects of Sleep Restrictions" Buildings 15, no. 17: 3021. https://doi.org/10.3390/buildings15173021
APA StyleZhu, H., Yang, D., Liao, Q., Yuan, D., Zhang, F., Ukai, M., & Ma, L. (2025). Cognitive Recovery of Young Males in Thermoneutral Indoor Environments: Effects of Sleep Restrictions. Buildings, 15(17), 3021. https://doi.org/10.3390/buildings15173021








